Abstract
Gastrointestinal stromal tumor is a rare mesenchymal tumor. Sorafenib is an effective medication in these tumors based on two phase II clinical trials and a retrospective analysis. We report a rare case of a 57-year-old male with acute hepatotoxicity from sorafenib. He was treated conservatively with IV fluids and prednisolone. Liver function tests improved over 2 months. We conclude that sorafenib could cause life-threatening hepatotoxicity and patients taking sorafenib need to be closely monitored.
Introduction
Mesenchymal tumors including gastrointestinal stromal tumors (GIST) are rare tumors of the GI tract and comprise less than 1% of primary GI tumors. These tumors can involve any part of the GI tract, omentum, and mesentery.1 The primary treatment is surgical resection of a tumor larger than 2 cm.2 The majority of tumors have C-kit mutation and some contain a platelet-derived growth factor receptor polypeptide gene (PDGFR-A) mutation.1 The kit mutation is thought to drive the tumor.3 The risk of progressive disease is high in a tumor larger than 2 cm and >5 mitoses per 50 microscopic high-power field (HPF) in tissue sections.2 There are 3 FDA-approved drugs for metastatic GIST: imatinib, sunitinib, and regorafenib.5 Sorafenib is a recommended treatment option based on National Comprehensive Cancer Network (NCCN) guidelines. We report a case of NCIC common toxicity criteria (CTC) grade 4 hepatotoxicity caused by sorafenib in a patient with GIST and review the literature for sorafenib-induced severe hepatotoxicity.
Case Report
A 57-year-old Vietnamese male with history of coronary artery disease status post-percutaneous coronary intervention 8 years ago with consequent systolic heart failure (ejection fraction of 35–40%) presented to the hospital with abdominal pain. He did not drink alcohol and his medications include metoprolol, quinapril hydrochloride, tamsulosin, aspirin, and atorvastatin. CT scan of the abdomen demonstrated small bowel obstruction resulting from a 9.9 × 6.4-cm mass arising from the small bowel. During emergent surgery, the tumor was removed with en bloc resection of small bowel, sigmoid colon, and portion of rectum. The pathologic specimen confirmed multifocal GIST with a high Ki-67. The tumor was C-kit (CD 117-stem cell factor receptor) positive.
He was offered adjuvant imatinib but he declined due to concerns for side effects. Surveillance CT scan 6 months later showed recurrence of disease. He was given imatinib, and, 1 month later, developed severe NCIC CTC grade 3 diarrhea and abdominal pain with normal liver function tests (LFTs). The imatinib was stopped.
Sunitinib is often used in patients who are resistant to or intolerant to imatinib, but can worsen underlying heart failure and was avoided in this patient. His LFTs were normal when he was prescribed sorafenib 200 mg twice daily. He reported feeling better after 1 month; side effects included grade 1 fatigue and dizziness but no diarrhea or hand-foot syndrome. His LFTs remained normal.
Two months later, he noticed darkening of urine color and worsening abdominal pain. He developed frank jaundice within a few days but no mental status alteration. He was admitted to the hospital for supportive care. Blood serology revealed normal alpha 1 antitrypsin, ceruloplasmin, and no evidence of viral hepatitis, Epstein-Barr virus, cytomegalovirus, or autoimmune hepatitis. Triple phase CT showed hepatic steatosis and pelvic masses consistent with his known recurrent GIST. Biopsy of the liver showed moderate acute hepatitis with parenchymal necrosis, prominent canalicular cholestasis, and lymphocytic infiltrate (Figure 1). His ALT and AST levels peaked to 1,193 U/L and 766 U/L, respectively, prior to total bilirubin peak at 23 mg/dL (direct bilirubin 20 mg/dL) after 2 weeks (Figure 2). His prothrombin time increased to 15.7 seconds and INR to 1.25. His alkaline phosphatase increased to 285 U/L.
Figure 1.
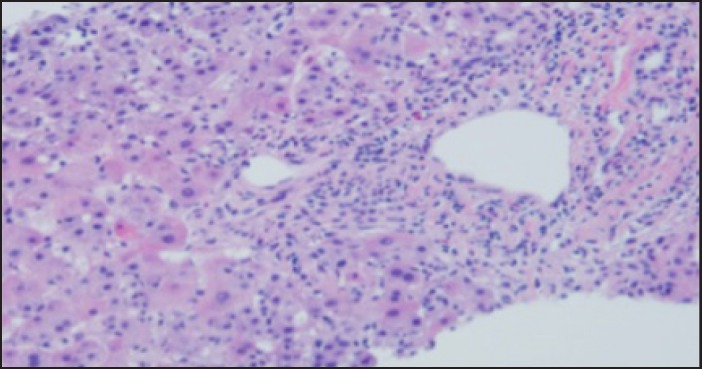
Morphology of core needle biopsy of the liver showed diffuse acute hepatitis with inflammatory infiltrate containing occasional eosinophils.
Figure 2.
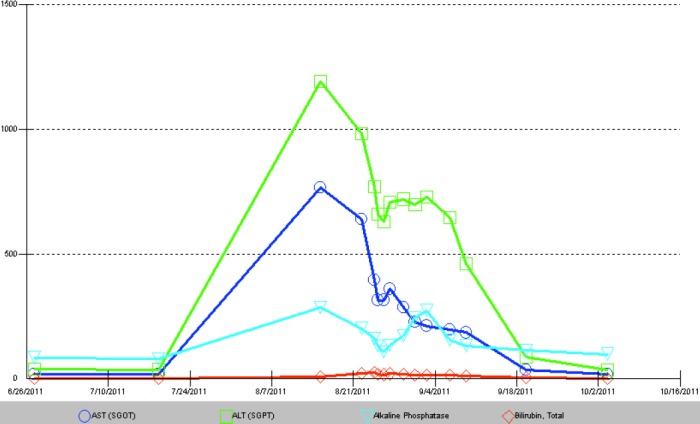
Graph of liver function tests showing increase in transaminases over 2 weeks, followed by slow recovery over 2 months. Total bilirubin peaked at 23 mg/dL, and was back to baseline in approximately 2 months after discontinuation of sorafenib. Aspartate aminotransferase (AST; blue circle); alanine aminotransferase (ALT; green square); alkaline phosphatase (turquoise triangle); and bilirubin (red diamond).
He was treated with IV fluids and prednisolone, and his sorafenib was discontinued. His liver function tests normalized over the course of 10 weeks. He subsequently was given sunitinib after complete normalization of his liver function tests.
Discussion
Sorafenib (Nexavar®) is a small molecule multi-tyrosine kinase inhibitor (TKI) that inhibits RAF kinase; vascular endothelial factor receptor 1, 2, and 3; and other tyrosine kinases.6 Sorafenib is metabolized primarily by oxidative metabolism in the liver (mediated by CYP3A4) and glucuronidation (mediated by UGT1A9).7 Common side effects (any grade in >30% of patients) are diarrhea, rash, fatigue, and hand-foot syndrome.6 Some of these side effects are dose limiting.
This agent is commonly used for patients with Child Pugh A and selected patients with Child Pugh B unresectable hepatocellular carcinoma (HCC)8 and metastatic renal cell carcinoma.6 Preclinical studies suggest sorafenib is active in imatinib-resistant GIST. This was thought to be due to activity of sorafenib against secondary Kit mutations.9 There was no NCI CTC grade IV hyperbilirubinemia or elevation of liver enzymes in the phase II study of sorafenib in resistant and refractory GIST patients,9 and this is the first case report of NCI CTC grade IV hepatotoxicity in patients with GIST.
In a small phase II trial of metastatic GIST resistant to imatinib or sunitinib, sorafenib has shown a disease control rate of 36%. The progression-free survival and overall survival was 4.9 months and 9.7 months, respectively.9 A retrospective analysis of sorafenib as third or fourth line treatment in 124 patients with advanced GIST showed a response rate of 10% and stabilization of disease in 57% of patients.10 In another phase II study, the use of sorafenib in 38 patients with imatinib and sunitinib-resistant GIST resulted in a disease control rate of 68%.11
In a randomized phase III trial of sorafenib in unresectable HCC, 1% of patients had grade IV liver toxicity, even though most of these patients had baseline Child Pugh A cirrhosis.8 Of patients with hepatocellular carcinoma who received Sorafenib in a large phase III trial, the incidence of grade IV increase in AST was 2%, ALT 1%, and hyperbilirubinemia 2%; however, the experimental arm (brivanib) had the same incidence of hepatotoxicity.12
Sorafenib is one of the FDA-approved TKI in renal cell carcinoma; in a pivotal phase III trial, no hepatotoxicity was documented.6 It is possible that these patients in this trial had relatively healthy livers compared to those with hepatocellular carcinoma. This explanation is supported by the absence of hepatotoxicity in a randomized phase III trial of axitinib versus sorafenib in metastatic renal cell cancer.14 Unlike these two large clinical trials, 2% of patients had grade IV increase in AST and ALT when sorafenib was compared to tivazonib.15 Nineteen percent of patients in the sorafenib arm had liver metastases, but the status of liver metastases in patients who had elevation of transaminases is not clear from publication.
Gupta-Abramson et al reported a phase II trial of sorafenib in advanced thyroid cancer. One patient developed worsening LFTs 8 weeks after commencement of treatment; despite stopping treatment and supportive care, the patient died 3 months later from liver failure. The patient refused liver biopsy and there were no reported drug–drug interactions or other clear etiology for the liver failure except sorafenib.13 One patient with metastatic renal cell carcinoma who had a normal liver with no metastases when he was given sorafenib as part of SORCE trial developed a severe idiosyncratic reaction 7 weeks after starting treatment. He later on died of fulminant hepatic failure. The autopsy revealed lobular hepatitis and hepatocyte necrosis.16 Our patient's liver toxicity also happened between 6-8 weeks after initiation of the drug.
This review of literature suggests relative increased risk of hepatotoxicity in patients with underlying liver damage by liver cirrhosis, hepatocellular carcinoma, or hepatic metastases.15 However, our case illustrates that hepatotoxicity is also possible in patients with normal liver function. It is not clear what increases the risk of hepatotoxicity, as the reported phase III RCC trials do not detail whether there were drug interactions, underlying liver metastases, or liver disease in patients who developed hepatotoxicity. Sorafenib is commonly used in hepatocellular and renal cell carcinoma, and we suggest careful, regular monitoring of liver function tests during treatment.
Disclosures
Author contributions: All authors contributed equally to the preparation of this article. W. Murad is the article guarantor.
Financial disclosure: No relevant potential conflict of interest or funding to disclose.
Informed consent was obtained for this case report.
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2.Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol. 2009;6(6):363–71. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80. [DOI] [PubMed] [Google Scholar]
- 4.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368(9544):1329–38. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clearcell renal cell carcinoma. N Engl J Med. 2007;356(2):125–34. [DOI] [PubMed] [Google Scholar]
- 7.Keating GM, Santoro A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–40. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: A phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30(6):2377–83. [DOI] [PubMed] [Google Scholar]
- 10.Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: A retrospective analysis. Eur J Cancer. 2013;49(5):1027–31. [DOI] [PubMed] [Google Scholar]
- 11.Kindler HL, et al. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): Final results of a University of Chicago Phase II Consortium trial. ASCO Meeting Abstracts. 2011;29(suppl 15). [Google Scholar]
- 12.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as firstline therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–24. [DOI] [PubMed] [Google Scholar]
- 13.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26(29):4714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Nosov D, Eisen T, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J Clin Oncol. 2013;31(30):3791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairfax BP, Pratap S, Roberts IS, et al. Fatal case of sorafenib-associated idiosyncratic hepatotoxicity in the adjuvant treatment of a patient with renal cell carcinoma. BMC Cancer. 2012;12:590. [DOI] [PMC free article] [PubMed] [Google Scholar]


