Abstract
Inflammatory fibroid polyps (IFPs) of the colon are very rare, reactive, non-neoplastic polyps that may grow to large sizes but do not carry any risk of malignancy. Because of their size, IFPs are usually treated with surgery; however, size alone should not be an indication for surgery. Depending on the location and morphology of the polyp, endoscopic resection should be considered. We here describe a case of a giant IFP that was successfully removed with endoscopy without complication or recurrence.
Introduction
Inflammatory fibroid polyps (IFPs) are rare, reactive non-neoplastic lesions with no documented malignant potential that involve the stomach (70%), the small intestine (20%), and, rarely, the colon.1 They usually contain blood vessels, fibroblasts, and an edematous stroma rich in eosinophils.2 Signs and symptoms depend mostly on their location and size. Bleeding, obstruction, or abdominal pain are potential manifestations of IFP. The diagnosis is usually made by imaging studies or endoscopy. Treatment options include surgical excision, in most cases, and endoscopic mucosal resection (Table 1).
Table 1.
Summary and Clinicopathological Features of Previously Reported Cases of Colonic IFP in the English Literature
| Case No. | Age, y | Sex | Location | Gross Description | Treatment | Ref | Year |
|---|---|---|---|---|---|---|---|
| 1 | 79 | M | Cecum | Less than 1 cm | None | 4 | 1952 |
| 2 | 37 | M | Cecum | 6.5 cm, pedunculated | Surgery | 16 | 1955 |
| 3 | 67 | M | Cecum | 3.5 cm, pedunculated | Surgery | 17 | 1960 |
| 4 | 4 | M | Transverse | 3.5 cm, pedunculated | Surgery | 18 | 1966 |
| 5 | 56 | M | Cecum | 7 cm | Surgery | 19 | 1977 |
| 6 | 69 | M | Transverse | 5 cm, pedunculated | Surgery | 20 | 1979 |
| 7 | 51 | M | Sigmoid | 3 cm, pedunculated, ulcer | Surgery | 9 | 1979 |
| 8 | 24 | M | Transverse | 5 cm | Surgery | 21 | 1983 |
| 9 | 8 | M | Rectum | 3 cm sessile | Surgery | 22 | 1984 |
| 10–14 | NS | NS | 4 Cecum 1 Ascending |
1.5–4 cm | 1 Cecum endopscopic 1 Ascending and 3 cecum surgery |
23 | 1984 |
| 15 | 71 | M | Cecum | 4 cm, pedunculated | Endoscopic | 24 | 1985 |
| 16 | 42 | M | Cecum | 3.5 cm | Surgery | 25 | 1992 |
| 17–20 | 24–72 | 3 M, 1 F | 3 Transverse 1 Cecum |
3.6–5 cm 2 pedunculated, 2 sessile |
NS | 26 | 1992 |
| 21 | 33 | F | Descending | 4 cm, pedunculated | Surgery | 27 | 1995 |
| 22 | 63 | M | Ascending | 3.5 cm, sessile, ulcer | Surgery | 28 | 1999 |
| 23 | 45 | F | Cecum | 0.5 cm, sessile, erosive | Endoscopic | 29 | 2000 |
| 24 | 66 | M | Cecum | 3.5 cm, sessile | Surgery | 30 | 2004 |
| 25 | 40 | M | Ascending | 3 cm, pedunculated | Endoscopic | 2 | 2005 |
| 26 | 45 | M | Transverse | 1.8 cm, depressed | Surgery | 31 | 2006 |
| 27 | 82 | M | Transverse | 0.6 cm, pedunculated | None | 32 | 2007 |
| 28 | 28 | M | Sigmoid | 4 cm, pedunculated | Endoscopic | 33 | 2007 |
| 29 | 23 | F | Descending | 4.5 cm, pedunculated, erosive | Endoscopic | 10 | 2008 |
| 30 | 66 | F | Cecum | 3 cm, sessile, ulcer | Endoscopic | 34 | 2008 |
| 31 | 63 | F | Cecum | 4 cm, pedunculated | Surgery | 35 | 2008 |
| 32 | 83 | M | Descending | 7 cm, pedunculated | Endoscopy | * | 2011 |
The case described in this article. NS = not specified.
Case Report
An 83-year-old male with a history of diabetes mellitus and coronary artery disease presented with intermittent hematochezia and abdominal pain for 6 months. Physical examination was unremarkable. Initial lab work showed a hemoglobin of 8.6 g/dL. CT scan of abdomen revealed a hypodense heterogeneous mass within the proximal descending colon with low internal density (Figure 1A). No other masses or significant lymphadenopathy were seen. Colonoscopy was performed and a giant 7-cm polypoid mass with a wide stalk and yellowish surface, nearly obstructing the lumen of the descending colon, was found (Figure 1B). Biopsies revealed an inflamed submucosal stroma with granulation tissue denuded of epithelium. Neither malignant cells nor features of an adenoma were present. Serum CEA was 1.3 ng/mL. Surgical resection was declined by the patient. He underwent a repeated colonoscopy with snare polypectomy. Despite using the largest snare available, we were unable to encircle the polyp due to its size, so it was removed in a piecemeal fashion and the site was tattooed (Figure 1C). Gross pathologic examination showed a 7.0 × 4.0 × 3.0 cm partially infarcted polypoid mass (Figure 1D). Microscopic examination revealed a polypoid lesion with a prominent fibrotic submucosal stroma, blood vessels, and scattered eosinophils. Immunostains performed on tumor tissue sections were positive for CD34 and negative for S-100 protein, desmin, and CD117 (Figure 2A). The findings were those of IFP. The patient tolerated the procedure well and a follow-up colonoscopy 7 months later did not show recurrence.
Figure 1.
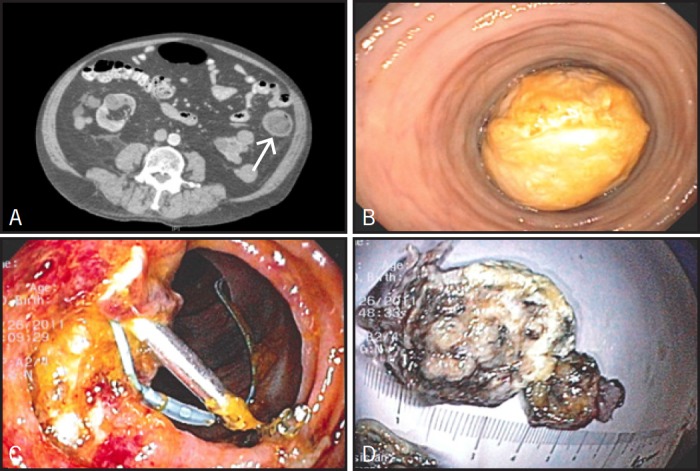
(A) CT scan showing large hypodense hetereogenous mass measuring 5 cm (arrow), occupying most of the descending colon. (B) Endoscopic view of the large colonic mass obstructing the lumen of the descending colon. (C) Endoscopic view of the post polypectomy site (endoloop and endoclips seen at the site). (D) Gross view of the colonic mass after endoscopic resection.
Figure 2.
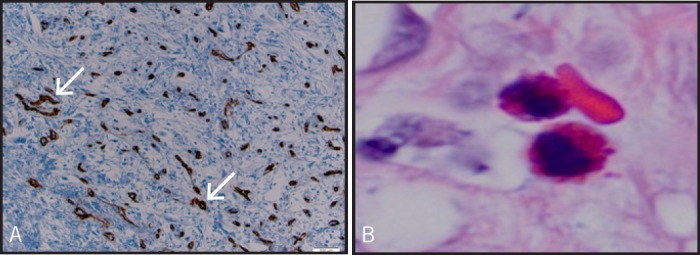
(A) Intermediate power view of the inflammatory fibroid polyp showing the rich vascular supply (arrows) highlighted by a CD34 immunostain. Tissue section. CD 34 immunostain. Original magnification ×100. (B) Eosinophils within the stroma. Inflammatory fibroid polyp. Tissue section, hematoxylin, and eosin; original magnification ×1,000.
Discussion
IFPs were first described in 1920 as smooth, usually solitary, submucosal proliferating growths with inflammatory eosinophilic (Figure 2B) and fibroblastic infiltration.3 The first case of colonic IFP was reported by Kofler in 1952.4 The etiology and pathogenesis are not well known. IFPs are found in all age groups, but most commonly in adults.5 They are mostly found in the stomach (70%) and the small intestine (20%). Colonic IFPs are rare and are most commonly located in the proximal colon, especially in the cecum.8 They can be sessile or pedunculated and usually contain blood vessels, fibroblasts, and an edematous stroma rich in eosinophils (Figure 3). Clinical presentation depends, in general, on their size and location.8 As they enlarge, they can cause abdominal pain, hematochezia, anemia, weight loss, diarrhea, and intussusceptions.9 Definitive diagnosis is made by histopathologic examination of tissue specimens obtained by surgery or endoscopy. Biopsies can be challenging because the epicenter of the lesion is often in the submucosa and the polyp is often covered by epithelial mucosa. Using immunohistochemistry studies, spindle cells of IFPs are generally positive for CD34 and negative for S-100 protein, P53, c-kit, and Bcl-2, which helps to differentiate IFP from gastrointestinal stromal tumor (GIST).2
Figure 3.
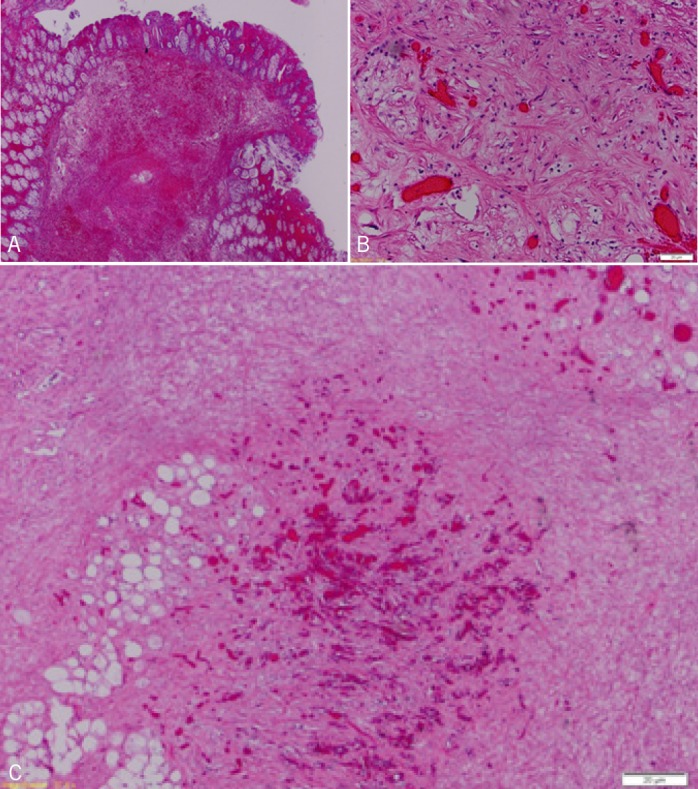
(A) Low power view. Submucosal stromal proliferation with superimposed hemorrhage. Inflammatory fibroid polyp, tissue section. Hematoxylin and eosin, original magnification ×20. (B) High power view of the inflammatory fibroid polyp displaying a rich vascularized area with branching capillaries surrounded by a collagenous stroma containing bland spindle cells. Tissue section. Hematoxylin and eosin, original magnification ×200. (C) Low power view of the deep aspect of the inflammatory fibroid polyp displaying rich vascularized areas. Tissue section. Hematoxylin and eosin, original magnification ×40.
PubMed, Medline, and Google Scholar were searched for English articles in reference to colonic IFP. All case reports of colonic IFP from 1952 to present were collected. The clinicopathological features and modality of removal were reviewed and summarized in Table 1. To our knowledge, only 31 cases of colonic IFP were reported in the English literature (Table 1). The size ranged between (0.5–7 cm) with a median diameter of 3.8 cm. There were 2 cases of small (<1 cm) polyps, 19 cases of large (1–4 cm) polyps, and only 5 cases of giant (>4 cm) polyps, as in our case; cases of unspecified size were not included in our calculations. Most of the polyps were found in the cecum (15 cases) accounting for 44% of cases, whereas only 2 cases of descending colon IFP were reported prior to our case. Males were affected more than females (72%). Fifteen cases (44 %) were pedunculated and 7 (20%) were sessile; the rest were unspecified.
Treatment approach was surgical in 20 cases (58%), while endoscopic resection was done in only 8 (23%). In addition to snare and cautery, other methods used for polypectomy included needle-knife assisted endoscopic polypectomy10 and endoclip-assisted polypectomy.2 The largest polyp treated with endoscopy, apart from our case, was 4.5 cm. There was no reported recurrence of IFP in the colon; however, there were 2 reported cases of recurrent gastrointestinal IFP in the small intestines7,12 and one in the stomach.13 Surgical resection has been the most common method of treatment for large and giant colonic IFP (Table 1). This is usually because of the technical difficulty of endoscopic polypectomy, which could be very challenging due to polyp size causing limited view and occupation of most of the lumen; the morphology (pedunculated with firm and wide stalk or sessile); the location at a flexure or sharp curve; and concerns regarding the curative role of endoscopic treatment and recurrence.1,14,15 Successful endoscopic resections have been reported in a smaller number of cases when the polyps were small and pedunculated. Due to the benign nature of IFPs and the low post-endoscopic resection recurrence rate,6 endoscopic polypectomy of an IFP could be an appropriate approach if technically possible, considering the size, location, and the morphology of the polyp. Moreover, endoscopic polypectomy is a valuable diagnostic method for providing tissue specimens for accurate histological assessment, and is a reasonable option for patients who are not surgical candidates or refuse surgery.
Disclosures
Author contributions: A. Toumeh and U. Ahmad conducted the literature review; L. De Las Casas provided the pathology description; A. Nawras supervised the article process; and A. Kayyali is the article guarantor.
Financial disclosure: The authors have no financial disclosures and no conflicts of interest to report.
References
- 1.Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: Endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997;46(1):53–7. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto T, Kato H, Okabe T, et al. A large inflammatory fibroid polyp of the colon treated by endoclip-assisted endoscopic polypectomy. Dig Liver Dis. 2005;37(12):968–72. [DOI] [PubMed] [Google Scholar]
- 3.Konjetzny GE. Uber magenfibrome. Beitr Klin Chir. 1920;119:53–61. [Google Scholar]
- 4.Kofler E. Uber die granulome des magen-darmashlauches. Virchows Arch. 1952;321(2):121–33. [DOI] [PubMed] [Google Scholar]
- 5.Helwig EB, Ranier A. Inflammatory fibroid polyps of the stomach. Surg Gynecol Obstet. 1953;96(3):335–67. [PubMed] [Google Scholar]
- 6.Goldman EL, Friedman NB. Neurogenic nature of so-called inflammatory fibroid polyps of the stomach. Cancer. 1967;20:134–43. [DOI] [PubMed] [Google Scholar]
- 7.Anthony PP, Morris DS, Vowles KD. Multiple and recurrent inflammatory fibroid polyps in three generations of a Devon family: A new syndrome. Gut. 1984;25(8):854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone JM, Morson BC. Inflammatory fibroid polyp of the gastrointestinal tract. Histopathology. 1978;2(5):349–61. [DOI] [PubMed] [Google Scholar]
- 9.Lifschitz O, Lew S, Witz M, Reiss R, Griffel B. Inflammatory fibroid polyp of sigmoid colon. Dis Colon Rectum. 1979;22(8):575–7. [DOI] [PubMed] [Google Scholar]
- 10.Kim BC, Cheon JH, Lee SK, et al. Needle knife–assisted endoscopic polypectomy for a large inflammatory fibroid polyp by making its stalk into an omega shape using endoloop. Yonsei Med J. 2008;49(4):680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doganavsargil B, Serin G, Akyldiz M, et al. Benign fibroblastic polyp of the colon: A case report. Turk J Gastroenterol. 2009;20(4):287–90. [DOI] [PubMed] [Google Scholar]
- 12.McGreevy P, Doberneck RC, McLeay JM, Miller FA. Recurrent eosinophilic infiltrate (granuloma) of the ileum causing intussusception in a two-year-old child. Surgery. 1967;61:280–284. [PubMed] [Google Scholar]
- 13.Zinkiewicz K, Zgodzinski W, Dabrowski A, et al. Recurrent inflammatory fibroid polyp of cardia: A case report. World J Gastroenterol. 2004;10(5):767–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eugene C, Penalba C, Gompel H, et al. Gastric eosinophilic granuloma: Value of endoscopic polypectomy. Apropos of 2 cases. Sem Hop. 1983;59(32):2249–2250. [PubMed] [Google Scholar]
- 15.Tada S, Iida M, Yao T, et al. Endoscopic removal of inflammatory fibroid polyps of the stomach. Am J Gastroenterol. 1991;86(9):1247–1250. [PubMed] [Google Scholar]
- 16.Vitolo RE, Rachlin SA. Inflammatory fibroid polyp of large intestine: Report of a case. J Int Coll Surg. 1955;23(6pt 1):700–9. [PubMed] [Google Scholar]
- 17.McGee HJ. Inflammatory fibroid polyp of the ileum and cecum. Arch Pathol. 1960;70:203–7. [Google Scholar]
- 18.Samter TG, Alstott DF, Kurlander GJ. Inflammatory fibroid polyps of the gastrointestinal tract. A report of 3 cases, 2 occurring in children. Am J Clin Pathol. 1966;45(4):420–36. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin SP, Hawk WA, Turnbull RB. Fibrous inflammatory polyps of the ileum and cecum: Review of five cases with emphasis on differentiation from mesenchymal neoplasm. Cancer. 1977;39(3):1300–5. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki S, Kikuchi K, Iwamura K, et al. A case of eosinophilic granuloma (inflammatory fibroid polyp) of the colon. Nippon Shokakibyo Gakkai Zasshi. 1979;76(1):126–32. [PubMed] [Google Scholar]
- 21.Ferin P, Skucas J. Inflammatory fibroid polyp of the colon simulating malignancy. Radiology. 1983;149(1):55–6. [DOI] [PubMed] [Google Scholar]
- 22.Pollice L, Bufo P. Inflammatory fibroid polyp of the rectum. Pathol Res Pract. 1984;178(5):508–12. [DOI] [PubMed] [Google Scholar]
- 23.Shimer GR, Helwig EB. Inflammatory fibroid polyps of the intestine. Am J Clin Pathol. 1984;81(6):708–14. [DOI] [PubMed] [Google Scholar]
- 24.Niv Y, Hurwitz A. Inflammatory fibroid polyp of the cecum, associated with adenomatous polyp and ovarian thecoma. Isr J Med Sci. 1985;21(7):624–6. [PubMed] [Google Scholar]
- 25.Merkel IS, Rabinovitz M, Deckker A. Cecal inflammatory fibroid polyp presenting with chronic diarrhea. A case report and review of the literature. Dig Dis Sci. 1992;37:133–6. [DOI] [PubMed] [Google Scholar]
- 26.Harned RK, Buck JL, Shekitka KM. Inflammatory fibroid polyps of the gastrointestinal tract: Radiologic evaluation. Radiology. 1992;182(3):863–6. [DOI] [PubMed] [Google Scholar]
- 27.Gooszen AW, Tjon A, Tham RT, et al. Inflammatory fibroid polyp simulating malignant tumor of the colon in a patient with multiple hamartoma syndrome (Cowden's disease). Am J Roentgenol. 1995;165(4):1012–3. [DOI] [PubMed] [Google Scholar]
- 28.de la Plaza R, Picardo A, Cuberes R, et al. Inflammatory fibroid polyps of the large intestine. Dig Dis Sci. 1999;44(9):1810–6. [DOI] [PubMed] [Google Scholar]
- 29.Nakase H, Mimura J, Kawasaki T, et al. Endoscopic resection of small inflammatory fibroid polyp of the colon. Intern Med. 2000;39(1):25–7. [DOI] [PubMed] [Google Scholar]
- 30.Ng C, Lam KY, Gupta TS, Ho YH. Inflammatory fibroid polyp of the cecum in a patient with neurofibromatosis. Ann Acad Med Singapore. 2004;33(6):797–9. [PubMed] [Google Scholar]
- 31.Iwamoto K, Sakashita M, Takashashi T, et al. Depressed type of inflammatory fibroid polyp of the colon. Int J Colorectal Dis. 2007;22(11):1409. [DOI] [PubMed] [Google Scholar]
- 32.Hiraskai S, Matsubara M, Ikeda F, et al. Inflammatory fibroid polyp occurring in the transverse colon diagnosed by endoscopic biopsy. World J Gastroenterol. 200721;13(27):3765–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park YB, Cheung DY, Kim JI, et al. A large inflammatory fibroid polyp in the sigmoid colon treated by endoscopic resection. Intern Med. 2007;46(19):1647–9. [DOI] [PubMed] [Google Scholar]
- 34.Peedikayil MC, Al Hindi HN, Rezeig MA. Inflammatory fobroid polyp of the cecum can be treated by endoscopic resection. Saudi J Gastroenterol. 2008;14(4):212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kan H, Suzaki H, Shinji S, et al. A case of inflammatory fibroid polyp of the cecum. J Nihon Med Sch. 2008;75(3):181–6. [DOI] [PubMed] [Google Scholar]


