Abstract
Sixteen non-ampullary duodenal neoplasms in 16 patients were treated with endoscopic submucosal dissection (ESD) performed by an endoscopist and an instrument assistant between February 2011 and November 2014. En bloc resection was performed in 15 cases (94%); lateral and vertical margins were pathologically free in 13 cases. Perforation occurred during submucosal dissection using a flex knife, but no perforations occurred in 15 cases using the hook knife only. No postoperative bleeding nor recurrence has been reported in any patient during the median 17-month follow-up period. Use of the hook knife as the main instrument and targeted training of the endoscopist and instrument assistant contributed to safe and effective duodenal ESD for non-ampullary duodenal neoplasms.
Introduction
Endoscopic submucosal dissection (ESD) has been widely used for the treatment of esophageal, gastric, and colorectal neoplasms associated with a minimal risk of regional lymph node and distant metastases. The use of ESD enables en bloc resection of neoplasms and detailed pathological assessment of resected specimens.1-4 ESD of the duodenum is difficult because of the thin wall of the duodenum, brunneromas in the submucosal layer, limited room for movement of the endoscope, and abundant digestive fluid. Because of the difficulty of the procedure, duodenal ESD is associated with a high risk of perforation. To date, there have been few reports of duodenal ESD.5-10 We aimed to investigate the safety and efficacy of ESD for non-ampullary duodenal neoplasms.
Case Report
Sixteen non-ampullary duodenal neoplasms in 16 patients were treated with ESD between February 2011 and November 2014. All duodenal neoplasms were classified into the following types on the basis of endoscopic findings: slightly elevated (0–IIa), flat (0–IIb), and slightly depressed without ulcer (0–IIc). Preoperative endoscopic biopsy was performed in all patients. Indications for ESD were: 1) superficial macroscopic appearance without ulceration and bank formation, 2) tubular adenoma or well-differentiated adenocarcinoma on the preoperative biopsy, and 3) lesions that could not be resected en bloc using endoscopic mucosal resection (EMR) because of the tumor size or scar formation secondary to previous biopsies.
An endoscopist (N.I.) and an instrument assistant (H.A.) performed all duodenal ESDs under general anesthesia or conscious sedation, based on the endoscopist's judgment of neoplasm size. The methods of ESD used in this study were similar to those previously reported.1-10 Images of the procedure are shown in Figure 1 and Video 1. Duodenal ESD was performed with a flex knife (KD-630L; Olympus Medical Systems, Tokyo, Japan)4 and/or a hook knife (KD-620LR; Olympus Medical Systems, Tokyo, Japan).1 A small-caliber-tip transparent hood (DH-15GR; Fujinon Toshiba ES Systems, Co., Saitama, Japan) was used to obtain a satisfactory view during ESD.3,5 The ulcer left after excision of the lesion was closed with hemoclips where possible (Figure 2). In some patients for whom complete closure could not be achieved with hemoclips, the post-ESD ulcer was shielded with polyglycolic acid (PGA) sheets (Neoveil; Gunze Co., Kyoto, Japan) and fibrin glue (Beriplast P Combi-set; CSL Behring Pharma, Tokyo, Japan) as described by Takimoto et al.6 For ESD in the second and third part of the duodenum, a duodenal tube was placed near the excision site after ESD and continuous aspiration (−10 cm H2O) was performed through the tube for 24 h with the intent of preventing delayed perforation caused by abundant digestive fluid. Octreotide acetate 300 μg was infused over a 24-h period after duodenal ESD.
Figure 1.

Endoscopic view showing (A) a slightly elevated type 0–IIa neoplasm in the second part of the duodenum, (B) marking dots made around the lesion, (C) difficult submucosal dissection because of brunneromas in the submucosal layer, and (D) the post-ESD ulcer.
Figure 2.
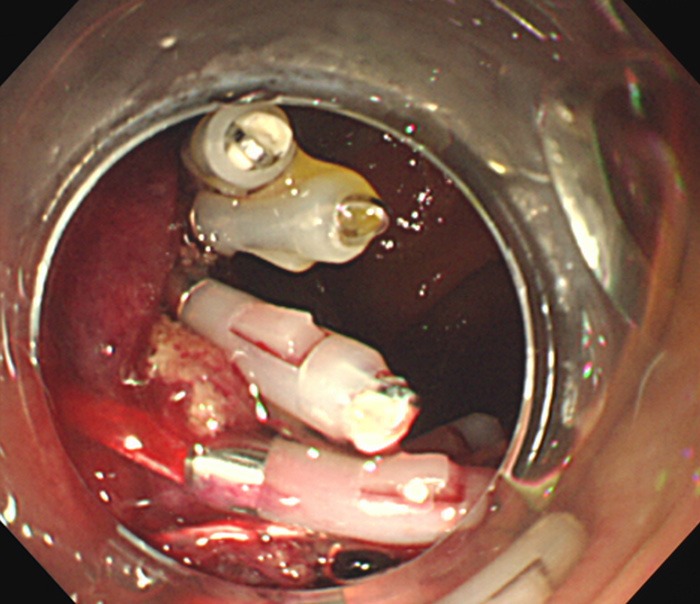
The ulcer left after excision of the lesion closed with hemoclips.
Video 1.
Duodenal ESD using the hook knife. Please view the video at:
The size of the neoplasms and the resected specimens (mm), ESD duration (time required from marking to complete resection; min), and adverse events such as perforation and postoperative bleeding were evaluated in each case. The rate of en bloc resection for the series was determined. A pathologist microscopically evaluated the ESD specimens (2-mm slices) for histopathological type, depth of invasion, and lateral and vertical resection margins (Figure 3). On the basis of preoperative endoscopic findings, all duodenal neoplasms except 1 lesion in the descending part were classified as slightly elevated types (0–IIa; Table 1). Endoscopic biopsy performed before ESD identified the neoplasms as low-grade adenoma (n=12) versus high-grade adenoma or well-differentiated adenocarcinoma (n=4). However, histopathological examinations of the resected specimens identified the neoplasms as brunneroma (n=1), low-grade adenoma (n=2), and high-grade adenoma or well-differentiated adenocarcinoma (n=13).
Figure 3.
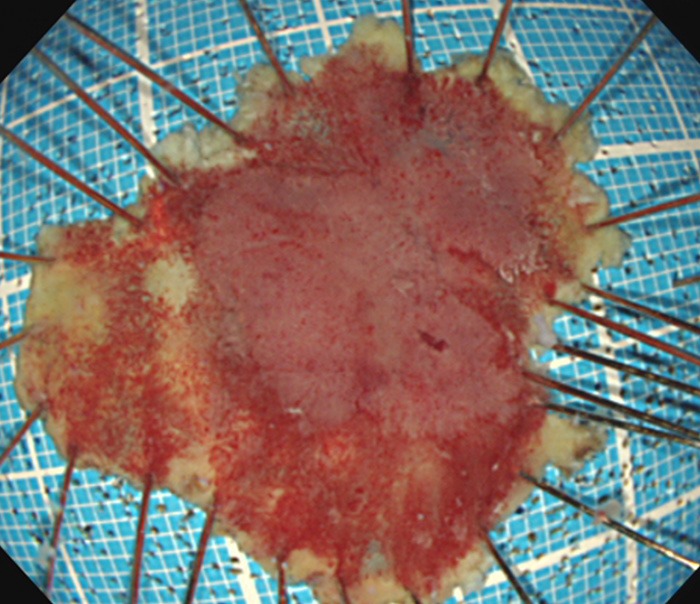
Resected specimen (32 × 28 mm).
Table 1.
Characteristics and Results of 16 Duodenal Neoplasms Treated With ESD
| Patient and Neoplasm Characteristics | Results |
|---|---|
| Gender: m/f | 10/6 |
| Median age, y (range) | 58 (44–78) |
| Location: first/second/third duodenal section | 2/12/2 |
| Macroscopic type: IIa/IIc | 15/1 |
| Histology of pre-op biopsies: LGA-HGA/IC | 12/4 |
| Conscious sedation/general anesthesia | 7/9 |
| Histology of resected specimens: Brunneroma/LGA-HGA/IC | 1/2/13 |
| Median neoplasm size, mm (range) | 13 (5–35) |
| Median ESD specimen size, mm (range) | 21 (15–40) |
| Median ESD duration, min (range) | 66 (35–255) |
| Rate of en bloc resection, % | 94 |
| Complete/incomplete closure with hemoclips | 10/6 |
| Rate of pathological free margins, % | 81 |
| Adverse events: bleeding/perforations | 0/1 |
| Median hospital duration, days (range) | 7 (6–36) |
ESD = endoscopic submucosal dissection; HGA = high-grade adenoma; IC = intramucosal carcinoma; IIa = slightly elevated type; IIc = slightly depressed type without ulcer; LGA = low-grade adenoma.
En bloc resection was performed in 15 patients; one patient had piecemeal resection for a neoplasm located in the second part of the duodenum that extended semi-circumferentially. Complete closure with hemoclips was achieved in 10 patients (63%; 9 neoplasms in the second part of the duodenum and 1 in the third part), but not in 6 patients (3 neoplasms in the first part of the duodenum and 3 in the second part). Shielding with PGA sheets and fibrin glue was performed for 2 cases in the second part of the duodenum.
Lateral and vertical margins were pathologically free in 13 patients who underwent en bloc resection. The lateral margin was positive in 2 patients with intramucosal carcinoma in the second part of the duodenum, and 1 patient underwent piecemeal resection. Perforation occurred during submucosal dissection using the flex knife in 1 patient with a neoplasm located in the third part of the duodenum and urgent surgery was performed after additional snare EMR. No perforation occurred in the 15 patients treated with the hook knife only. Median hospital duration was 7 days (range: 6–36). However, the patient who received urgent surgery needed a longer hospital stay (36 days) due to postoperative partial small bowel obstruction. There was no postoperative bleeding and no recurrence in any patient during the median follow-up period of 17 months (range: 2–47).
Discussion
EMR has played an important role in the management of non-ampullary duodenal neoplasms, though recently, ESD has been performed for these neoplasms.5-10 Although the rates of en bloc resection and free pathological margins for ESD were higher than those for EMR, ESD was time-consuming and carried a higher risk of perforation.5-10
We evaluated the safety and efficacy of ESD for non-ampullary duodenal neoplasms. One of the most important factors contributing to the safe completion of duodenal ESD is careful dissection of the submucosal layer to prevent perforation. The hook knife is useful for accurate dissection of the submucosal layer.1 However, the endoscopist and the instrument assistant need to be highly trained in the use of the hook knife, which is used to gradually and cautiously hook up the submucosal layer enabling precise dissection, avoiding perforation. In our study, the only case of perforation occurred with the flex knife.
Some endoscopic methods, such as over-the-scope clipping and shielding with PGA sheets and fibrin glue, have been used to manage the post-ESD ulcer.6,7 In our study, endoscopic closure with hemoclips was attempted first, but shielding with PGA sheets and fibrin glue was used for 2 neoplasms that could not be adequately closed with hemoclips. Better methods for supporting post-ESD ulcers, along with postoperative management such as continuous aspiration and injection of octreotide acetate, may be the focus of future research. Although the number of patients was small and the study was retrospective, the use of the hook knife as the main instrument and targeted training of the endoscopist and instrument assistant contributed to safe and effective duodenal ESD for non-ampullary duodenal neoplasms.
Disclosures
Author contributions: N. Ishii, H. Akiyama, and K. Suzuki designed the study, acquired, analyzed, and interpreted the data, and drafted the manuscript. Y. Fujita supervised the study. N. Ishii is the article guarantor.
Financial disclosure: None to report.
This study was approved by the ethical committee of our hospital, and informed consent was obtained from all patients.
References
- 1. Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;37( suppl 1): S67–70. [DOI] [PubMed] [Google Scholar]
- 2. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48(2):225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35(8):690–694. [DOI] [PubMed] [Google Scholar]
- 4. Yahagi N, Fujishiro M, Imagawa A, et al. Endoscopic submucosal dissection for the reliable en bloc resection of colorectal mucosal tumors. Dig Endosc. 2004;16( suppl 1): 89–92. [Google Scholar]
- 5. Honda T, Yamamoto H, Osawa H, et al. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21(4):270–274. [DOI] [PubMed] [Google Scholar]
- 6. Takimoto K, Toyonaga T, Matsuyama K.. Endoscopic tissue shielding to prevent delayed perforation associated with endoscopic submucosal dissection for duodenal neoplasms. Endoscopy. 2012;44( suppl 2): 414–415. [DOI] [PubMed] [Google Scholar]
- 7. Mori H, Shintaro F, Kobara H, et al. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2013;25(4):459–461. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto S, Miyatani H, Yoshida Y.. Endoscopic submucosal dissection for duodenal tumors: A single-center experience. Endoscopy. 2013;45(2):136–137. [DOI] [PubMed] [Google Scholar]
- 9. Inoue T, Uedo N, Yamashina T, et al. Delayed perforation: A hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26(2):220–227. [DOI] [PubMed] [Google Scholar]
- 10. Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47(2):129–135. [DOI] [PubMed] [Google Scholar]


