Abstract
A 3-year-old female presented with splenomegaly and hypersplenism manifestations, including anemia, thrombocytopenia, and abdominal pain/distention. All common metabolic, hematologic, gastrointestinal, and infectious etiologies for splenomegaly were excluded. Diagnosis of idiopathic splenic peliosis was made and splenectomy was recommended. History revealed that the patient's mother had acquired a nonspecific skin infection during a visit to the Middle East 2 years prior. Serum antibodies and confirmatory PCR testing for visceral leishmaniasis were positive. After treatment with liposomal amphotericin, at 6-month follow-up her hypersplenism manifestations had resolved and her splenomegaly had significantly decreased. Visceral leishmaniasis should be considered in cases of marked splenomegaly, anemia, and thrombocytopenia, especially with a history of visiting an endemic area.
Introduction
Leishmaniasis is a neglected tropical disease (NTD) that is rarely encountered in the United States; therefore, it is not considered a common cause of splenomegaly. There are several clinical presentations of this parasitic infection. The most common is cutaneous and characterized by skin sores or visceral leishmaniasis that affects internal organs (usually spleen, liver, and bone marrow) and presents with systemic manifestations such as fever, lymphadenopathy, and hepatosplenomegaly. People from unaffected areas may contract the disease after visiting endemic areas, so detailed history can be very helpful in making the diagnosis.
Case Report
A 3-year-old Caucasian female was referred for evaluation of splenomegaly. History revealed that symptoms started 6 months ago with a febrile illness, cough, mouth sores, cervical lymphadenopathy, and splenomegaly. A bacterial infection was suspected, for which she was prescribed a course of amoxicillin and cefdinir, followed by azithromycin. Antibiotics resulted in resolution of the fever and malaise, but the splenomegaly persisted. Her splenomegaly progressed causing abdominal distention, pain, and evidence of hypersplenism (anemia and thrombocytopenia).
Initial evaluations for infectious agents such as HIV, CMV, and EBV, and proliferative etiologies such as leukemia or metastatic malignancy were negative. Gastrointestinal and metabolic causes were also excluded. Imaging by ultrasonography and magnetic resonance radiography showed mildly prominent liver with diffuse multiple hypoechoic lesions and a markedly enlarged spleen with non-enhancing structures at the splenic hilum (Figure 1). Due to these findings, a diagnosis of splenic peliosis was considered and a splenic biopsy with splenectomy proposed. A second opinion was sought at our institution.
Figure 1.
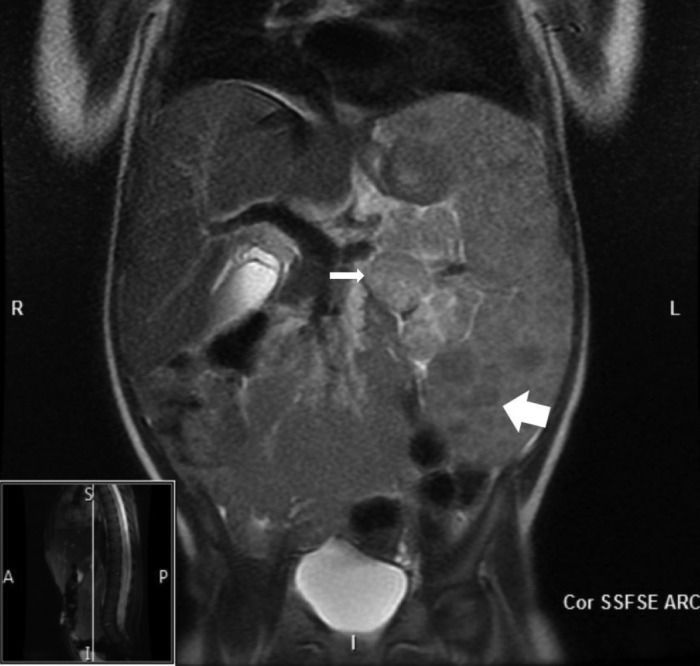
MR abdomen demonstrates marked splenomegaly with diffusely low intensity lesions (thick arrow). Rounded soft tissue structures at the splenic hilum containing numerous low intensity lesions (thin arrow).
On presentation, the patient was playful without signs of distress. Physical exam was normal except for distended abdomen, splenomegaly extending to the superior iliac crest, and an umbilical hernia. Outside evaluation excluded hemolytic etiology with normal hemolytic anemia panel, hemoglobin electrophoresis, red blood cell enzymes, and osmotic fragility tests. Malignant infiltration was excluded by bone marrow aspirate/biopsy and brain/spine MRI. Medical genetics work-up excluded metabolic etiology with normal karyotype 46, XX, urine/serum organic acids, serum amino acids, plasma acylcarnitine profile, and arylsulfatase B activity (a test for mucopolysaccharidosis VI). A lysosomal disease panel was normal. Immune deficiency disorders and other infectious etiologies like Toxocara, Brucella, Bartonella, and M. tuberculosis were excluded. Finally, portal hypertension and splenic vein thrombosis were excluded by a Doppler abdominal ultrasound (US) showing patency of vessels. The US also confirmed splenomegaly with diffuse hypoechoic lesions (Figure 2).
Figure 2.
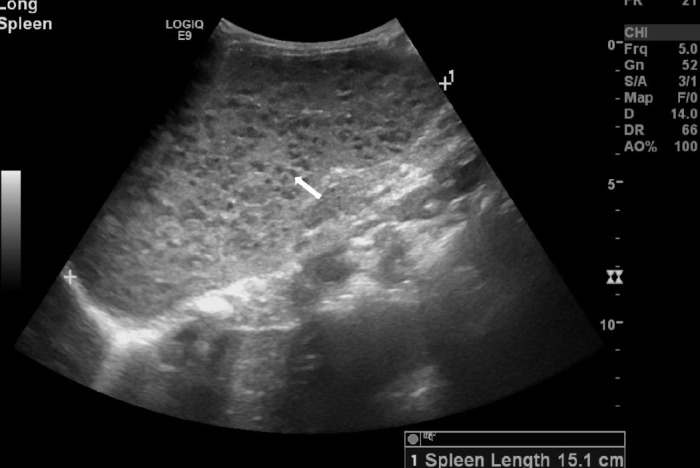
Splenomegaly with diffuse innumerable hypoechoic foci.
Further detail regarding travel history revealed that the patient visited Aleppo, Syria, and Dubai, United Arab Emirates, about 2 years ago. At that time, the patient's mother had a nonspecific skin infection that resolved spontaneously. The area they visited in the Middle East is endemic for Leishmania. Therefore, serological testing for Leishmania antibodies was performed and was positive. Confirmation with PCR testing was performed and a diagnosis of visceral leishmaniasis was made. Our patient was treated with liposomal amphotericin B with rapid reduction in her spleen size. Her energy level improved and her hypersplenism manifestations (anemia and thrombocytopenia) resolved.
Discussion
Peliosis is a rare condition characterized by multiple cystlike, blood-filled spaces within the parenchyma of solid organ. Isolated splenic peliosis is a unique phenomenon most commonly affecting the liver and, less frequently, the spleen and other hematolymphoid organs. Patients with this condition are often asymptomatic. However, it can be a lethal condition if spontaneous organ rupture occurs.1 Studies showed that peliosis can be due to injury of endothelial lining of sinusoids. Factors involved in peliosis are malignancy, tuberculosis, acquired immune deficiency, diabetes, drugs and parasites.2–4
Splenic peliosis is usually found incidentally by finding splenomegaly on routine physical examination or by laboratory work showing changes consistent with hypersplenism. In the United States splenomegaly in children is most commonly due to the body's response to different infectious agents. Other common etiologies include disorders of immune regulation or abnormal destruction of red blood cells. Less common etiologies include infiltration with neoplastic diseases, storage diseases, portal hypertension, or space-occupying lesions, which also must be considered. The differential diagnosis includes liver fibrosis, abnormal blood flow due to splenic vein thrombosis or portal vein obstruction, and liver cirrhosis due to chronic viral hepatitis B or C, fatty liver, and alcohol use. All the common etiologies were excluded in our patient, but her travel history triggered an extended work-up to investigate parasitic etiologies such as Leishmania, which are otherwise unusual to the United States.
Leishmaniasis is a parasitic disease endemic to the tropics, subtropics, and southern Europe. It is classified as a neglected tropical disease (NTD). Leishmaniasis is caused by infection with Leishmania parasites, which are spread by phlebotomine sand flies. Leishmaniasis is rarely encountered in the United States; therefore, it is not considered a common cause of splenomegaly. There are several clinical presentations of this parasitic infection. The most common is cutaneous and characterized by skin sores or visceral leishmaniasis, also known as Kala-azar. This also affects internal organs (usually spleen, liver, and bone marrow) and presents with systemic manifestations such as fever, lymph-adenopathy, and hepatosplenomegaly.5,6 People from unaffected areas may contract the disease after visiting endemic areas. Factors that increase the risk of clinical development of the disease include malnutrition, immunosuppression, and HIV infection.7 The clinical presentation of visceral leishmaniasis lacks specificity, thus the diagnosis is based on a high index of suspicion and serological and PCR confirmatory testing. The best diagnostic test is organism isolation from the patient tissue (lymph node or spleen). The risk of bleeding from a splenic biopsy may preclude the need for organism isolation and therefore reliance on antibody testing is safer. A 30% false positive rate of antibody testing necessitates confirmation with PCR for organism identification. A correct diagnosis is essential to avoid administration of potentially toxic therapeutic agents.8,9
Conclusion
The diagnosis of visceral leishmaniasis was made in this setting due to the careful history of travel. As human population travel and migration increase worldwide, it is essential to consider infection by unusual organisms from endemic areas in the differential diagnosis of splenomegaly and hypersplenism.
Disclosures
Author contributions: Y. Ghazzawi gathered data and completed the initial draft. I. Absah reviewed the paper, completed the literature search and citations, and is the article guarantor.
Financial disclosure: Neither author received support for this article, and neither has financial disclosures to report.
References
- 1.Celebrezze JP Jr, Cottrell DJ, Williams GB. Spontaneous splenic rupture due to isolatedsplenic peliosis. South Med J. 1998;91(8):763. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos M, Püschel K. Isolated peliosis of the spleen: Report of 2 autopsy cases. Am J For Med Pathol. 2004;25(3):251–4. [DOI] [PubMed] [Google Scholar]
- 3.Diebold J, Audouin J. Peliosis of the spleen. Report of a case associated with chronic myelomonocytic leukemia, presenting with spontaneous splenic rupture. Am J Surg Pathol. 1983;7(2):197–204. [PubMed] [Google Scholar]
- 4.Garcia RL, Khan MK, Berlin RB. Peliosis of the spleen with rupture. Hum Pathol. 1982;13(2):177–9. [DOI] [PubMed] [Google Scholar]
- 5.Desjeux P. Human leishmaniases: Epidemiology and public health aspects. World Health Stat Q. 1992;45(2-3):267–75. [PubMed] [Google Scholar]
- 6.Guerin PJ, Olliaro P, Sundar S, et al. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2(8):494–501. [DOI] [PubMed] [Google Scholar]
- 7.Murray HW. Kala-Azar: Progress against a neglected disease. N Engl J Med. 2002;347(22):1793–1794. [DOI] [PubMed] [Google Scholar]
- 8.Chappuis F, Sundar S, Hailu A, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–82. [DOI] [PubMed] [Google Scholar]
- 9.Buyukasik Y, Ileri NS, Haznedaroglu IC, et al. Fever, hepatosplenomegaly and pancytopenia in a patient living in the Mediterranean region. Postgrad Med J. 1998;74(870):237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


