Abstract
We present a case report of an 80-year-old woman with volume overload thought initially to be secondary to heart failure, but determined to be amiodarone-induced acute and chronic liver injury leading to submassive necrosis and bridging fibrosis consistent with early cirrhosis. Her histopathology was uniquely absent of steatosis and phospholipidosis, which are commonly seen in AIC.
Introduction
Amiodarone has become one of the most frequently prescribed antiarrhythmics in the United States. It possesses a significant side effect profile that includes hepatotoxicity and cirrhosis. Amiodarone-induced cirrhosis (AIC) is a rare and challenging diagnosis, and established mortality rates for AIC can be as high as 60% at 5 months.1 The most common reported symptoms of AIC are generalized weakness, abdominal pain, and distention, with nearly one-quarter of patients presenting with some degree of abdominal distention or ascites.1
Case Report
An 80-year-old white woman presented with complaints of nausea, fatigue, anorexia, 4.5 kg of weight gain, and lower extremity and abdominal swelling over the last 4 weeks. Her past medical history was significant for coronary artery disease, ischemic cardiomyopathy, amiodarone-induced hypothyroidism, atrial fibrillation, and chronic kidney disease. Noteworthy medications were amiodarone and furosemide. She had an estimated cumulative amiodarone dosage of 412.5 g over 3.5 total years. During this time period, she was appropriately followed by current prescribing recommendations that suggest AST and ALT monitoring every 6 months with medication discontinuation if levels reach 2 times the upper limit of normal.2 She had a distant history of tobacco use, without any alcohol or illicit drug use. She denied exertional dyspnea, orthopnea, or paroxysmal nocturnal dyspnea. She was admitted to the hospital for concern of heart failure exacerbation and acute-on-chronic kidney injury. Her exam was notable for a blood pressure of 70/44 mm Hg, a paced heart rate of 78 beats per minute, pitting edema in her bilateral lower extremities, flat neck veins, and a benign cardiac and pulmonary examination. She had a firm, distended, non-tender abdomen, and fluid wave. A chest radiograph revealed a possible small right pleural effusion. Admission labs were notable for pro-brain natriuretic peptide (BNP) 2812 pg/mL, urinalysis with 1+ protein, creatinine 3.4 mg/dL, fractional excretion of sodium of 0.64%, and an abnormal hepatic panel (Table 1). Echocardiogram was normal, and right heart catheterization revealed low-normal filling pressures and normal cardiac output. She was started on intravenous fluids and her creatinine improved.
Table 1.
Trend of Laboratory Values
| Day of Admission | 1 Week Prior to Admission | 7 Months Prior to Admission | Normal Range | |
|---|---|---|---|---|
| Total protein, g/dL | 5.0 | 6.0 | 6.3 | 5.8-7.8 |
| Albumin, g/dL | 2.1 | 2.6 | 3.6 | 3.5-4.8 |
| Total bilirubin, mg/dL | 1.9 | 2.3 | 1.0 | 0.4-1.5 |
| Alkaline phosphatase, U/L | 200 | 252 | 100 | 24-110 |
| AST, U/L | 94 | 110 | 43 | 15-41 |
| ALT, U/L | 54 | 63 | 32 | 14-54 |
| Creatinine, mg/dL | 3.4 | 2.1 | 1.4 | 0.4-1.0 |
ALT = alanine transaminase; AST = aspartate transaminase.
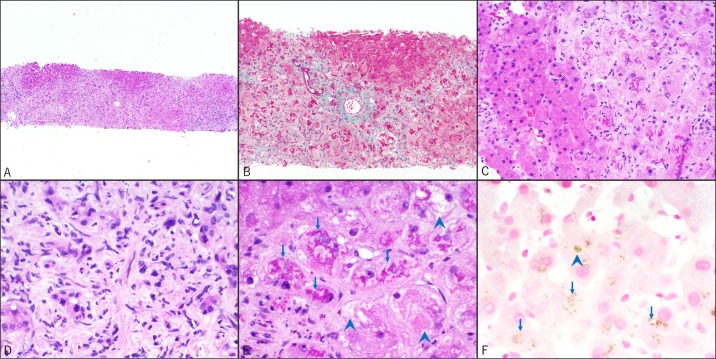
Work-up for elevated liver enzymes revealed negative hepatitis A, B, and C viral serologies and normal transferrin level. A liver ultrasound revealed normal echogenicity of the liver and a moderate amount of ascites, and paracentesis revealed a serum albumin-ascites gradient (SAAG) >1.3 with total ascitic protein of <1.0 g/dL. Cytology from the ascitic fluid was negative for malignancy. Non-contrast abdominal CT scan revealed a liver diffusely high in attenuation, suggesting possible amiodarone toxicity. A transjugular liver biopsy revealed extensive areas of hepatocyte injury and loss leading to lobular collapse with extensive, relatively recent sinusoidal fibrosis as well as bands of mature bridging fibrosis suggestive of early cirrhosis. Notably, there was a near complete absence of an inflammatory response except for the neutrophils associated with proliferating ductules seen in the fibrotic portal areas (Figure 1).
Figure 1.
Liver biopsies, all H&E stains, except where indicated. Original magnification is shown for each photomicrograph. (A) Lobular collapse due to hepatocyte loss. (B) Relatively thin bundles of recent periportal and sinusoidal fibrosis along with some bands of darker staining, more mature bridging fibrosis. Moderate ductular proliferation is seen but ductular bile is absent. (C) Lack of inflammation in areas of injured hepatocytes. (D) Neutrophils associated with proliferating ductules seen in areas of fibrosis. (E) The injured hepatocytes show abundant Mallory hyaline (arrows) and ballooning degeneration (arrowheads). (F) Prussian blue (iron) stain is negative for increased hemosiderin deposition but highlights occasional canalicular bile plugs (arrowhead) and lipofuschin pigment in hepatocytes (arrows).
These histological findings with the patient's clinical presentation were most consistent with an amiodarone-induced acute and chronic drug reaction. Amiodarone was immediately discontinued. Given her failure to improve with aggressive medical management, the patient and her family ultimately decided to pursue hospice care, and the patient died 2 days after discharge.
Discussion
It has been suggested that a cumulative dose of at least 380 g of amiodarone is necessary to induce cirrhosis,3 but a more recent examination of available cases suggests courses as short as 1 to 2 years, and that total doses as low as 55 g and averaging only 280 g may suffice.1 The only definitive treatment is cessation of the drug. Amiodarone has a large apparent volume of distribution, and even after discontinuation, total body stores do not decline for several weeks.4 While most adverse effects are reversible after drug cessation and fatal reactions remain rare, routine screening may be underutilized in practice.2 This idea, coupled with the growing trend of amiodarone prescriptions in the United States, has the propensity to make serious adverse effects more common.5
Amiodarone-induced hepatotoxicity is characterized by histologic steatosis, inflammation, fibrosis, and phospholipidosis.6 Phospholipidosis can be present without evidence of cirrhosis, and should be considered more as an indication of drug ingestion rather than hepatotoxicity.7 The combination of histopathologic findings is often referred to as pseudoalcoholic cirrhosis, given the near-classic findings of alcohol-induced cirrhosis, but with an absent history of alcohol use. Amiodarone-induced hepatotoxicity with a histological absence of steatosis and phospholipidosis has been reported.7-9 However, the authors suggested that tissue preparation may have obscured lysosomal changes.8 Our patient's biopsy lacked steatosis and phospholipidosis despite years of amiodarone ingestion. It remains uncertain what accounts for this unique presentation.
The rarity of this condition and vagueness of presenting symptoms make amiodarone-induced liver injury and cirrhosis a challenging diagnosis. It is important that physicians are aware of current monitoring guidelines of the medications they prescribe and understand that adherence does not preclude the development of serious adverse effects. Physicians should not rely heavily on cumulative dosing or time course to rule out AIC. Our case suggests that the absence of formerly recognized common histopathologic features such as steatosis and phospholipidosis should not exclude the diagnosis.
Disclosures
Author contributions: All authors contributed equally to the preparation of the manuscript. J. Buggey is the article guarantor.
Financial disclosure: None to report.
Informed consent: The authors were unable to obtain informed consent from the patient as the manuscript was drafted postmortem. Several attempts were made to contact the patient's next of kin including telephone calls and email correspondence. At the time of publication no response had been received, but the authors feel that all identifying information has been adequately removed and the patient would not object to publication.
References
- 1.Hussain N, Bhattacharyya A, Prueksaritanond S. Amiodarone-induced cirrhosis of liver: What predicts mortality? ISRN Cardiol. 2013;2013: 617943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassallo P, Trohman RG. Prescribing amiodarone: An evidence-based review of clinical indications. JAMA. 2007;298(11):1312–1322. [DOI] [PubMed] [Google Scholar]
- 3.Adams PC, Bennett MK, Holt DW. Hepatic effects of amiodarone. Br J Clin Pract Suppl. 1986;44: 81–95. [PubMed] [Google Scholar]
- 4.Klotz U.Antiarrhythmics: Elimination and dosage considerations in hepatic impairment. Clin Pharmacokinet. 2007;46(12):985–996. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, LaPointe NM, Curtis LH, et al. Outpatient prescribing of antiarrhythmic drugs from 1995 to 2000. Am J Cardiol. 2003;91(1):91–94. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62(6):481–492. [DOI] [PubMed] [Google Scholar]
- 7.Guigui B, Perrot S, Berry JP, et al. Amiodarone-induced hepatic phospholipidosis: A morphological alteration independent of pseudoalcoholic liver disease. Hepatology. 1988;8(5):1063–1068. [DOI] [PubMed] [Google Scholar]
- 8.Cimic A, Sirintrapun J. Amiodarone hepatotoxicity with absent phospholipidosis and steatosis: A case report and review of amiodarone toxicity in various organs. Case Rep Pathol. 2013;2013: 201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis JH, Mullick F, Ishak KG, et al. Histopathologic analysis of suspected amiodarone hepatotoxicity. Hum Pathol. 1990;21(1):59–67. [DOI] [PubMed] [Google Scholar]