ABSTRACT
Both DICER and DROSHA are RNase III enzymes involved in the biogenesis of small noncoding RNAs. DROSHA cleaves the stem-loop portion of the primary miRNAs and produces precursor miRNAs in the nucleus, whereas DICER processes double-stranded RNA precursors into mature miRNAs and endogenous small interference RNAs in the cytoplasm. Selective inactivation of Dicer in growing oocytes of primary follicles leads to female infertility due to oocyte spindle defects. However, it remains unknown if oocyte Dicer expression in the fetal ovary is required for proper follicular development in the postnatal ovary. Moreover, the role of Drosha in folliculogenesis has never been investigated. Here, we report that conditional knockout of Dicer in prophase I oocytes of the fetal ovary led to compromised folliculogenesis, premature ovarian failure, and female infertility in the adult ovary, whereas selective inactivation of Drosha in oocytes of either the fetal or the developing ovary had no effects on normal folliculogenesis and female fertility in adulthood. Our data indicate that oocyte DICER expression in the fetal ovary is required, and oocyte DROSHA is dispensable, for postnatal follicular development and female fertility in adulthood.
Keywords: fertility, meiosis, oocyte, oogenesis, ovary, ovulation, posttranscriptional regulation, premature ovarian failure, primordial germ cells, small RNA, spindle
INTRODUCTION
Sexual reproduction requires gametes from both sexes. Male and female gametes (i.e., sperm and oocytes) are both derived from primordial germ cells (PGCs) [1]. During embryonic development, both male and female PGCs multiply themselves through mitosis and then adopt different differentiation pathways: prospermatogonia in the testis remain in G0 phase until the onset of pubertal testicular development, whereas oogonia in the ovary enter meiosis prophase I and become oocytes followed by a late prophase I arrest at birth [2, 3]. The number of available primordial follicles at birth, which is believed to represent the total follicular reserve for the entire reproductive lifespan in a female, is determined by the proliferation rate of the female PGCs and also the rate of PGCs committing meiosis during embryonic ovarian development [4]. Upon puberty, mature oocytes are produced via folliculogenesis, a process through which primordial follicles develop into primary, secondary, and eventually antral follicles competent for ovulation. During folliculogenesis, both oocytes and follicular cells are subject to complex regulation of gene expression, which can occur at multiple levels, including transcriptional, posttranscriptional, and posttranslational [5]. In oocytes, posttranscriptional regulation is prominent because numerous maternal transcripts need to be produced and stored for usage during fertilization and during postfertilization development [6]. RNA-binding proteins (RBPs) have long been suggested to be involved in the control of mRNA fate, and thus research efforts have been focused on oocyte-expressed RBPs for the past several decades [6]. Small noncoding RNAs (sncRNAs) represent another critical component in the complex machinery of posttranscriptional regulation [7–9]. Among numerous sncRNAs, miRNAs and endogenous small interference RNAs (endo-siRNAs) are well studied and both function to affect stability and translational efficiency through binding 3′ untranslated regions of their target mRNAs [7, 10]. The biogenetic pathways of these two types of sncRNAs have been defined, both of which involve a cytoplasmic RNase III, DICER, and miRNA production also requires another nuclear RNase III, i.e., DROSHA [11–14].
Similar to mRNA genes, miRNA genes are transcribed into primary miRNA transcripts (pri-miRNAs) with 5′ caps, 3′ polyA tails, and a stem-loop region containing the mature miRNA sequences [15]. The stem-loop portion of pri-miRNAs can be recognized by the microprocessor complex, which contains DROSHA, a nuclear RNase III, and its cofactor DGCR8, and cleaves to form precursor miRNAs (pre-miRNAs), which are then exported from the nucleus to the cytoplasm by a RAN-binding protein called EXPORTIN5 [16, 17]. Once in the cytoplasm, DICER recognizes the double-stranded RNA in the stem-loop region of pre-miRNAs and cleaves out two mature miRNAs [15]. Endo-siRNAs are processed by DICER in the cytoplasm using naturally formed double-stranded RNAs (dsRNAs), which can be derived from two mRNAs with partial segments that are complementary [15]. Endo-siRNAs have been identified in male and female germ cells as well as in embryonic stem cells [15]. Because endo-siRNAs are processed in the cytoplasm, its biogenesis requires DICER, but not DROSHA [15–17].
The early embryonic lethality phenotype (i.e., embryo dies at Embryonic Day 6.5 [E6.5]–E7.5) in global Dicer, Dgcr8, or Drosha knockout (KO) mice highlights the necessity of miRNAs and endo-siRNAs in development [11–14]. Studies using Dicer or Drosha conditional KO (cKO) mice have demonstrated that miRNAs and endo-siRNAs play critical roles in both the development and functions of almost all organs and cell types [18]. Dicer plays an essential role in both male and female germ cell development [19–27]. In the male, selective inactivation of Dicer or Drosha in postnatal spermatogenic cells causes disrupted spermatogenesis characterized by severe depletion of spermatocytes and spermatids, azoospermia, and complete male infertility [21, 23–27]. Although selective inactivation of Dicer in growing oocytes of primary follicles does not cause obvious disruptions in folliculogenesis, the Dicer-deficient oocytes fail to mature properly and display aberrant spindle integrity and misalignment of chromosomes [20]. Consequently, those Zp3-Dicer cKO females are completely infertile. This finding was initially interpreted to reflect the necessity of miRNAs in oocyte maturation, but later noted as an effect of endo-siRNA depletion due to DICER deficiency because miRNA depletion by Dgcr8 inactivation in growing oocytes of primary follicles does not disrupt folliculogenesis, and Dgcr8-null oocytes develop normally and are fertile [28]. These earlier studies suggest that it is endo-siRNAs, but not miRNAs, that are essential for oocyte maturation and thus female fertility [29]. In this study, we aimed to answer the following questions: 1) Does Dgcr8 inactivation phenocopy Drosha inactivation? 2) Is Dicer or Drosha expression in early prophase I oocytes needed for follicular development in the developing and adult ovaries? 3) Is DROSHA required for folliculogenesis and female fertility? Here, we report that inactivation of oocyte Dicer in the fetal ovary led to compromised folliculogenesis and premature ovarian failure in the adult ovary, whereas inactivation of Drosha in oocytes of either fetal or developing ovaries did not affect folliculogenesis and female fertility in adulthood.
MATERIALS AND METHODS
Animal Use and Generation of cKO Mice
The Institutional Animal Care and Use Committee of the University of Nevada, Reno, approved all animal use protocols. All mice were housed and maintained under specific pathogen-free conditions with a temperature- and humidity-controlled animal facility in the University of Nevada, Reno. All flox and Cre lines used in this study were purchased from the Jackson Laboratory. All mouse lines were backcrossed for five generations to get onto the C57B6/6J background. Adult Dicerflox/flox female mice [30] were bred with adult Ddx4-Cre male mice [31] to generate Ddx4-Cre; Dicer+/flox offspring, which were further crossed with Dicerflox/flox mice to obtain Ddx4-Cre; Dicerflox/flox female mice. Droshaflox/flox male mice [14] were bred with Zp3-Cre female mice [32] to generate Zp3-Cre; Drosha+/flox mice, which were then further crossed with Droshaflox/flox mice to obtain Zp3-Cre; Droshaflox/flox females for this study. To generate Drosha global KO mice, Zp3-Cre; Drosha+/flox female mice were mated with wild-type (WT) male mice to obtain Drosha+/del mice, which were then further intercrossed to generate Droshadel/del (global KO) mice.
Fertility Test and Oocyte Collection
For fertility test, six 6-wk-old Ddx4-Cre; Dicerflox/flox or Zp3-Cre; Droshaflox/flox female mice were bred with adult WT males of proven fertility for a period of 10 wk. To collect fully grown, germinal vesicle (GV)-intact, cumulus-enclosed oocytes, 4- to 6-wk-old WT, Ddx4-Cre; Dicerflox/flox, and Zp3-Cre; Droshaflox/flox female mice were primed with equine chorionic gonadotropin (eCG; 5 IU/mouse) via i.p. injection. The primed mice were killed 46–48 h after eCG treatment, and the ovaries were dissected in M2 medium to release GV-stage oocytes. For mature (metaphase II [MII] stage) oocytes, mice were first injected with eCG (5 IU/mouse, i.p.) and subsequently with human chorionic gonadotropin (hCG; 5 IU/mouse, i.p.) 48 h after eCG treatment, followed by collecting MII-stage oocytes with cumulus cells from oviduct 14–16 h after hCG treatment. The GV- and MII-stage oocytes were treated with 0.1% bovine testicular hyaluronidase in M2 medium at 37°C for 2–3 min to remove cumulus cells. The cumulus-free GV and MII oocytes were washed three times in M2 medium and used for subsequent molecular analyses.
Mouse Genotyping
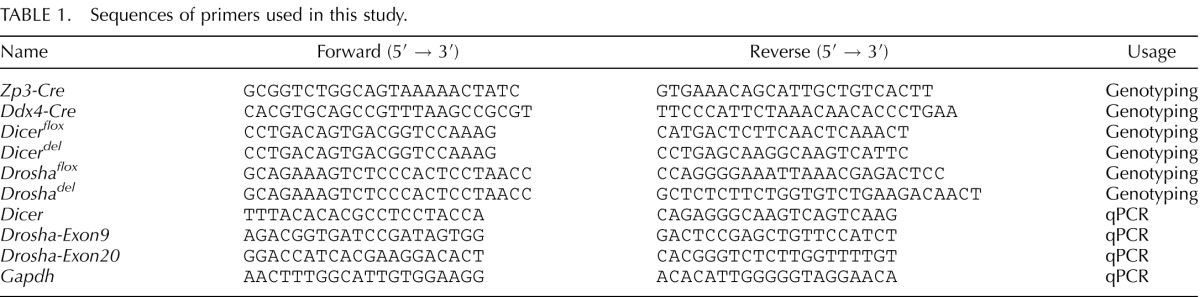
Mouse DNA was prepared by digestion of tail snips in a lysis buffer (40 mM NaOH, 0.2 mM EDTA) for 2 h at 95°C, followed by neutralization using the same volume of neutralizing buffer (40 mM Tris-HCl). PCR reactions were conducted in a 20-μl volume with tail DNA (1 μl), H2O (7 μl), 2× GoTaqGreen master mix (10 μl) (Promega, Cat. No. M7122), and 10 μM of forward and reverse primer (1 μl each). The primers used for genotyping are listed in Table 1. The PCR conditions were 95°C 2 min; (95°C 30 sec, 60°C 30 sec, 72°C 1 min) × 33 cycles; 72°C 5 min.
TABLE 1.
Sequences of primers used in this study.
Dissection of Early Mouse Embryos
The presence of vaginal plugs in female Drosha+/del mice mated with Drosha+/del males was marked as E0.5. At E6.5, E7.5, and E8.5, the pregnant females were euthanized and the embryos were dissected as described [33]. After photography, a portion of the embryo was washed three times in PBS, and then put into individual PCR tubes, each containing 30 μl of embryo lysis buffer (1 mM Tris-HCl, pH 8.4, 50 mM KCl, 2 mM MgCl2, 0.45% NP-40, 0.45% Tween20, and 180 μg/ml protease K), followed by incubation at 55°C for 5 h and boiling 10 min at 95°C. An aliquot of 5 μl embryo lysates was used for PCR genotyping using primer sets described in Table 1.
Histology and Immunohistochemistry
Ovaries were dissected and fixed in Bouin fixative overnight at 4°C followed by paraffin embedding. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E) for histological examination. For oocyte immunostaining, oocytes of different genotypes were fixed in 4% paraformaldehyde in M2 medium for 1 h and washed three times in 0.1 M glycine with 0.3 mg/ml bovine serum albumin (BSA) at room temperature (RT), followed by permeabilization with 0.2% Triton X-100 in PBS for 15 min and blocking with a buffer (2% BSA in PBS) for 1 h at RT. The oocytes were then incubated with the following antibodies diluted in blocking buffer for 1 h at RT: rabbit anti-DROSHA polyclonal antibody (Cat. No. ab12286; 1:500 dilution; Abcam) and mouse anti-beta-tubulin (Cat. No. T5293; 1:500 dilution; Sigma). After three washes with the blocking buffer, oocytes were incubated with fluorescence-conjugated, species-specific secondary antibodies (Alexa Fluor 594 goat anti-mouse IgG [H + L] and Alexa Fluor 568 goat anti-rabbit IgG [H + L]; 1:2000 dilution; Invitrogen Molecular Probes) for 1 h at RT. Finally, the oocytes were counterstained with 4′, 6-diamidino-2-phenylindole dilactate (DAPI; Sigma) for indirect immunofluorescent assays using a fluorescence microscope (AxioVision; Carl Zeiss).
Quantitative Analyses of Follicles
Quantitative analyses of various follicles in cKO and WT control ovaries at Postnatal Day 30 (P30), P40, and P120 were conducted as described [34]. Briefly, serial sections (8 μm) of the ovarian paraffin blocks were cut, and every 10th section was mounted onto slides, followed by H&E staining. The numbers of primordial, primary, secondary, and antral follicles were then counted under a microscope. To avoid counting follicles twice, only follicles with a visible nucleolus in the oocyte were counted. The number of follicles on each slide was multiplied by 10 and subsequently multiplied by 8 to obtain the estimated total number of follicles in each ovary.
Single-Oocyte Quantitative PCR Analyses
Single GV or MII oocytes were collected from WT, Ddx4-Dicer cKO, and Zp3-Drosha cKO females and treated with hyaluronidase to remove cumulus cells. Single oocytes were then individually put into PCR tubes containing 5 μl CellDirect 2× Reaction Mix lysis buffer (One-Step quantitative PCR [qPCR] kit; Invitrogen) and immediately frozen in liquid nitrogen and stored at −80°C. Complementary DNA synthesis and specific target amplification using single oocytes were performed according to the manufacturer's instructions (One-Step qPCR kit). The qPCR analyses were conducted using Drosha- or Dicer-specific primers, and Gapdh was used as a reference gene (Table 1).
Statistical Analyses
Data are presented as mean ± SEM, and statistical differences between datasets were assessed by one-way ANOVA or t-test using SPSS 16.0 software. P < 0.05 and P < 0.01 were considered as significant and highly significant differences, respectively.
RESULTS
Global Inactivation of Drosha Leads to Embryonic Lethality
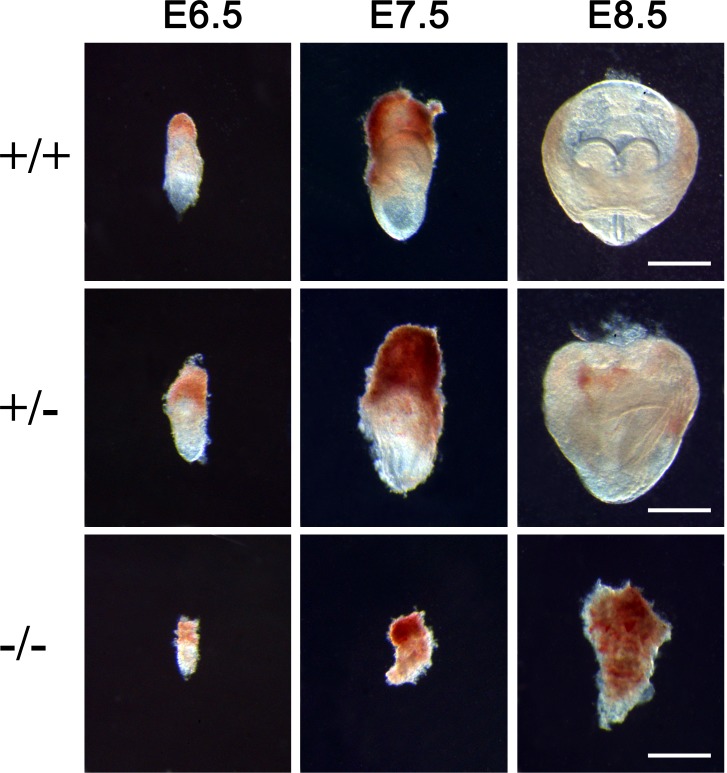
Global ablation of DGCR8, a cofactor of DROSHA in miRNA biogenesis, led to embryonic lethality at E6.5 [12, 13]. Because DGCR8 acts as an essential cofactor for DROSHA RNase III activity during pre-miRNA production [15, 29], Drosha global KO mice should, in theory, phenocopy DGCR8 KOs. Indeed, among 61 pups produced in eight litters by four heterozygous breeding pairs (Drosha+/del X Drosha+/del), only WT (19/61, 31%) and heterozygotes (42/61, 69%) were obtained, and no homozygous pups were ever found, suggesting a potential embryonic lethality phenotype. To determine the timing of embryonic lethality, we collected the embryos from Drosha heterozygous females mated with heterozygous males at E6.5, E7.5, and E8.5 and examined the morphology (Fig. 1). Drosha−/− embryos were much smaller than WT or heterozygous embryos at E6.5 and degenerated between E7.5 and E8.5 (Fig. 1), suggesting that Drosha-null embryos also die at ∼E6.5.
FIG. 1.
Drosha−/− embryos died at E6.5. WT (+/+) and Drosha heterozygous (+/−) embryos developed normally between E6.5 and E8.5, whereas Drosha-null (−/−) embryos ceased growth at E6.5 and degenerated by E8.5. Bar = 200 μm.
Generation of Ddx4-Dicer cKO and Zp3-Drosha cKO Mice
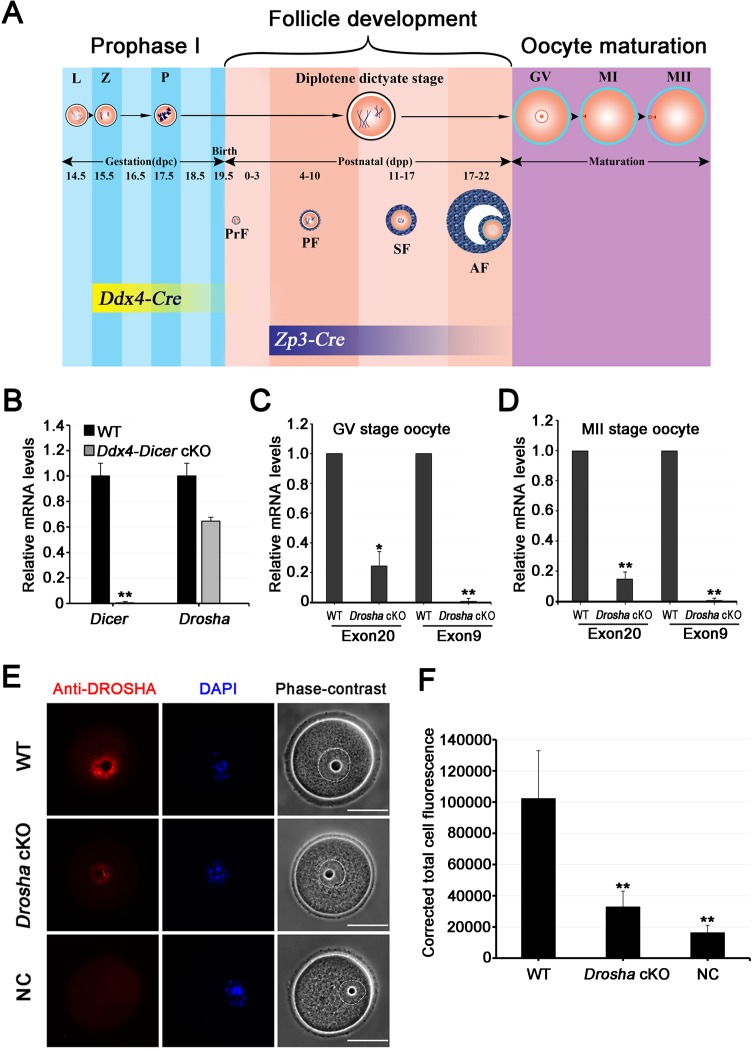
A previous study has demonstrated that conditional inactivation of Dicer in growing oocytes of developing follicles causes spindle defects and female infertility in mice [20]. However, it remains unknown whether inactivation of Dicer in oocytes of fetal ovaries will affect follicular development in postnatal ovaries. To address this question, we crossed Dicerflox/flox female mice with male Ddx4-Cre mice. The Dicer conditional allele contains two loxp sites flanking exon 23 of the Dicer gene, which encodes for a critical portion of the catalytic domain [30], whereas the Ddx4-Cre line expresses Cre recombinase exclusively in PGCs starting at E15.5 (Fig. 2A) [31]. Thus, the Ddx4-Cre; Dicerflox/flox female mice should lack DICER in oocytes of fetal ovaries at E15.5 and thereafter. We also crossed Droshaflox/flox mice with a Zp3-Cre deleter line, which starts to express Cre exclusively in growing oocytes of primary follicles (Fig. 2A) [32, 35, 36]. The Drosha conditional allele has two loxp sites flanking exon 9, and the deletion of exon 9 leads to frame shift and multiple premature stop codons [14].
FIG. 2.
Selective inactivation of Dicer or Drosha in prophase I oocytes of the fetal ovary or in growing oocytes of developing follicles. A) Schematic timeline of Cre expression during oocyte and follicular development. The Ddx4-Cre deleter line starts to express CRE in early prophase I oocytes at E15.5, whereas Zp3-Cre mice display CRE activity in growing oocytes of developing follicles. L, leptotene; Z, zygotene; P, pachytene; MI, metaphase I; PrF, primordial follicles; PF, primary follicle; SF, secondary follicle; AF, antral follicle. B) The qPCR analyses of Dicer and Drosha mRNA levels in WT and Ddx4-Dicer cKO oocytes. C) The qPCR analyses of levels of exon 9- and exon 20-containing Drosha mRNAs in WT and Zp3-Drosha cKO GV oocytes. D) The qPCR analyses of levels of exon 9- and exon 20-containing Drosha mRNAs in WT and Zp3-Drosha MII oocytes. Note that in all qPCR analyses (B–D), relative mRNA levels were determined using the ΔΔCT method. Data are presented as means ± SEM (n = 6). *P < 0.05; **P < 0.01. E) Immunofluorescent staining of DROSHA in WT and Zp3-Drosha cKO GV-stage oocytes. DNA was visualized with DAPI (blue), and morphology was shown by the phase-contrast images. Dashed lines circle the nuclear membrane. All images were taken using the same exposure time. Bar = 50 μm. F) Quantitative analyses of immunofluorescent intensity of DROSHA in WT and Zp3-Drosha cKO GV-stage oocytes. Data are presented as means ± SEM (n = 10). **P < 0.01.
To confirm the inactivation of Dicer or Drosha, we performed qPCR analyses using oocytes collected from Ddx4-Cre; Dicerflox/flox (herein called Ddx4-Dicer cKO) and Zp3-Cre; Droshaflox/flox (herein called Zp3-Drosha cKO) female mice. In Ddx4-Dicer cKO oocytes, no expression of exon 23-containing Dicer mRNAs was detected, whereas Drosha mRNA levels appeared to be slightly reduced (Fig. 2B). Similarly, exon 9-carrying Drosha transcripts were completely absent, whereas the exon 20 transcripts persisted despite drastically decreased levels in both GV and MII oocytes collected from Zp3-Drosha cKO females (Fig. 2, C and D). Further immunofluorescent staining detected markedly reduced levels of DROSHA in Drosha cKO GV-stage oocytes (Fig. 2, E and F). These results indicate that although Drosha mRNAs were still transcribed after the loss of exon 9, production of the truncated DROSHA appeared to be minimal. Therefore, Dicer and Drosha were indeed inactivated in the Ddx4-Dicer and Zp3-Drosha cKO oocytes, respectively.
Inactivation of Oocyte Dicer in the Fetal Ovary Leads to Compromised Folliculogenesis and Premature Ovarian Failure in the Adult Ovary
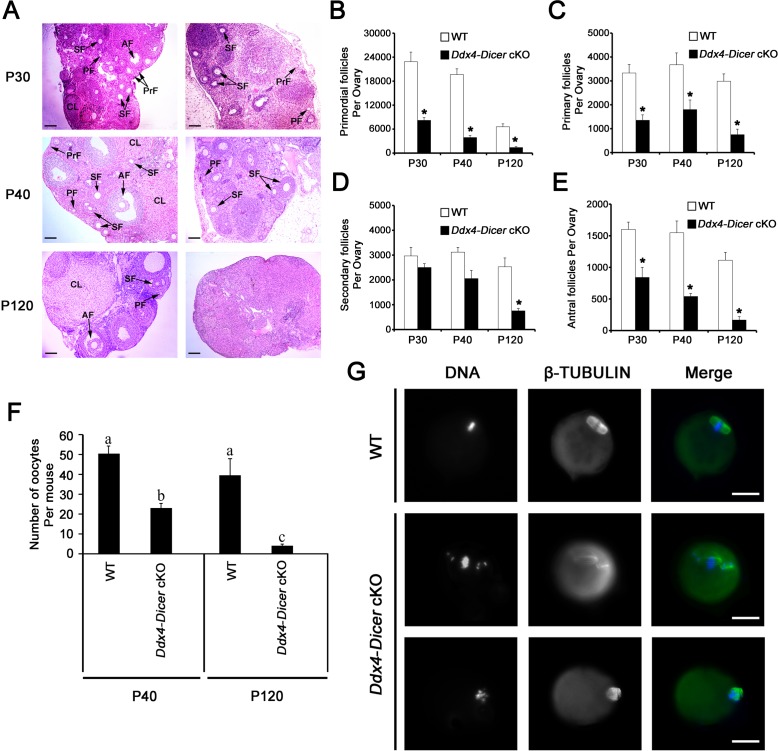
Ddx4-Dicer cKO female mice produced no offspring during a 10-wk fertility test by breeding with males of proven fertility, suggesting that Ddx4-Dicer cKO females are infertile. To explore potential ovarian defects, we examined the histology of Ddx4-Dicer cKO ovaries at P30, P40, and P120. At P30 and P40, WT ovaries contained all types of follicles, including primordial, primary, secondary, and antral follicles, whereas primordial and antral follicles were rarely seen in Ddx4-Dicer cKO ovaries (Fig. 3A). At P120, WT ovaries displayed dynamic folliculogenesis with all types of follicles and corpora lutea present, whereas Ddx4-Dicer cKO ovaries appeared to lack any developing follicles (Fig. 3A), suggesting a progressive loss of follicles.
FIG. 3.
Lack of Dicer in early prophase I oocytes leads to compromised folliculogenesis, premature ovarian failure, and oocyte maturation defects in the adult ovary. A) Ovarian histology of WT and Ddx4-Dicer cKO females at P30, P40, and P120. H&E-stained cross sections of WT (left panels) ovaries show normal folliculogenesis at all three age groups. In Ddx4-Dicer cKO (right panels) ovaries, primordial follicles (PrF), and antral follicles (AF) are rarely seen at P30 and P40, and follicles are largely lacking at P120 (right lower panel). PF, primary follicle; SF, secondary follicle; CL, corpus luteum. Bar = 100 μm. B–E) The numbers of PrF (B), PF (C), SF (D), and AF (E) in WT and Ddx4-Dicer cKO ovaries at P30, P40, and P120. Bars represent means ± SEM. *P < 0.05 (n = 3 for both WT and Ddx4-Dicer cKO at P30; n = 3 for WT and n = 4 for Ddx4-Dicer cKO at P40; n = 3 for both WT and Ddx4-Dicer cKO at P120). F) The number of GV-stage oocytes collected from WT and Ddx4-Dicer cKO females after superovulation at P40 and P120. Bars represent means ± SEM (n = 12 for WT, n = 4 for Ddx4-Cre-Dicer cKO at P40; n = 8 for WT, n = 3 for Ddx4-Dicer cKO at P120). Data marked with different letters are statistically significant (P < 0.05). G) Spindle defects in Ddx4-Dicer cKO oocytes revealed by immunofluorescent analyses. The spindle was stained with β-tubulin antibody, and DNA was counterstained with DAPI. Representative images are shown. Bar = 50 μm.
To determine the process of follicle depletion in Ddx4-Dicer cKO ovaries, we counted various follicles in WT and Ddx4-Dicer cKO ovaries at P30, P40, and P120 (Fig. 3, B–E). The number of primordial follicles was significantly smaller in Ddx4-Dicer cKO ovaries than in WT ovaries at all three age groups (Fig. 3B). Despite a slight increase in the number of primary follicles in Ddx4-Dicer cKO ovaries from P30 to P40, the primary follicle number was much smaller than that in WT ovaries at all three time points (Fig. 3C). Interestingly, although the number of secondary follicles was drastically decreased between P40 and P120 in Ddx4-Dicer cKO ovaries, WT and Ddx4-Dicer cKO ovaries appeared to contain comparable numbers of secondary follicles at both P30 and P40 (Fig. 3D). Similar to primary follicles, antral follicles were significantly fewer in Ddx4-Dicer cKO ovaries than in WT ovaries at all three time points (Fig. 3E). Overall, these results suggest that premature ovarian failure in Ddx4-Dicer cKO females most likely results from defects at multiple steps during follicular development, including the reduced primordial follicle formation and enhanced depletion of developing follicles during folliculogenesis. Consistent with the histological observation, superovulation experiments indicated that the numbers of oocytes recovered from Ddx4-Dicer cKO mice were only ∼50% and ∼10% of those from WT females at P40 and P120, respectively (Fig. 3F). These data suggest that folliculogenesis in Ddx4-Dicer cKO females is compromised, and the primordial follicle pool is exhausted by the age of ∼4 mo, resembling premature ovarian failure in humans.
Earlier studies have shown that although folliculogenesis appears to be normal in Zp3-Cre; Dicerflox/flox females, oocytes from these mice display spindle defects [20]. To determine whether oocytes collected from Ddx4-Dicer cKO females also had similar defects, we performed immunofluorescent staining of the spindle using β-tubulin antibodies (Fig. 3G). Consistent with the previous report [20], oocytes of Ddx4-Dicer cKO females frequently displayed multiple spindles with misaligned chromosomes strewn around the spindle axes, whereas WT oocytes formed a single barrel-shaped spindle with integrated and congregated chromosomes at the metaphase plate (Fig. 3G). Taken together, our data suggest that loss of oocyte Dicer in the fetal ovary leads to defective follicular development in the postnatal and adult ovaries.
Oocyte Drosha Is Dispensable for Follicular Development
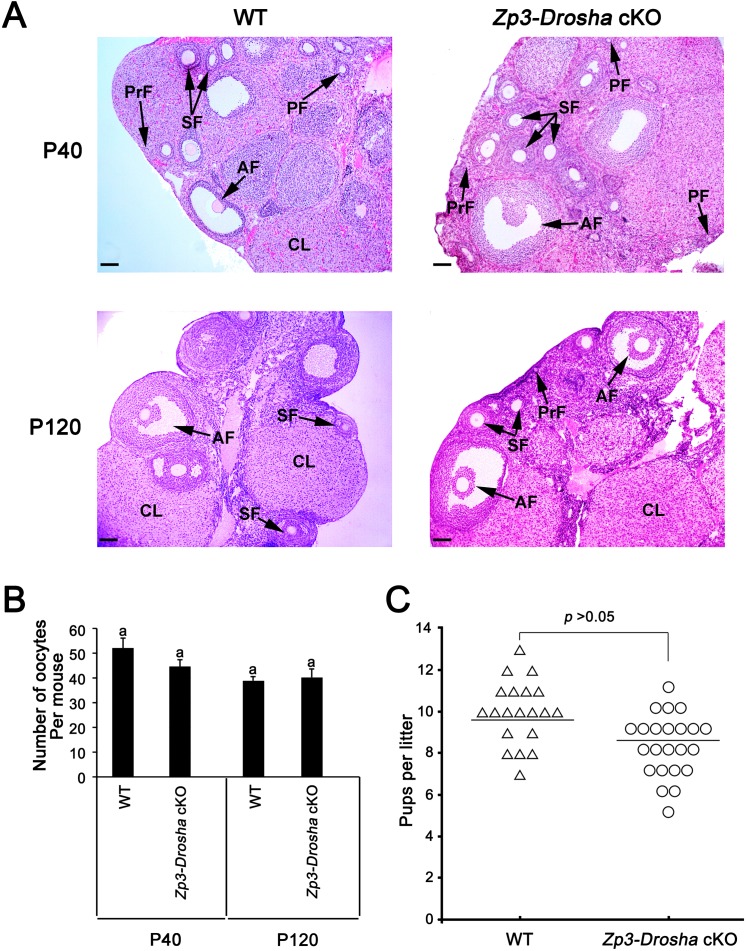
Histology of Zp3-Drosha cKO ovaries was indistinguishable from that of WT controls at P40 and P120 (Fig. 4A). Similar numbers of oocytes were recovered from both Zp3-Drosha cKO and WT females (Fig. 4B). A 10-wk fertility test by breeding the Zp3-Drosha cKO females with fertility-proven WT male mice revealed normal fertility (Fig. 4C). Similar to Zp3-Drosha cKO female mice, Ddx4-Cre; Droshaflox/flox female mice that we generated were completely fertile (data not shown). Moreover, our breeding records showed no fertility decrease in either Ddx4-Drosha cKO or Zp3-Drosha cKO female mice older than 6 mo, suggesting that Drosha deficiency does not cause latent phenotype in aging female mice. Together, these data suggest that oocyte Drosha is dispensable for normal follicular development.
FIG. 4.
Oocyte Drosha is dispensable for folliculogenesis. A) Ovarian histology of WT and Zp3-Drosha cKO females at P40 and P120. H&E-stained cross sections of WT (left panels) and Zp3-Drosha cKO (right panels) ovaries show normal folliculogenesis at both P40 and P120. PrF, primordial follicles; PF, primary follicle; SF, secondary follicle; AF, antral follicle; CL, corpus luteum. Bar = 100 μm. B) The number of GV-stage oocytes recovered from WT and Zp3-Drosha cKO females after superovulation at P40 and P120. Bars represent means ± SEM (n = 12 for WT, n = 6 for Zp3-Drosha cKO at P40; n = 8 for WT, n = 9 for Zp3-Drosha cKO at P120). Data marked with different letters are statistically significant (P < 0.05). C) Zp3-Drosha cKO females display normal fertility. Adult Zp3-Drosha cKO females mated with adult WT males produced a litter size comparable to that of WT control mating pairs. Both individual and mean values are shown (n = 20 for WT and n = 23 for Zp3-Drosha cKO mice).
DISCUSSION
DICER is required for the production of both miRNAs and endo-siRNAs, whereas DROSHA is essential for miRNA biogenesis, but dispensable for endo-siRNA production [15, 29]. Several reports have suggested that both DICER and DROSHA may have yet-to-be-defined, sncRNA-independent roles [16, 37, 38]. Although miRNA and/or endo-siRNA deficiency is most likely the cause for the phenotype observed in Ddx4-Dicer cKO females, disruptions of other DICER-dependent cellular functions couldn't be excluded. We therefore confined this report to phenotypic characterization without sncRNA transcriptome analyses.
The DGCR8-DROSHA complex functions as a microprocessor required for pre-miRNA production during miRNA biogenesis [15, 29]. The function was initially established based on data mostly from in vitro experiments using cultured cell lines [38]. If DGCR8 and DROSHA are equally important for pre-miRNA production in vivo, then Drosha global KO females should phenocopy Dgcr8 global KO females. Dgcr8 global ablation leads to embryonic lethality at E6.5 and cKO of Dgcr8 in growing oocytes of developing follicles abolishes most of the miRNA production in oocytes, but causes no discernible phenotype [12]. Interestingly, Drosha global KO mice also die at E6.5. Similar to Zp3-Dgcr8 cKO females [12], Zp3-Drosha cKO females display no discernible phenotype. Moreover, conditional inactivation of Drosha in oocytes of the fetal ovary at E15.5 (Ddx4-Cre; Droshaflox/flox mice) has no effects on follicular development in postnatal and adult ovaries or on female fertility. Together, these data support the notion that DROSHA and DGCR8 play a similar role, and both are required for embryonic development beyond E6.5, but their expression in oocytes is dispensable for follicular development.
Dicer inactivation in growing oocytes of primary follicles appears to be compatible with folliculogenesis because no abnormalities in ovarian histology and normal superovulation outcome were observed [20]. However, those Dicer-deficient oocytes failed to mature properly, as evidenced by aberrant spindle formation [20], which may have been directly caused by DICER deficiency or have indirectly resulted from disrupted production of DICER-dependent miRNAs and/or endo-siRNAs. In this report, we have demonstrated that oocyte Dicer expression in the fetal ovary is essential for postnatal follicular development. The fact that Ddx4-Dicer cKO ovaries contain many fewer primordial follicles but display no significantly accelerated primordial recruitment at young (P30 and P40) and adult (P120) ages suggests that the premature ovarian failure in Ddx4-Dicer cKO females is likely caused by reduced primordial follicle formation. Moreover, the significantly reduced number of primary follicles suggests suppressed recruitment of primordial follicles and/or enhanced depletion of primary follicles, and the drastically reduced number of antral follicles may be due to a block in secondary follicle stage given that the number of secondary follicles appeared to decrease at a slower pace from P30 to P40. Together, our data indicate that oocyte Dicer expression in the fetal ovary is required for postnatal follicular development both quantitatively and qualitatively. Conditional knockout of Drosha in oocytes of either the fetal (Ddx4-Drosha cKO) or the postnatal (Zp3-Drosha cKO) ovary causes no disruptions in folliculogenesis, which is in sharp contrast to the Ddx4-Dicer cKO females showing compromised folliculogenesis, premature ovarian failure, and female infertility. The phenotypic differences between Dicer and Drosha cKO females reflect most likely the contribution of their nonoverlapping functions, e.g., endo-siRNA production, or other unknown, DICER-dependent and DROSHA-independent cellular functions. Nevertheless, it remains puzzling that Drosha, despite its essential role in miRNA production and the abundant expression of miRNAs in both developing and mature oocytes, is dispensable for oocyte development and fertility.
In summary, our data provide physiological evidence showing that proper Dicer expression in early prophase I oocytes of the fetal ovary is essential for normal follicular development in the postnatal ovary. Like DGCR8, DROSHA is essential for early embryonic development, but its expression in oocytes is dispensable for postnatal follicular development. Further study is needed to fully understand the differential roles of oocyte DICER and DROSHA in sncRNA biogenesis and in other sncRNA-independent functions during folliculogenesis.
ACKNOWLEDGMENT
The authors would like to thank Ms. Tingting Zhu for her help with figure preparation.
Footnotes
Supported in part by NIH grants (HD060858, HD071736, and HD074573 to W.Y.) and funds from the University of Nevada School of Medicine.
REFERENCES
- Buehr M. The primordial germ cells of mammals: some current perspectives. Exp Cell Res. 1997;232:194–207. doi: 10.1006/excr.1997.3508. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Bendel-Stenzel M, Anderson R, Heasman J, Wylie C. The origin and migration of primordial germ cells in the mouse. Semin Cell Dev Biol. 1998;9:393–400. doi: 10.1006/scdb.1998.0204. [DOI] [PubMed] [Google Scholar]
- Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4:223–241. doi: 10.1101/cshperspect.a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Cheung CK, Wang Y, Tsang BK. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci. 2003;8:d222–d237. doi: 10.2741/949. [DOI] [PubMed] [Google Scholar]
- Bettegowda A, Smith GW. Mechanisms of maternal mRNA regulation: implications for mammalian early embryonic development. Front Biosci. 2007;12:3713–3726. doi: 10.2741/2346. [DOI] [PubMed] [Google Scholar]
- Gomes AQ, Nolasco S, Soares H. Non-coding RNAs: multi-tasking molecules in the cell. Int J Mol Sci. 2013;14:16010–16039. doi: 10.3390/ijms140816010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye D, Hossain MM, Schellander K. The noncoding genome: implications for ruminant reproductive biology. Soc Reprod Fertil Suppl. 2010;67:73–93. [PubMed] [Google Scholar]
- Schmittgen TD. Regulation of microRNA processing in development, differentiation and cancer. J Cell Mol Med. 2008;12:1811–1819. doi: 10.1111/j.1582-4934.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RP, Kotaja N. Small RNAs in spermatogenesis. Mol Cell Endocrinol. 2014;382:498–508. doi: 10.1016/j.mce.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP. Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16:223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Park CY, Choi YS, McManus MT. Analysis of microRNA knockouts in mice. Hum Mol Genet. 2010;19:R169–R175. doi: 10.1093/hmg/ddq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen HM, Meikar O, Yadav RP, Papaioannou MD, Romero Y, Da Ros M, Herrera PL, Toppari J, Nef S, Kotaja N. Dicer is required for haploid male germ cell differentiation in mice. PLoS One. 2011;6:e24821. doi: 10.1371/journal.pone.0024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Song R, Ortogero N, Zheng H, Evanoff R, Small CL, Griswold MD, Namekawa SH, Royo H, Turner JM, Yan W. The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis. J Biol Chem. 2012;287:25173–25190. doi: 10.1074/jbc.M112.362053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou MD, Lagarrigue M, Vejnar CE, Rolland AD, Kuhne F, Aubry F, Schaad O, Fort A, Descombes P, Neerman-Arbez M, Guillou F, Zdobnov EM, et al. Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice Mol Cell Proteomics 2011. 10:M900587MCP900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GJ, Georg I, Scherthan H, Merkenschlager M, Guillou F, Scherer G, Barrionuevo F. Dicer is required for Sertoli cell function and survival. Int J Dev Biol. 2010;54:867–875. doi: 10.1387/ijdb.092874gk. [DOI] [PubMed] [Google Scholar]
- Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, Schaad O, Vejnar CE, Kuhne F, Descombes P, Zdobnov EM, McManus MT, Guillou F, et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol. 2009;326:250–259. doi: 10.1016/j.ydbio.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Y, Meikar O, Papaioannou MD, Conne B, Grey C, Weier M, Pralong F, De Massy B, Kaessmann H, Vassalli JD, Kotaja N, Nef S. Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One. 2011;6:e25241. doi: 10.1371/journal.pone.0025241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod. 2008;79:696–703. doi: 10.1095/biolreprod.108.067827. [DOI] [PubMed] [Google Scholar]
- Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, Blelloch R. MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr Biol. 2010;20:271–277. doi: 10.1016/j.cub.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N, Blelloch R. Small RNAs in early mammalian development: from gametes to gastrulation. Development. 2011;138:1653–1661. doi: 10.1242/dev.056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- Andras Nagy MG, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. 5. [Google Scholar]
- Tomic D, Brodie SG, Deng C, Hickey RJ, Babus JK, Malkas LH, Flaws JA. Smad 3 may regulate follicular growth in the mouse ovary. Biol Reprod. 2002;66:917–923. doi: 10.1095/biolreprod66.4.917. [DOI] [PubMed] [Google Scholar]
- Lira SA, Kinloch RA, Mortillo S, Wassarman PM. An upstream region of the mouse ZP3 gene directs expression of firefly luciferase specifically to growing oocytes in transgenic mice. Proc Natl Acad Sci U S A. 1990;87:7215–7219. doi: 10.1073/pnas.87.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- Tijsterman M, Plasterk RH. Dicers at RISC; the mechanism of RNAi. Cell. 2004;117:1–3. doi: 10.1016/s0092-8674(04)00293-4. [DOI] [PubMed] [Google Scholar]
- Macias S, Plass M, Stajuda A, Michlewski G, Eyras E, Caceres JF. DGCR8 HITS-CLIP reveals novel functions for the microprocessor. Nat Struct Mol Biol. 2012;19:760–766. doi: 10.1038/nsmb.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]