Abstract
We present a case of a 61-year-old woman with end-stage renal disease (ESRD) who developed painless hematochezia following initiation of anticoagulation. Work-up revealed a large ulceration in the sigmoid colon, and histologic images revealed sevelamer crystals embedded in the colonic mucosa, consistent with sevelamer crystal-mediated injury. This is a novel cause of gastrointestinal hemorrhage that has not previously been described in the literature. Physicians should be aware of the potential for sevelamer-induced injury.
Introduction
Non-absorbable drugs such as sequestering agents exert their effect in the gastrointestinal lumen by binding a target molecule and forming an insoluble complex preventing absorption. This class of drugs includes many commonly used medications such as sodium polystyrene sulfonate, cholestyramine, and sevelamer. While the potential for mucosal injury of the gastrointestinal tract has been demonstrated in the literature with the use of cholestyramine and sodium polystyrene sulfate, there have been few reports of sevelamer-associated mucosal injury.
Case Report
A 61-year-old African-American woman with a history of end-stage renal disease (ESRD) requiring chronic hemodialysis presented to our hospital for management of a thrombosed arteriovenous graft. After mechanical thrombectomy, the patient was started on an IV heparin infusion with transition to warfarin. Two days post-operatively, she developed painless hematochezia. Her medications included calcium acetate, cinacalcet, diltiazem, glipizide, hydroxyzine, losartan, omeprazole, pravastatin, and sevelamer 2,400 mg by mouth 3 times daily. She denied any recent nonsteroidal anti-inflammatory drug use. She denied having problems with constipation. Prior to surgery, the patient received a brief course of high-dose steroids as premedication for a contrast allergy.
Physical examination revealed a hemodynamically stable obese woman in no acute distress. Her abdomen was soft, non-tender, and non-distended. Rectal exam revealed dark red blood with no hemorrhoids or anal fissures. Laboratory evaluation revealed hemoglobin 10.8 g/dL (baseline: 11.1 g/dL). Colonoscopy revealed a 5-cm semicircumferential ulceration in the sigmoid colon 25 cm from the anal verge (Figure 1 and Figure 2). The ulcer was not actively bleeding, so no endoscopic intervention for hemostasis was performed. The ulcer border was biopsied, and histology revealed an ulcer bed with attached fibrin, hemorrhage, and entrapped pale yellow-to-orange crystals, which is a finding associated with sevelamer-induced mucosal injury (Figure 3). Sevelamer was discontinued with no recurrence of hematochezia, and a heparin drip was restarted 6 hours after the colonoscopy without any subsequent gastrointestinal bleeding. After discussion with the vascular surgery team, the patient was switched to clopidrogel for long-term anticoagulation. Repeat endoscopy is planned 6 months after this bleeding episode to check for mucosal healing after discontinuation of sevelamer.
Figure 1.
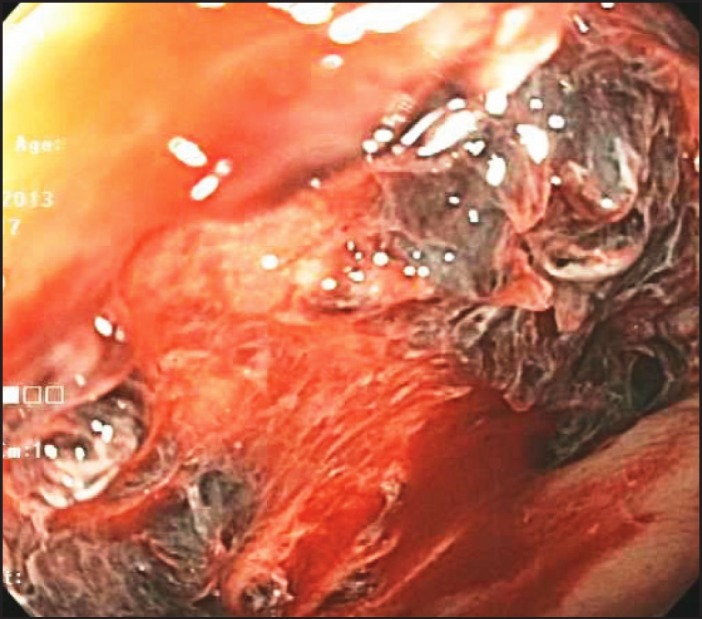
A 5-cm semi-circumferential ulceration in the sigmoid colon 25 cm from the anal verge revealed by colonoscopy.
Figure 2.
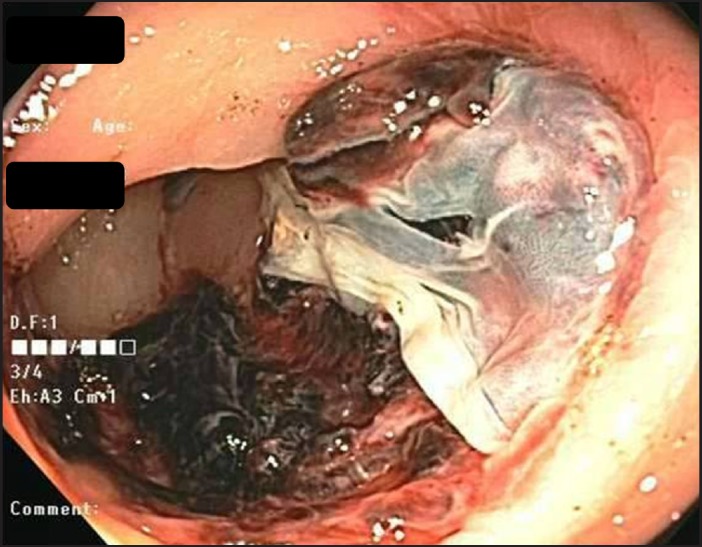
Ulcer in the sigmoid colon.
Figure 3.
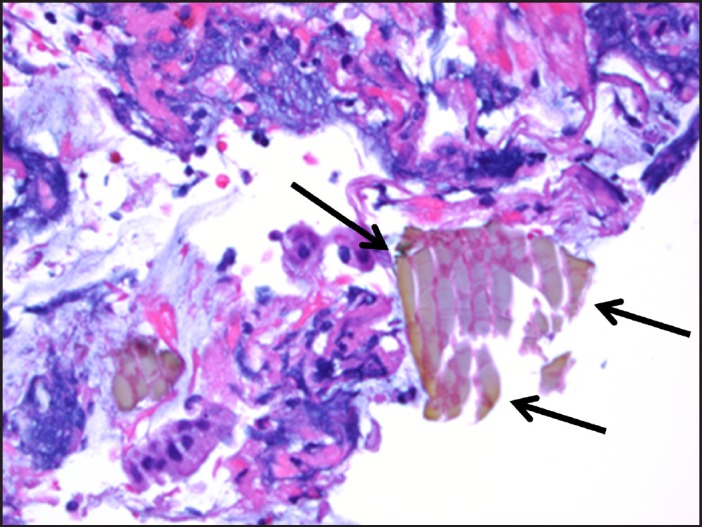
Hematoxylin and eosin stain of ulcer bed with sevelamer crystals.
Discussion
Sevelamer is an anion exchange resin that is approved for use in the United States and Europe to treat hyperphosphatemia in patients with ESRD who are on hemodialysis. It is a nonabsorbable polymer that dissociates from its anion (either carbonate in Renvela® or hydrochloride in Renagel®) and binds to dietary phosphate within the gastrointestinal tract preventing reabsorption.1 The most common side effects associated with sevelamer use are gastrointestinal, including vomiting (22%), nausea (20%), diarrhea (19%), dyspepsia (16%), and constipation (8%). The most concerning adverse effect is metabolic acidosis, which is seen with sevelamer hydrochloride (Renagel®) but not with sevelamer carbonate (Renvela®). Sevelamer use is contraindicated in the presence of bowel obstruction. Sevelamer crystals are non-polarizable and are characterized as having broad, curved, irregular fish scales. Sevelamer crystal deposition in the colon may contribute to mucosal inflammation and ulcer formation. On hematoxylin and eosin stain, the crystals are two-toned in appearance with bright pink accentuations on a rusty yellow background.2
There is little existing literature regarding sevelamer crystal-mediated mucosal injury. A prospective series identified 15 pathology specimens from throughout the gastrointestinal tract containing sevelamer crystals, of which 14 had abnormal background mucosal findings.2 While some of these cases were felt to include incidental sevelamer crystal deposition in the context of other comorbidities, there were also patients with findings suggestive of direct sevelamer crystal-mediated injury as evidenced by acute ulcerations in various parts of the gastrointestinal tract. They also found a dose-dependent relationship with an increased degree of mucosal injury in patients receiving higher doses of sevelamer.
Madan et al described an incident of sevelamer use causing lower gastrointestinal bleeding through the formation of a stercoral ulcer. Stercoral ulceration is defined as an ulcer caused by pressure necrosis from a fecaloma. In this case, sevelamer caused severe constipation, which in turn resulted in formation of a fecaloma and subsequently a stercoral ulceration in the rectum.3 In contrast to this report, our patient did not report constipation and her ulcer was in the sigmoid colon, thus stercoral ulceration unlikely to be the cause of her colonic mucosal injury.
With the growing use of sevelamer in patients with ESRD, it is important to recognize the potential clinical impact of mucosal injury associated with sevelamer crystal deposition. Unfortunately, at this time, there are no known strategies described in the literature to prevent sevelamer-associated mucosal injury. Our case suggests that there may be an association between sevelamer use and mucosal injury, but further evidence is necessary to indicate causation. Sodium polysyterene sulfonate is a cation-exchange resin used in the treatment of hyperkalemia that is believed to be directly toxic to the colonic mucosa, resulting in colonic ischemia and ulceration. We postulate that sevelamer potentially mediates mucosal injury under a similar mechanism, and that sevelamer crystals deposit in the mucosa of the colon, where they are directly toxic and result in ischemic changes and subsequent ulcer formation.
Disclosures
Author contributions: P. Chintamaneni and R. Das acquired and interpreted the data, and drafted the manuscript. SF Kuan and TR Kermanshahi acquired and interpreted the data. JG Hashash interpreted the data, and drafted and critically revised the manuscript for important intellectual content. P. Chintamaneni is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Grinfeld J, Inaba A, Hutchinson A. Update and critical appraisal of sevelamer in the management of chronic renal failure. Open Access J Urol. 2010;2:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson BJ, Limketkai BN, Liu TC, et al. Sevelamer crystals in the gastrointestinal tract: A new entity associated with mucosal injury. Am J Surg Pathol. 2013;37(11):1686–93. [DOI] [PubMed] [Google Scholar]
- 3.Madan P, Bhayana S, Chandra P, Hughes JI. Lower gastrointestinal bleeding: Association with sevelamer use. World J Gastroenterol. 2008;14(16):2615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


