Abstract
Green tea extract is a popular ingredient in herbal weight loss supplements. There have been reports of hepatotoxicity associated with the use of dietary supplements, some of these cases lead to fatal outcomes. To our knowledge, we report the first case of fulminant hepatic failure requiring orthotopic liver transplantation caused by SlimQuick™ (Wellnx Life Sciences, Wilmington, DE), a widely available weight loss supplement containing green tea extract.
Introduction
Green tea extract is a relatively ubiquitous herbal product long celebrated for its antioxidant properties; the proven health benefits create the illusion of benignity.1 Its reported fat-burning ability has led to its inclusion in many herbal weight loss supplements. Since its introduction into a largely unregulated weight loss market, several cases of hepatotoxicity have arisen, yet it still remains a key ingredient in many supplements.2
Case Report
A 52-year-old woman presented to the emergency department with 1 week of vomiting and progressive jaundice. Three weeks before presentation, she drank SlimQuick™ for 2 days while fasting. Past medical, surgical, and family histories were unremarkable. Medication history included only metoprolol. Physical examination revealed normal mental status, icteric sclera, mild abdominal distension, and lower extremity edema. Initial laboratory values revealed total bilirubin 16.5 mg/dL, aspartate transaminase (AST) 1507 IU/L, alanine transaminase (ALT) 945 IU/L, alkaline phosphatase 210 IU/L, and international normalized ratio (INR) 2.82. Abdominal computed tomography (CT) showed nodular liver with small amount of ascites (Figure 1). Serological tests for viral hepatitis, autoimmune hepatitis, Wilson disease, and primary biliary cirrhosis were negative. Liver biopsy was consistent with confluent hepatic necrosis with collapse (Figure 2), and prednisone 60 mg was initiated.
Figure 1.
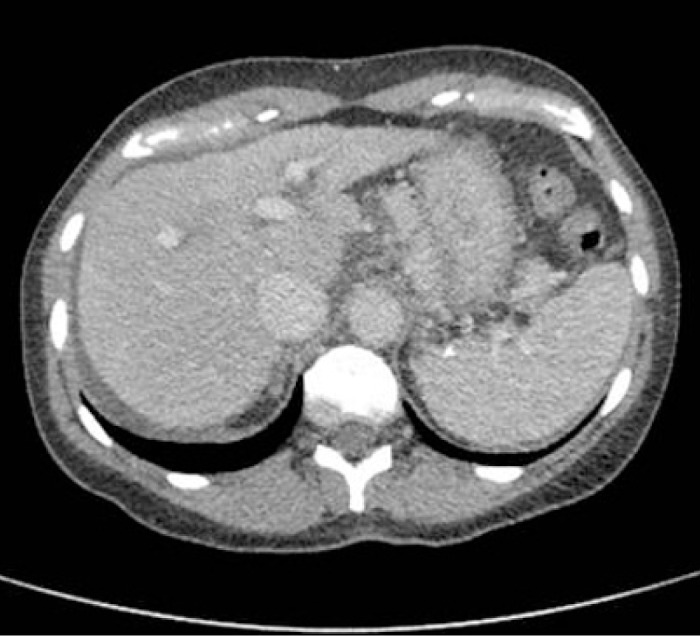
Abdominal CT revealing nodular liver with small amount of ascites.
Figure 2.
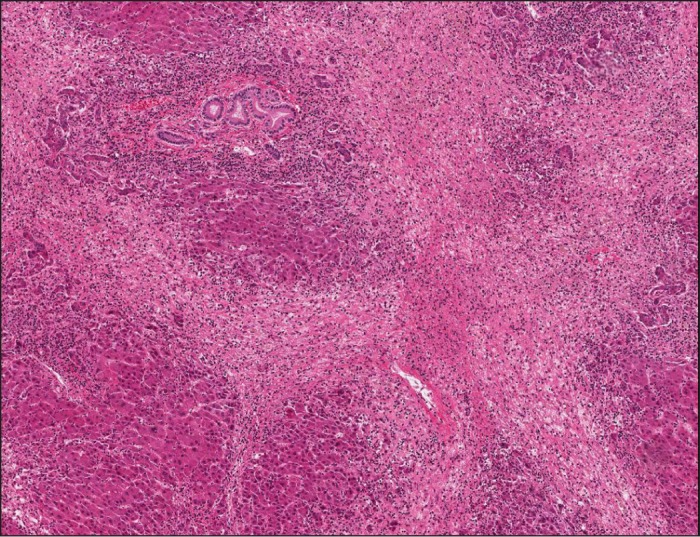
Liver biopsy showing confluent hepatic necrosis with collapse.
Soon after, the patient developed slurred speech and asterixis and required intubation with transfer to the ICU, where the patient was expeditiously evaluated and listed for liver transplant. She underwent liver transplantation 2 days later. The patient was discharged home post-operative day 8. Explant biopsy revealed extensive centrilobular and confluent necrosis with zonal distribution of necrosis favoring drug/toxin-related injury.
Discussion
Green tea extract is derived from the herb Camellia sinensis. Polyphenolic compounds called catechins are present in high concentrations, and are responsible for green tea extract's well-known anti-carcinogenic and anti-inflammatory properties.3 It has been linked to weight loss due to the thermogenic properties of catechins, particularly epigallocatechin-3-gallate (EGCG).4 Case reports of green tea extract hepatotoxicity are linked to higher concentrations of EGCG, which exerts its toxic effects through generation of reactive oxygen species, mitochondrial damage, and depletion of glutathione in hepatocytes.2–6 In animal models, consumption of EGCG while fasting leads to greater morbidity due to higher plasma concentrations, a troubling fact since many weight loss supplements are taken with a low-calorie diet.7
Despite reports of hepatotoxicity and attempts to quantify safe amounts of catechin consumption, catechins have a continued presence in the market in products such as Slimquick™. Exolise® (Laboratoires Arkopharma, Carros, France), a European herbal weight loss product, was withdrawn from Spanish and French markets in 2003 after being linked to 13 cases of hepatotoxicity, one of which required orthotopic liver transplantation.2 Exolise® was 25% by content catechins and mostly EGCG.8 In 2008, the United States Pharmacopeia reviewed 34 cases of hepatotoxicity linked to green tea extract and studies that examined the safety of catechin dosage in humans and animals; they concluded that green tea extract does have potential for hepatic injury.2 Patel et al reported a case of hepatotoxicity in an adolescent who ingested Applied Nutrition Green Tea Fat Burner® (Applied Nutrition, Los Angeles, CA) in combination with other supplements; it was felt that prolonged ingestion of catechins caused his injury.9 Boehm et al found increasing adverse effects with catechin consumption exceeding 250 mg daily.10 Makers of SlimQuick™, which contains 135 mg EGCG, recommend twice daily dosing with a 1,350 calorie per day diet for maximum weight loss results.11
Our patient's ingestion of green tea extract while fasting likely led to her precipitous deterioration. While beta-adrenergic antagonists, particularly labetalol, have been linked to hepatotoxicity, our patient began her beta-blocker months before without incident.12 Weinstein et al reported a case of hepatotoxicity from Slimquick™ in a woman with the alpha-1-antitrypsin MZ phenotype, suggesting that in seemingly healthy patients, phenotypic variations may explain their predisposition to hepatic injury with drug or supplement ingestion.1 No studies yet exist that correlate the MS phenotype, which our patient had, with increased risk of liver injury.13
Our case highlights many important facts about herbal supplemental use and its implications for patients and health care providers. Herbal supplement medicinal use, particularly for weight loss, is common in the United States.14 According to U.S. Drug-Induced Liver Injury Network (DILIN), between 2003 and 2011, 16% of drug-induced liver injury was attributed to herbal supplements, with 26% of these cases involving weight loss supplements.15 In 2009, Americans spent nearly $3 million on green tea extract products alone,16 and many patients may be unknowingly consuming catechins in other herbal supplements. Navarro et al analyzed over 90 supplements implemented in 47 cases of drug-induced liver injury (DILI) to determine the hidden presence of catechins.5 Nearly 40% of supplements analyzed contained catechins despite non-disclosure on supplement labels.5 There is a need for providers to counsel on the safety of green tea extract and remind patients of the dangers of herbal supplement use.
Disclosures
Author contributions: M. Whitsett and D. Halegoua-De Marzio researched the literature, and drafted and critically reviewed the manuscript. D. Halegoua-De Marzio is the article guarantor. S. Rossi critically reviewed the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Weinstein DH, Twaddell WS, Raufman JP, et al. . SlimQuick-associated hepatotoxicity in a woman with alpha-1 antitrypsin heterozygosity. World J Hepatol. 2012; 4( 4): 154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarma DN, Barrett ML, Chavez ML, et al. . Safety of green tea extracts: A systematic review by the US Pharmacopeia. Drug Saf. 2008; 31( 6): 469–84. [DOI] [PubMed] [Google Scholar]
- 3.Molinari M, Watt KD, Kruszyna T, et al. . Acute liver failure induced by green tea extracts: Case report and review of the literature. Liver Transpl. 2006; 12( 12): 1892–5. [DOI] [PubMed] [Google Scholar]
- 4.Shixian Q, VanCrey B, and Shi J, et al. . Green tea extract thermogenesis-induced weight loss by epigallocatechingallate inhibition of catechol-O-methyltransferase. J Med Food. 2006; 9( 4): 451–8. [DOI] [PubMed] [Google Scholar]
- 5.Navarro VJ, Bonkovsky HL, Hwang SI, et al. . Catechins in dietary supplements and hepatoxicity. Dig Dis Sci. 2013; 58( 9): 2682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006; 40( 4): 570–80. [DOI] [PubMed] [Google Scholar]
- 7.Isbruker RA, Edwards JA, Wolz E, et al. . Safety studies on epigallocatechingallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006; 44( 5): 636–50. [DOI] [PubMed] [Google Scholar]
- 8.Gloro R, Hormand-Olliver I, Mosquet B, et al. . Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Gastroenterol Hepatol. 2005; 17( 10): 1135–7. [DOI] [PubMed] [Google Scholar]
- 9.Patel S, Beer S, Carter B. Green tea extract: A potential cause of acute liver failure. World J Gastroenterol. 2013; 19( 31): 5174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm K, Borrelli F, Ernst E, et al. . Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009; 3: CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.How SlimQuick works. SlimQuick Website. http://www.myslimquick.com/faq.php Published 2013 Accessed December 3rd, 2013.
- 12.Halegoua-De Marzio D, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In: Kaplowitz N, DeLeve LD, eds. Drug-Induced Liver Disease. 3rd ed.Amsterdam: Elsevier; 2013: 522–6. [Google Scholar]
- 13.Graziadei IW, Joseph JJ, Wiesner RH, et al. . Increased risk of chronic liver failure in adults with heterozygous alpha1-antitrypsin deficiency. Hepatology. 1998; 28( 4): 1058–63. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy J.Herb and supplement use in the US adult population. Clin Ther. 2005; 27( 11): 1847–58. [DOI] [PubMed] [Google Scholar]
- 15.Navarro V, Barnhart H, Bonkovsky H, et al. . Herbal and dietary supplement induced hepatotoxicity in the U.S. Gastroenterology. 2012; 142( 5suppl 1): S41. [Google Scholar]
- 16.Cavaliere C, Rea P, Lynch ME, Blumenthal M. Herbal supplement sales rise in all channels in 2009. HerbalGram Website. http://cms.herbalgram.org/herbalgram/issue86/article3530.html?ts=1387243983&signature=4d8c7fb0b25877efd0facf92a61e3ac3 Accessed December 16, 2013.


