Abstract
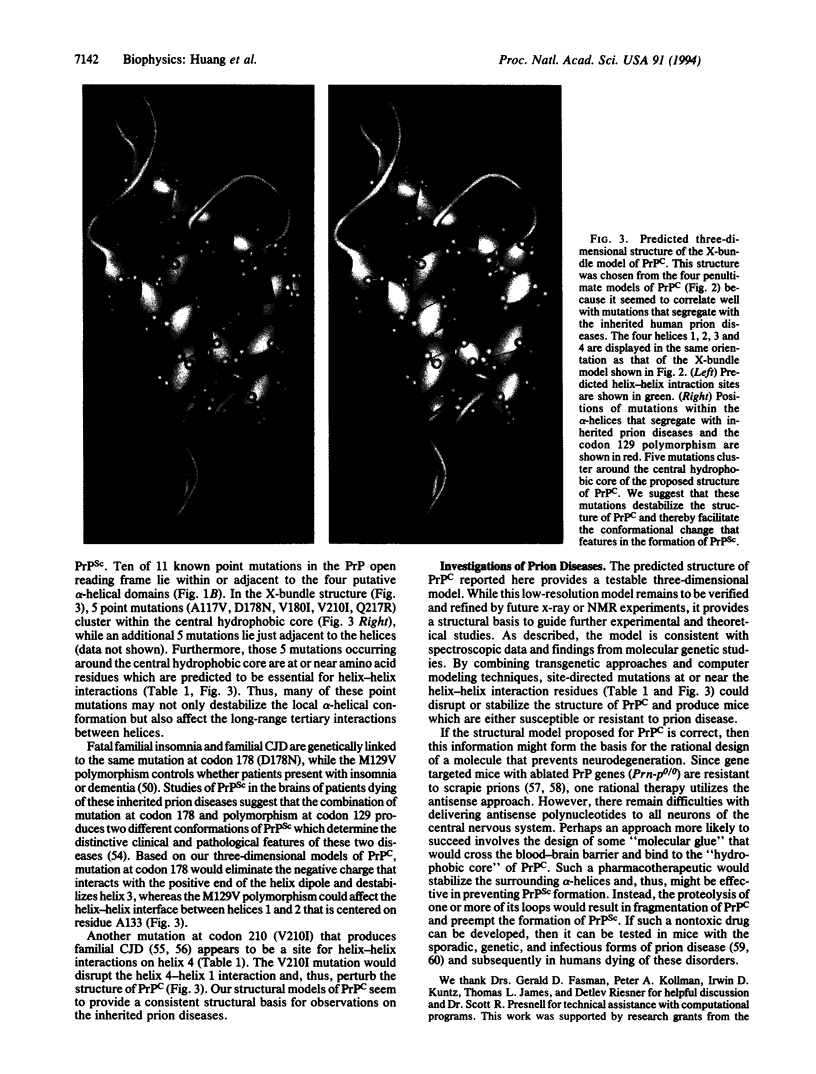
Prion diseases are a group of neurodegenerative disorders in humans and animals that seem to result from a conformational change in the prion protein (PrP). Utilizing data obtained by circular dichroism and infrared spectroscopy, computational studies predicted the three-dimensional structure of the cellular form of PrP (PrPc). A heuristic approach consisting of the prediction of secondary structures and of an evaluation of the packing of secondary elements was used to search for plausible tertiary structures. After a series of experimental and theoretical constraints were applied, four structural models of four-helix bundles emerged. A group of amino acids within the four predicted helices were identified as important for tertiary interactions between helices. These amino acids could be essential for maintaining a stable tertiary structure of PrPc. Among four plausible structural models for PrPc, the X-bundle model seemed to correlate best with 5 of 11 known point mutations that segregate with the inherited prion diseases. These 5 mutations cluster around a central hydrophobic core in the X-bundle structure. Furthermore, these mutations occur at or near those amino acids which are predicted to be important for helix-helix interactions. The three-dimensional structure of PrPc proposed here may not only provide a basis for rationalizing mutations of the PrP gene in the inherited prion diseases but also guide design of genetically engineered PrP molecules for further experimental studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Gerloff D. Patterns of divergence in homologous proteins as indicators of secondary and tertiary structure: a prediction of the structure of the catalytic domain of protein kinases. Adv Enzyme Regul. 1991;31:121–181. doi: 10.1016/0065-2571(91)90012-b. [DOI] [PubMed] [Google Scholar]
- Boissel J. P., Lee W. R., Presnell S. R., Cohen F. E., Bunn H. F. Erythropoietin structure-function relationships. Mutant proteins that test a model of tertiary structure. J Biol Chem. 1993 Jul 25;268(21):15983–15993. [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Caughey B. W., Dong A., Bhat K. S., Ernst D., Hayes S. F., Caughey W. S. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry. 1991 Aug 6;30(31):7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Structure of proteins: packing of alpha-helices and pleated sheets. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4130–4134. doi: 10.1073/pnas.74.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Kosen P. A., Kuntz I. D., Epstein L. B., Ciardelli T. L., Smith K. A. Structure-activity studies of interleukin-2. Science. 1986 Oct 17;234(4774):349–352. doi: 10.1126/science.3489989. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Richmond T. J., Richards F. M. Protein folding: evaluation of some simple rules for the assembly of helices into tertiary structures with myoglobin as an example. J Mol Biol. 1979 Aug 15;132(3):275–288. doi: 10.1016/0022-2836(79)90260-2. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Presnell S. R., Srinivasan S., Sassenfeld H., Klinke R., Jeffery E., Cosman D., March C. J., Cohen F. E. Experimental and theoretical studies of the three-dimensional structure of human interleukin-4. Proteins. 1991;11(2):111–119. doi: 10.1002/prot.340110204. [DOI] [PubMed] [Google Scholar]
- Dekker N., Cox M., Boelens R., Verrijzer C. P., van der Vliet P. C., Kaptein R. Solution structure of the POU-specific DNA-binding domain of Oct-1. Nature. 1993 Apr 29;362(6423):852–855. doi: 10.1038/362852a0. [DOI] [PubMed] [Google Scholar]
- Diederichs K., Boone T., Karplus P. A. Novel fold and putative receptor binding site of granulocyte-macrophage colony-stimulating factor. Science. 1991 Dec 20;254(5039):1779–1782. doi: 10.1126/science.1837174. [DOI] [PubMed] [Google Scholar]
- Dlouhy S. R., Hsiao K., Farlow M. R., Foroud T., Conneally P. M., Johnson P., Prusiner S. B., Hodes M. E., Ghetti B. Linkage of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease to the prion protein gene. Nat Genet. 1992 Apr;1(1):64–67. doi: 10.1038/ng0492-64. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Gabizon R., Rosenmann H., Meiner Z., Kahana I., Kahana E., Shugart Y., Ott J., Prusiner S. B. Mutation and polymorphism of the prion protein gene in Libyan Jews with Creutzfeldt-Jakob disease (CJD). Am J Hum Genet. 1993 Oct;53(4):828–835. [PMC free article] [PubMed] [Google Scholar]
- Gabriel J. M., Oesch B., Kretzschmar H., Scott M., Prusiner S. B. Molecular cloning of a candidate chicken prion protein. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9097–9101. doi: 10.1073/pnas.89.19.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Garnier J. Protein structure prediction. Biochimie. 1990 Aug;72(8):513–524. doi: 10.1016/0300-9084(90)90115-w. [DOI] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Fletterick R. J., Prusiner S. B. Perturbation of the secondary structure of the scrapie prion protein under conditions that alter infectivity. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):1–5. doi: 10.1073/pnas.90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Lloyd D. H., Gabriel J. M., Holtzman D. M., Cohen F., Fletterick R., Prusiner S. B. Predicted alpha-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10940–10944. doi: 10.1073/pnas.89.22.10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb L. G., Petersen R. B., Tabaton M., Brown P., LeBlanc A. C., Montagna P., Cortelli P., Julien J., Vital C., Pendelbury W. W. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science. 1992 Oct 30;258(5083):806–808. doi: 10.1126/science.1439789. [DOI] [PubMed] [Google Scholar]
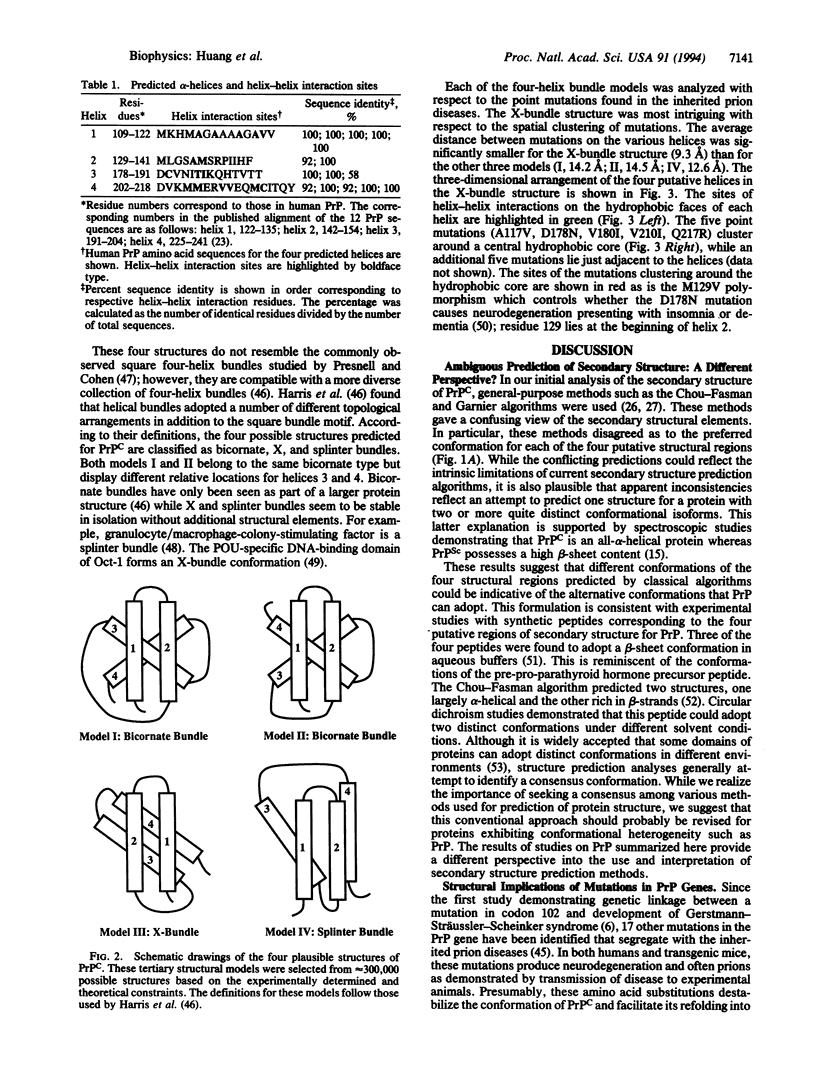
- Harris N. L., Presnell S. R., Cohen F. E. Four helix bundle diversity in globular proteins. J Mol Biol. 1994 Mar 11;236(5):1356–1368. doi: 10.1016/0022-2836(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Hsiao K. K., Scott M., Foster D., Groth D. F., DeArmond S. J., Prusiner S. B. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science. 1990 Dec 14;250(4987):1587–1590. doi: 10.1126/science.1980379. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J. Human prion diseases with variant prion protein. Philos Trans R Soc Lond B Biol Sci. 1994 Mar 29;343(1306):391–398. doi: 10.1098/rstb.1994.0034. [DOI] [PubMed] [Google Scholar]
- Kneller D. G., Cohen F. E., Langridge R. Improvements in protein secondary structure prediction by an enhanced neural network. J Mol Biol. 1990 Jul 5;214(1):171–182. doi: 10.1016/0022-2836(90)90154-E. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Bessen R. A., Lehmann S., Hartsough G. R. Epidemiological and experimental studies on a new incident of transmissible mink encephalopathy. J Gen Virol. 1991 Mar;72(Pt 3):589–594. doi: 10.1099/0022-1317-72-3-589. [DOI] [PubMed] [Google Scholar]
- Medori R., Tritschler H. J., LeBlanc A., Villare F., Manetto V., Chen H. Y., Xue R., Leal S., Montagna P., Cortelli P. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med. 1992 Feb 13;326(7):444–449. doi: 10.1056/NEJM199202133260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monari L., Chen S. G., Brown P., Parchi P., Petersen R. B., Mikol J., Gray F., Cortelli P., Montagna P., Ghetti B. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2839–2842. doi: 10.1073/pnas.91.7.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottonen J., Strand A., Symersky J., Sweet R. M., Danley D. E., Geoghegan K. F., Gerard R. D., Goldsmith E. J. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992 Jan 16;355(6357):270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Owen F., Poulter M., Collinge J., Crow T. J. Codon 129 changes in the prion protein gene in Caucasians. Am J Hum Genet. 1990 Jun;46(6):1215–1216. [PMC free article] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. B., Tabaton M., Berg L., Schrank B., Torack R. M., Leal S., Julien J., Vital C., Deleplanque B., Pendlebury W. W. Analysis of the prion protein gene in thalamic dementia. Neurology. 1992 Oct;42(10):1859–1863. doi: 10.1212/wnl.42.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocchiari M., Salvatore M., Cutruzzolá F., Genuardi M., Allocatelli C. T., Masullo C., Macchi G., Alemá G., Galgani S., Xi Y. G. A new point mutation of the prion protein gene in Creutzfeldt-Jakob disease. Ann Neurol. 1993 Dec;34(6):802–807. doi: 10.1002/ana.410340608. [DOI] [PubMed] [Google Scholar]
- Presnell S. R., Cohen B. I., Cohen F. E. MacMatch: a tool for pattern-based protein secondary structure prediction. Comput Appl Biosci. 1993 Jun;9(3):373–374. doi: 10.1093/bioinformatics/9.3.373. [DOI] [PubMed] [Google Scholar]
- Presnell S. R., Cohen F. E. Topological distribution of four-alpha-helix bundles. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6592–6596. doi: 10.1073/pnas.86.17.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D., Serban A., Koehler R., Foster D., Torchia M., Burton D., Yang S. L., DeArmond S. J. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Hsiao K. K. Human prion diseases. Ann Neurol. 1994 Apr;35(4):385–395. doi: 10.1002/ana.410350404. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Richards F. M. Packing of alpha-helices: geometrical constraints and contact areas. J Mol Biol. 1978 Mar 15;119(4):537–555. doi: 10.1016/0022-2836(78)90201-2. [DOI] [PubMed] [Google Scholar]
- Ring C. S., Cohen F. E. Modeling protein structures: construction and their applications. FASEB J. 1993 Jun;7(9):783–790. doi: 10.1096/fasebj.7.9.8330685. [DOI] [PubMed] [Google Scholar]
- Ripoll L., Laplanche J. L., Salzmann M., Jouvet A., Planques B., Dussaucy M., Chatelain J., Beaudry P., Launay J. M. A new point mutation in the prion protein gene at codon 210 in Creutzfeldt-Jakob disease. Neurology. 1993 Oct;43(10):1934–1938. doi: 10.1212/wnl.43.10.1934. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M., Beaudette N. V., Fasman G. D. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B., Sander C. Jury returns on structure prediction. Nature. 1992 Dec 10;360(6404):540–540. doi: 10.1038/360540b0. [DOI] [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem. 1993 Sep 25;268(27):20276–20284. [PubMed] [Google Scholar]
- Safar J., Roller P. P., Gajdusek D. C., Gibbs C. J., Jr Thermal stability and conformational transitions of scrapie amyloid (prion) protein correlate with infectivity. Protein Sci. 1993 Dec;2(12):2206–2216. doi: 10.1002/pro.5560021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. J., Redfield C., Boyd J., Lawrence G. M., Edwards R. G., Smith R. A., Dobson C. M. Human interleukin 4. The solution structure of a four-helix bundle protein. J Mol Biol. 1992 Apr 20;224(4):899–904. doi: 10.1016/0022-2836(92)90457-u. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Teplow D. B., Hood L., Gibson B. W., Burlingame A. L., Prusiner S. B. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993 Mar 2;32(8):1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Westaway D., DeArmond S. J., Cayetano-Canlas J., Groth D., Foster D., Yang S. L., Torchia M., Carlson G. A., Prusiner S. B. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell. 1994 Jan 14;76(1):117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Westaway D., Zuliani V., Cooper C. M., Da Costa M., Neuman S., Jenny A. L., Detwiler L., Prusiner S. B. Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes Dev. 1994 Apr 15;8(8):959–969. doi: 10.1101/gad.8.8.959. [DOI] [PubMed] [Google Scholar]
- Wilesmith J. W., Wells G. A. Bovine spongiform encephalopathy. Curr Top Microbiol Immunol. 1991;172:21–38. doi: 10.1007/978-3-642-76540-7_2. [DOI] [PubMed] [Google Scholar]