ABSTRACT
After mating, many female mammals store a subpopulation of sperm in the lower portion of the oviduct, forming a reservoir. The reservoir lengthens sperm lifespan, regulates sperm capacitation, controls polyspermy, and selects normal sperm. It is believed that sperm bind to glycans on the oviduct epithelium to form the reservoir, but the specific adhesion molecules that retain sperm are unclear. Herein, using a glycan array to test 377 glycans for their ability to bind porcine sperm, we found two glycan motifs in common among all glycans with sperm-binding ability: the Lewis X trisaccharide and biantennary structures containing a mannose core with 6-sialylated lactosamine at one or more termini. Binding to both motifs was specific; isomers of each motif did not bind sperm. Further work focused on sialylated lactosamine. Sialylated lactosamine was found abundantly on the apical side of epithelial cells collected from the oviduct isthmus, among N-linked and O-linked glycans. Sialylated lactosamine bound to the head of sperm, the region that interacts with the oviduct epithelium. After capacitation, sperm lost affinity for sialylated lactosamine. Receptor modification may contribute to release from the reservoir so that sperm can move to the site of fertilization. Sialylated lactosamine was required for sperm to bind oviduct cells. Simbucus nigra agglutinin or an antibody specific to sialylated lactosamine with a preference for Neu5Acalpha2-6Gal rather than Neu5Acalpha2-3Gal reduced sperm binding to oviduct isthmic cells, as did occupying putative receptors on sperm with sialylated biantennary glycans. These results demonstrate that sperm binding to oviduct 6-sialylated biantennary glycans is necessary for normal adhesion to the oviduct.
Keywords: capacitation, cell adhesion, glycans, oviduct, sialic acid, Sperm, sperm reservoir
A specific glycan motif found in the oviduct binds porcine sperm very specifically and is required for normal sperm binding to oviduct cells.
INTRODUCTION
Sperm storage in the female reproductive tract after mating is used by a variety of animals to maintain fertility, particularly in species in which mating and ovulation are poorly synchronized [1]. Sperm storage has been documented in salamanders [2], snakes, turtles [3], many birds, and mammals [1, 4], including a species of bat in which ovulation and fertilization occur months after mating [5]. In many mammals, the major location of the sperm reservoir is the lower oviduct, the isthmus, and utero-tubal junction [6–11]. After mating, small fractions of sperm enter the oviduct in a regulated process that requires specific sperm proteins [12, 13]. In the lower oviduct, the epithelial cell lining retains some sperm, forming a reservoir [9, 10, 14]. This reservoir lengthens the fertile lifespan of sperm [15, 16] and appears to regulate capacitation to provide a supply of fertile cells to the site of fertilization [17–20]. The sperm reservoir also controls the number of sperm at the site of fertilization to limit the opportunity for polyspermy [21]. Finally, the oviduct appears to be able to select sperm with normal acrosome morphology: those that are capable of fertilizing oocytes [22, 23].
The oviduct can affect fertilization broadly by altering the fluid environment [24, 25] or by regulating adhesion of sperm to its epithelium, which is the focus of this report. Sperm bind to epithelial cells lining the oviduct in a cell-restricted manner [10, 26–28]. Binding to the oviduct maintains sperm viability and motility [29, 30]. The ability to maintain sperm viability is not a common property of all cells [17], and requires direct membrane contact between sperm and oviduct epithelial cells [18, 31, 32].
The identity of the molecules that mediate sperm binding to the oviduct is controversial. It appears that different species may use different adhesion molecules. Using bovine tissues, one group found that two oviduct proteins, the chaperones GRP78 and HSP60, bound to sperm [33]. In contrast, a second group, also using bovine sperm, proposed that oviduct plasma membrane annexins containing fucose bind to accessory gland proteins deposited on sperm at ejaculation [34]. Studies of porcine sperm also implicated accessory gland secretions added to sperm in oviduct binding [35, 36]. However, the observation that epididymal sperm, not exposed to accessory gland proteins, are fertile is in conflict with a requirement of receptors of accessory gland origin for fertility [37].
There is evidence in several species that sperm binding to the isthmus is mediated by glycans found on oviduct epithelial cells [38, 39]. A considerable number of early studies suggested that oviduct glycans bind sperm [39–42]. These conclusions were based on the ability of a competitor to bind to one of a pair of coreceptors and inhibit the normal interaction. Unfortunately, these assays tested a limited number of monosaccharides and small oligosaccharides, making their physiological relevance uncertain.
To define the molecular components of the porcine oviduct sperm reservoir, we used a novel and more global approach to detect glycans that bind sperm, with an array of 377 glycans, including simple and more complex glycans [43, 44]. This array was originally developed to identify glycans to which purified lectins would bind, but we found that it was useful for motile cells in suspension. We also determined if glycans with the ability to bind uncapacitated sperm were present within the oviduct ampulla and isthmus, and localized the region of sperm to which they bound. Finally, we investigated the biological importance of the glycans of interest by blocking both the glycan in the oviduct and its putative receptor(s) on sperm.
MATERIALS AND METHODS
Collection and Processing of Sperm
For each of the four replicates, ejaculated semen samples (Prairie State Semen, Inc., Champaign, IL) were collected from three to five different mature boars and diluted in extender. Samples were stored at 16°C–18°C up to 24 h prior to use. Pooled extended semen (3 ml) was washed through Percoll gradients containing 5.4 ml Percoll, 0.6 ml 10× HBS (1.3 M NaCl, 40 mM KCl, 10 mM CaCl2, 5 mM MgCl2), and 4 ml dmTALP (2.1 mM CaCl2, 3.1 mM KCl, 1.5 mM MgCl2, 100 mM NaCl, 0.29 mM KH2PO4, 0.36% lactic acid, 26 mM NaHCO3, 0.6% BSA, 1 mM pyruvic acid, 20 mM Hepes [pH 7.3], 10 U/ml penicillin, 10 μg/ml streptomycin) at 800 × g for 10 min. Sperm were washed with 5 ml dmTALP and pelleted for 5 min at 600 × g. Samples with greater than 80% motility were used immediately for experiments. Sperm concentration was determined by hemocytometer and adjusted according to the experiment.
Glycan Array Analysis
Washed sperm were stained with 200 nM Syto-16 for 15 min, placed in a culture dish, and a glycan array slide (Version 3.1) was floated, glycan-side down, on the surface of the sperm suspension at 39°C for 15 min. The slide was removed carefully, rinsed gently or more vigorously thrice, and dried for analysis. Fluorescence on each glycan spot was quantitated by the Consortium for Functional Glycomics, as previously described [43, 44]. Background fluorescence was subtracted from the value for each glycan.
Collection of Oviduct Epithelial Cells
For each experiment, the isthmus of 15–20 oviducts (Meadowbrooke Farms, Rantoul, IL; Momence Packing Company, Momence, IL; Calihan Pork Producers, Peoria, IL; and Rantoul Foods, Rantoul, IL) were collected from pre- and postpubertal females and transported in PBS in a sterile 50-ml conical tube on ice. After 2–20 h on ice, the oviducts were processed at the lab. The isthmus was trimmed and the edge of a microscope slide was used to apply pressure to the outside of the oviduct and strip sheets of oviduct epithelial cells from the isthmus. Epithelial sheets in PBS were transferred to a 15-ml conical tube and centrifuged at 100 × g for 1 min. After removing the supernatant, the cells were deaggregated by passage through a 1-ml pipette tip 10 times. After bringing the volume to 15 ml with PBS, the suspension was centrifuged again. The partially deaggregated cells in the pellet were passed through a 22-gauge needle 10 times. After adjusting the volume to 12 ml with dmTALP, the cells were divided evenly into three 100-mm tissue culture dishes. Cells were allowed to reaggregate for 90–120 min at 39°C. Spherical aggregates that were 100–200 μm in diameter were selected for experiments.
Mass Spectrometry Analysis of Oviduct Glycans
Protein powder was prepared from oviduct epithelial sheets as described previously [45]. Briefly, oviduct isthmic epithelial cells were homogenized and delipidated in a solvent mixture with a final ratio of 4:8:3 (chloroform:methanol:water). The extracted material was allowed to incubate for 6 h at room temperature. The precipitated protein material was collected by centrifugation, and the resulting protein pellet was re-extracted with fresh solvent. The precipitated protein pellet was washed with ice-cold 20% acetone and then dried under a gentle nitrogen stream at 45°C.
Glycosphingolipids were analyzed as previously described [46, 47]. In brief, lipid extracts containing glycosphingolipids were combined and dried under a nitrogen stream. Glycerolipids were removed by incubating with 0.5 M NaOH in methanol. The reaction mixture was neutralized with acetic acid and desalted on a Sep-Pak C18 cartridge column. The column was preconditioned with methanol and water. The sample was adjusted to 50% aqueous methanol and loaded onto a column. Salt was removed by washing the column with water, and glycosphingolipids were eluted with methanol and dried under a nitrogen stream. Glycosphingolipids were permethylated, dissolved in 50 μl of 1 mM NaOH in methanol/water (1/1) for infusion, and analyzed by nanospray ionization mass spectrometry (MS).
N-linked glycans were prepared from tryptic/chymotryptic digests of protein powder as previously described [45]. Following enzymatic release with peptide-N-glycosidase F (Prozyme, San Leandro, CA), liberated N-linked glycans were permethylated [48] and analyzed by nanospray ionization, ion trap MS (NSI-LTQ/Orbitrap; Thermo Fisher).
O-Glycans were released using reductive β-elimination as reported previously [49]. Briefly, protein powder was resuspended with 1 M NaBH4 in 100 mM NaOH and incubated at 45°C for 18 h. The reaction mixture was neutralized with 10% acetic acid on ice and then loaded onto a column of AG 50W-X8 cation-exchange resin (Bio-Rad, Hercules, CA) for desalting. The released oligosaccharides were eluted from the column with 3 volumes of 5% acetic acid and taken to dryness using a SpeedVac. Borate was removed by adding 10% acetic acid in methanol and drying under a nitrogen stream at 45°C. The sample was then resuspended in 5% acetic acid and loaded onto a C18 cartridge column (JT Baker, Phillipsburg, NJ) that was previously washed with acetonitrile and pre-equilibrated with 5% acetic acid. Flow-through from the column was collected after loading; the column was washed with 3 ml of 5% acetic acid. The flow-through and washes were combined and evaporated to dryness.
Released oligosaccharides were permethylated to facilitate their analysis by MS as reported previously [45]. Sulfated permethylated glycans were fractionated on a Sep-Pak C18 [50]. For MS of sulfated glycans in negative ion mode, permethylated sulfated glycans were reconstituted in 50 μl of methanol/2-propanol/1-propanol/13 mM aqueous ammonium acetate (16:3:3:2 by volume) for infusion. For MS of permethylated sulfated and nonsulfated glycans in positive ion mode, permethylated glycans were dissolved in 50 μl of 1 mM NaOH in methanol/water (1:1) for infusion. Both preparations were analyzed by infusion into a linear ion trap mass spectrometer (LTQ; Thermo Fisher Scientific, Waltham, MA) using a nanoelectrospray source at a syringe flow rate of 0.40 μl/min and capillary temperature set to 210°C [45, 46, 48, 51].
The total ion mapping (TIM) function of the Xcalibur software package was used to detect and quantify the prevalence of all detected glycan structures. Through the application of TIM, MS/MS spectra were acquired within overlapping collection windows that spanned 2.8 mass units. This method was used to scan the m/z range from 200 to 2000. Following characterization of glycan structures observed from the resulting TIM scan, the relative abundance of either individual glycans or groups of isobaric glycans was determined. The relative abundance of the observed glycan structures was calculated based upon the peak area from a single glycan structure relative to the total area of all observed glycan structures.
Localization of Glycan Binding to Sperm
Semen samples were washed through Percoll and incubated in capacitating conditions at 10–20 million cells/ml in dmTALP at 39°C for 4 h. These conditions promote sperm capacitation, as assessed by increased tyrosine phosphorylation and the ability to undergo the acrosome reaction [52, 53]. As a control, sperm were incubated at 39°C for 4 h in dmTALP in which sodium bicarbonate was replaced by an equimolar quantity of Hepes, and BSA was replaced by an equal mass concentration of polyvinylpyrrolidone (dmTALP-NC). This formulation does not allow capacitation [53]. Specific fluorescein-conjugated glycans on a polyacrylamide core were prepared, as previously described [54, 55], and incubated with sperm at a final concentration of 50 μg/ml for 30 min at 39°C. Sperm were immobilized with 0.01% formaldehyde (final concentration), placed on a slide under a coverslip, and observed by fluorescence microscopy and Nomarski optics. In each experiment, at least 100 sperm were observed and counted in each group.
Assay of Sperm Binding to Oviduct Epithelial Cells
To test the effect of glycans, lectins, or antibodies on sperm-oviduct cell adhesion, an assay to detect sperm binding to oviduct cell aggregates was used. Spherical oviduct cell aggregates were selected and washed twice in 100-μl drops of fresh dmTALP. A Stripper Pipette (MidAtlantic Diagnostics, Inc., Mount Laurel, NJ) with a 250-μm internal diameter tip was used to collect oviduct epithelial cell aggregates and wash them. Depending upon the experiment, either the cell aggregates were pretreated with lectins and antibodies that bind sialylated lactosamine, or the sperm were pretreated with sugars to block lectins. Cell aggregates were preincubated for 30 min at 39°C in 45–49 μl of Simbucus nigra agglutinin lectin (SNA; Vector Laboratories, Burlingame, CA), Maackia amurensis lectin II (MAL II, Vector Laboratories), GL7 monoclonal antibody (BD Biosciences, Franklin Lakes, NJ), or IgM control (BD Biosciences), for a final concentration of 4–250 μg/ml (the graphed concentration) in dmTALP-NC. Sperm in dmTALP-NC were added to bring the total volume to 50 μl (final concentration of 0.5 × 106 cells/ml). In other experiments, sperm were preincubated with 8–200 μg/ml of different glycoconjugates in 47 μl dmTALP-NC for 30 min (the graphed concentration) at 39°C. Oviduct cell aggregates were then added in 3 μl to preincubated sperm for a total volume of 50 μl. At least 10 aggregates were added to each droplet in triplicate droplets. Sperm and oviduct cell aggregates were coincubated at 39°C for 15 min to allow sperm to bind to aggregates. After coincubation, free and loosely attached sperm were removed by washing with 30 μl of dmTALP-NC. Aggregates were transferred onto a microscope slide in a volume of 3 μl. Each droplet with 10 aggregate sperm complexes was considered an experimental unit for statistical analysis. Images were captured using a Zeiss Axioskop and AxioCam HRc digital camera (Carl Zeiss, Thornwood, NY). The number of sperm bound to the periphery of each aggregate was enumerated and the circumference of the aggregate calculated using AxioVision V 4.5 software (Carl Zeiss). The number of sperm bound per millimeter circumference was calculated for each aggregate. The average of more than 10 aggregates for each droplet was used for statistical analysis.
For statistical analysis of sperm-oviduct cell binding data, we used SAS software v. 9.1 (SAS Institute, Inc., Cary, NC) to run a one-way analysis of variance using GLM (General Linear Models) procedure in accordance with the general model: Yij = μ + αi + εij (where Yij is the jth sample observation from population i; μ is the overall mean; α is an effect due to population, i; and ε is the random deviation of Yij about the ith population mean). Results are presented as means ± SEM. Differences were considered to be significant at P < 0.05 using the Tukey test for multiple comparisons.
Collection and Fixation of Oviducts for Localization of Sialylated Lactosamine
Oviducts were collected and sorted by reproductive stage at Momence Packing Company, Inc. (Momence, IL). The stages included were follicular (>10 antral follicles on both ovaries combined of 7–12 mm diameter and no corpora lutea >6 mm), luteal (>12 corpora lutea, which were >6 mm), and anestrous (no follicles >3 mm and no corpora lutea >5 mm), as previously described [56]. Oviducts were from four sows in the follicular phase, five sows in the luteal phase, and two sows in anestrous. After collection, the oviducts were transported, on ice, back to the laboratory and washed gently in PBS. Portions of the oviduct (1 cm) were excised and placed in a 1.5-ml microfuge tube. Six sections per oviduct were used, the upper, middle, and lower isthmus and the upper, middle, and lower ampulla. Each section was fixed overnight in 4% paraformaldehyde. Tissues were washed 3× in PBS for 5 min each. Oviducts were dehydrated for long-term storage with 10%, 20%, and 30% sucrose solutions (1× PBS) in subsequent overnight incubations.
Samples of these regions were placed into tissue embedding molds in a 1:1 mixture of 30% sucrose solution and OCT compound. Rapid freezing to avoid crystallization within the sample was accomplished by dipping the embedding molds with the sample and sucrose-OCT mixture into isopentane chilled with liquid nitrogen. After freezing, tissue blocks were kept on dry ice or stored at −80°C.
Samples were cryosectioned and stained with 10 μg/ml fluorescein-labeled SNA (Vector Laboratories), which detects sialic acid attached to galactose in an α-2,6 linkage, but not sialic acid attached to galactose in an α-2,3 linkage [44, 57]. Slides were examined under fluorescence optics using a compound Zeiss microscope and AxioCam with AxioVision software. Observations were made at 100×, 400×, and 630×. Comparisons for each stage of the estrous cycle were made.
RESULTS
Analysis of Glycans that Bind Sperm Using the Glycan Array
Previous studies of carbohydrates proposed to form the mammalian oviduct sperm reservoir have investigated a very limited number of glycans. To conduct a much broader survey of glycans that might be involved in porcine sperm binding, we used a glycan array that was first developed to assess which glycans purified lectins would bind [43, 44]. Version 3.1 contained 377 glycans linked covalently to a glass slide. We used this as an affinity matrix for sperm. By floating the slide, glycan-side down, on a suspension of uncapacitated sperm, binding of only motile cells that could swim up in the medium and collide with glycans on the array was assessed. Prior to incubation with the array, sperm were labeled with the fluorochrome Syto-16 for detection of sperm bound to the array. After allowing 15 min for sperm binding, the slides were washed. Similar results were obtained whether moderate or less-stringent washing conditions were performed after sperm array incubation. Bound sperm were detected by fluorescence (Fig. 1).
FIG. 1.
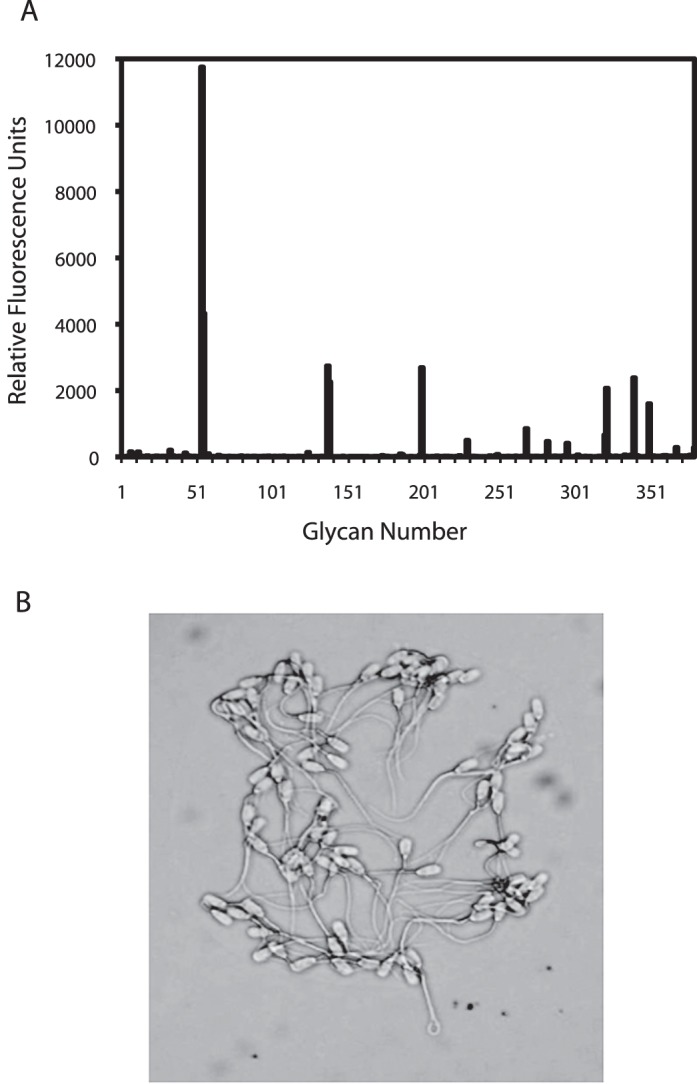
Sperm bound to specific glycans covalently arrayed on a microscope slide. Porcine sperm were labeled and incubated with a slide containing 377 glycans. Fluorescence corresponding to bound sperm was detected for each glycan spot. A small number of glycans bound sperm (A). The glycan number and identification are given in Figure 2. Results are an average of four independent experiments. Sperm bound to a single glycan spot are shown in B. Sperm bound to specific spots of glycans and not to areas outside of the spot. Original magnification ×630.
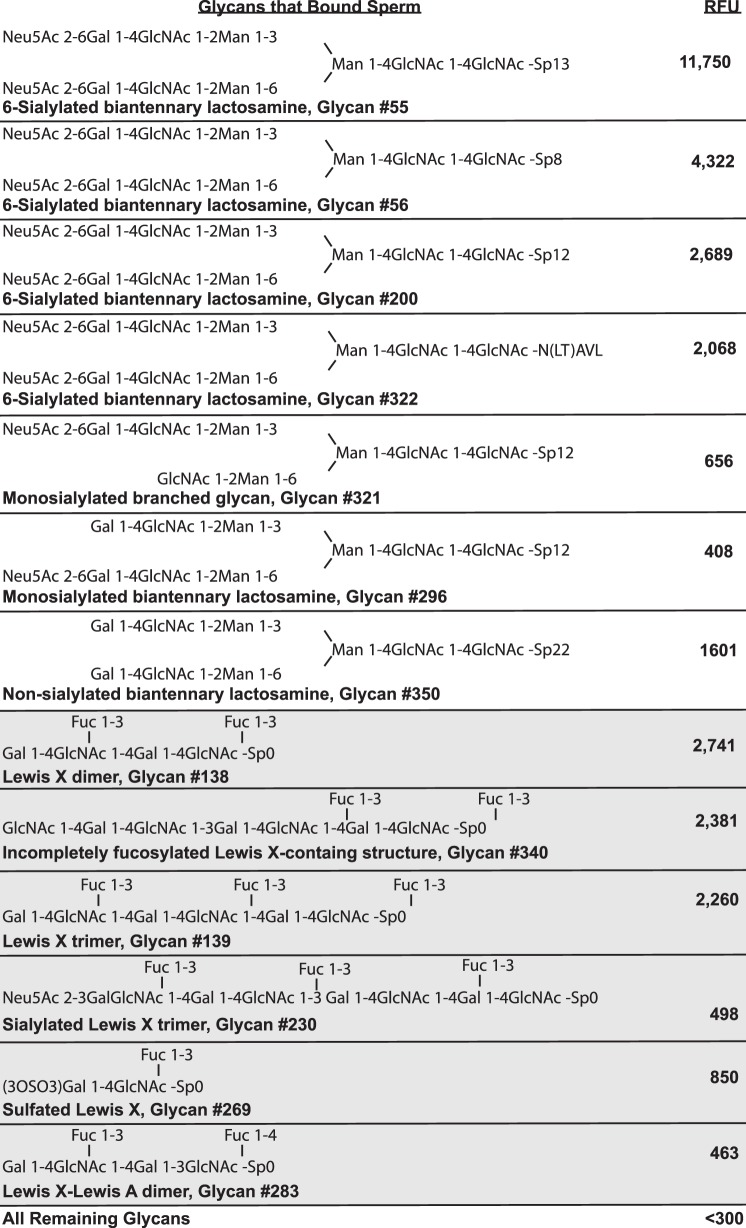
All the glycans to which sperm bound contained one of two glycan motifs, either a Lewis X trisaccharide (LeX; Fig. 1 and shaded part of Fig. 2) or a branched structure with core mannose and antennae terminating in the sialylated lactosamine trisaccharide or, in some cases, terminating in simply lactosamine (Fig. 1 and unshaded part of Fig. 2). Four of the glycan-spacer combinations that bound the most sperm differed only in the spacer used (Glycans 55, 56, 200, and 322); the glycan was the same. The other three glycans sharing the branched lactosamine motif were sialylated on just one antenna or on neither antenna (Glycans 321, 296, 350). In all sialic acid-containing structures that bound sperm, sialic acid was linked to the 6 position of galactose rather than the 3 position, demonstrating that a specific linkage was necessary to bind sperm. Furthermore, the branched structure on a mannose core was required. Single sialylated lactosamine trisaccharides (Neu5Acα2-6Galβ1-4GlcNAc) did not bind sperm.
FIG. 2.
Glycans that bound porcine uncapacitated sperm. Glycans containing either biantennary sialylated lactosamine (unshaded) or LeX structures (shaded) bound sperm. Within each group, glycans are grouped by structure and ranked by the number of sperm bound, as indicated by relative fluorescence units (RFU). A description of the glycan and the glycan ID number used in Figure 1 are in bold. RFU is the average of four independent replicates. In several cases, the glycans were the same, but the spacer arm (Sp) used to link the glycan to the array was different. Sp13 = Gly; Sp8 = −CH2CH2CH2NH2; Sp12 = asparagine; Sp22 = Asp-Ser-Thr; Sp0 = −CH2CH2NH2; (3OSO3) = sulfate group added at the 3 carbon of galactose.
The LeX motif was found in six glycans that bound sperm. Four sperm-binding glycans contained an LeX dimer or trimer. One sperm-binding glycan was simply the sulfated version of LeX monomer. Finally, one sperm-binding glycan was a dimer of two trisaccharides, LeX linked to LeA, the structurally related Lewis A trisaccharide (a positional isomer; the linkage of Gal and Fuc to GlcNAc is switched between these Lewis structures). Fucose substitution on LeX was necessary because sulfated or unsulfated Galβ1-4GlcNAc (N-acetyllactosamine) did not bind sperm. In total, these results indicated that uncapacitated porcine sperm bind very specifically to glycans containing LeX or branched sialylated lactosamine motifs.
Profile of Oviduct Glycans
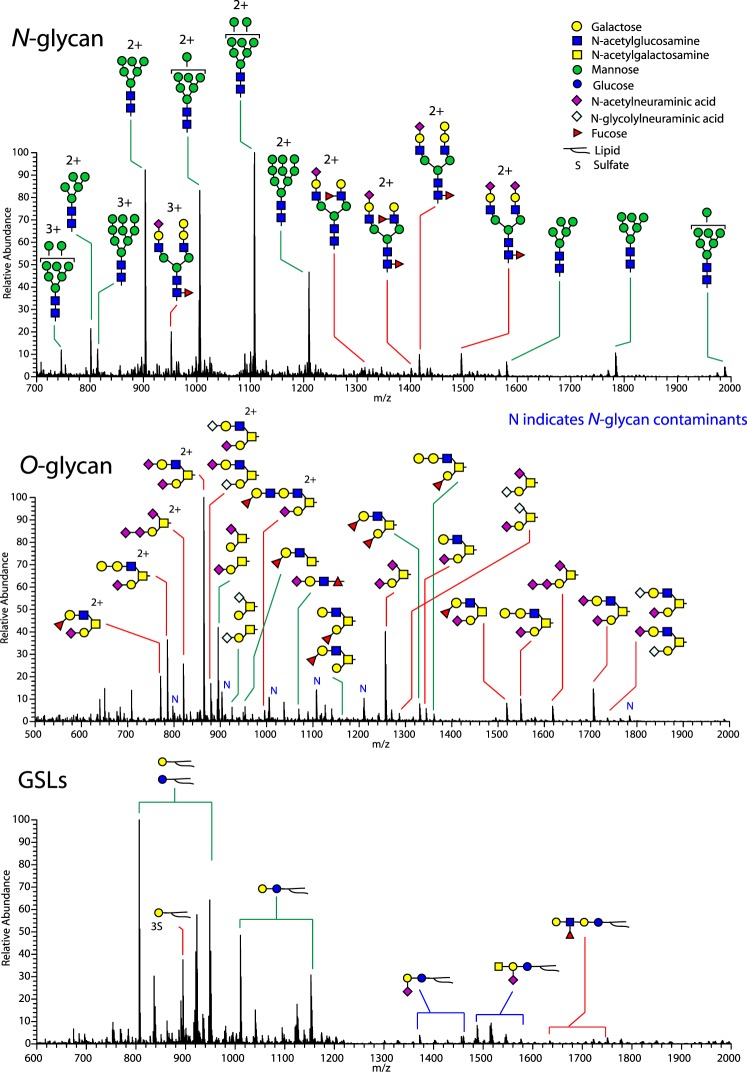
To confirm that the oviduct isthmus produces glycans with motifs that bind sperm and to identify the entire structures of the sperm-binding glycans, we collected epithelial sheets from the isthmus of postpubertal females, digested the proteins enzymatically, and analyzed the remaining glycan components of glycoproteins by spray MS/MS. N-glycans were released using peptide-N-glycosidase F. As expected, in preparations from cell lysates, there was an abundance of incompletely processed oligomannose glycan structures (Fig. 3, upper panel). Most of the complex-type oligosaccharides were biantennary and had a sialyl residue on at least one nonreducing terminus. Some biantennary glycans had sialyl residues on each terminus and, interestingly, some had a sialyl residue on one terminus and a Lewis structure on the second. Oviduct epithelial cells had numerous O-linked glycans, most of which were Core 2-derived oligosaccharides (Fig. 3, middle panel). Many O-linked glycans were branched, with at least one terminus containing a sialyl residue. Several had a sialylated lactosamine trisaccharide on the nonreducing terminus of one antenna. A series of sulfated O-linked glycans was detected in oviduct epithelial cells, but none of these were among those found to bind sperm (Fig. 1 and Supplemental Fig. S1; all supplemental data are available online at www.biolreprod.org). One of the glycans from oviduct epithelial cells glycosphingolipids contained a terminal Lewis structure, but it was not abundant (Fig. 3, lower panel and Supplemental Fig. S2). Thus, both N- and O-linked glycans and glycolipids contained Lewis structures and/or biantennary glycans with terminal sialylated lactosamine, and most of the glycan motifs that bound the greatest number of sperm were found among glycans linked to glycoproteins.
FIG. 3.
Oviduct epithelial cells contain glycans with sialylated lactosamine structures and/or Lewis structures at the nonreducing termini. Oviduct epithelial cell sheets were lysed and glycans analyzed by nanospray MS/MS. N-glycan structures are shown in the upper panel. Those structures that contained sperm binding motifs are identified with a red line linking the peak to the structure. Structures of the most abundant glycans are displayed. As expected of cell lysates, there were many unprocessed high-mannose oligosaccharides. Most of the complex-type oligosaccharides were biantennary and had at least one terminus with a sialyl residue (N-acetylneuraminic acid). Some glycans had two sialic acid-containing termini, and some glycans contained a sialyl residue on one terminus and a Lewis structure on the second terminus. O-glycan structures are shown in the middle panel. Oviduct epithelial cells had numerous O-linked glycans, most of which were Core 2-derived oligosaccharides. Several structures (labeled by red lines) had sialyl residues attached to galactosyl residues on at one nonreducing terminus and were branched. Tandem MS analysis determined the majority of the structure at 895 m/z was the linear glycan. Glycosphingolipid structures are shown in the lower panel. The blue lines indicate gangliosides, red lines indicate glycans with a sulfate group or Lewis structure, and the green lines indicate neutral glycosphingolipids. A Lewis structure was detected on one glycosphingolipid.
6-Sialylated Lactosamine Is Abundant in the Oviduct Epithelium
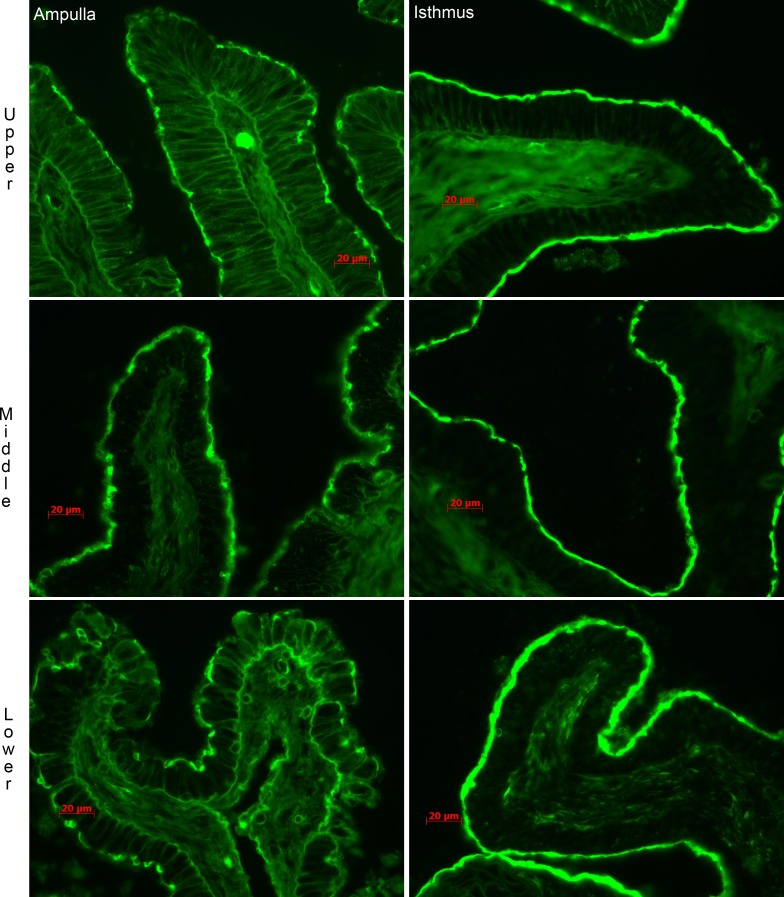
The remainder of this study was focused on sialylated lactosamine. To localize sialylated lactosamine structures in the oviduct, tissue from the upper, middle, and lower ampulla and isthmus was fixed and stained with fluorescein-conjugated SNA, which binds to sialic acid attached to galactose in an α-2,6 linkage preferentially, and not sialic acid attached to galactose in an α-2,3 linkage [44, 57]. Abundant sialylated lactosamine was detected on the epithelium of all parts of the ampulla and isthmus, including ciliated and nonciliated cells (Fig. 4). Similar results were obtained if the sections were stained with the GL7 monoclonal antibody that detects sialic acid attached to galactose in an α-2,3 linkage (data not shown) [58]. The sections shown were collected from animals that had more than 10 antral follicles in total on both ovaries, 7–12 mm diameter and no corpora lutea >6 mm, animals expected to be in proestrus or estrus [56]. However, similar staining was observed in oviducts collected from sows in diestrus or just prior to puberty (data not shown).
FIG. 4.
Localization of 6-sialylated lactosamine structures to the luminal epithelium of regions of the oviduct. Oviduct tissues from the upper, middle, and lower portion of the ampulla and isthmus were stained with fluoresceinated SNA. Sialylated lactosamine was detected on the epithelium of each portion of the examined oviduct. Bars = 20 μm.
Biantennary Glycans with 6-Sialylated Lactosamine Bind to the Sperm Head
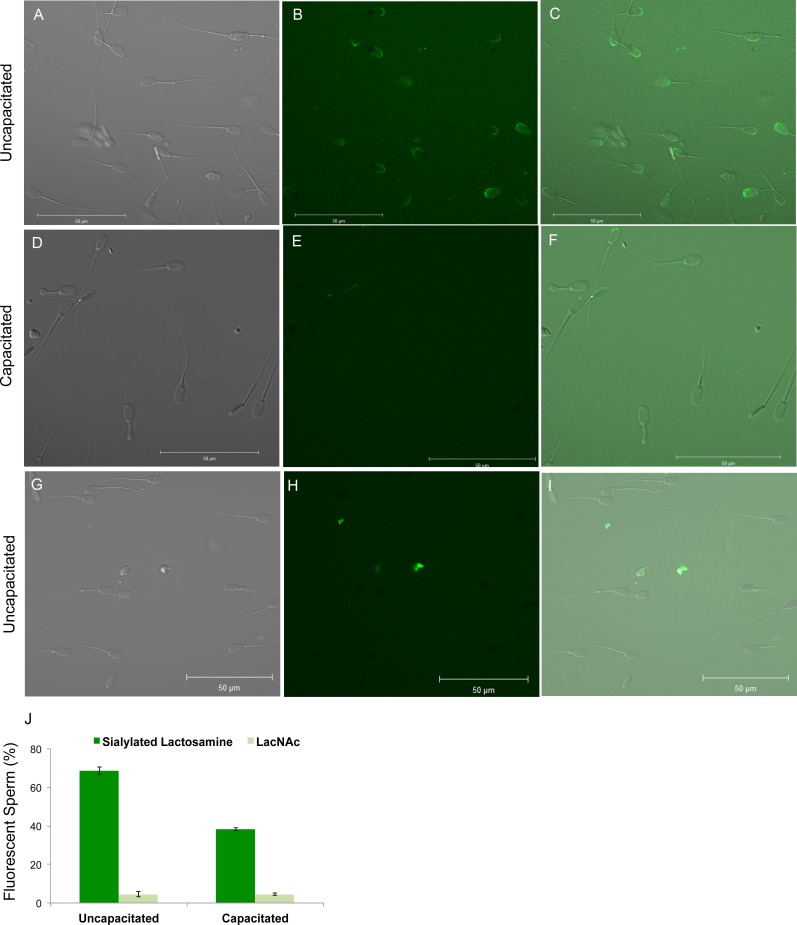
The portion of sperm that binds to the oviduct epithelium is the head [59]. If branched sialylated lactosamine motifs on the oviduct epithelium have an important role in binding sperm, we hypothesized that receptors for these motifs would be found on the sperm head. To test this, sperm were incubated with 6-sialylated lactosamine on a mannose biantennary core linked to a chain of polyacrylamide that was directly labeled with fluorescein [54, 55]. Labeled 6-sialylated lactosamine biantennary glycan bound to the anterior head of nearly 70% of the sperm and preferentially to the apical edge of the head overlying the acrosome (Fig. 5, A–C, J). In some experiments, propidium iodide was also added to sperm to identify membrane-compromised sperm. Nearly all the sperm that bound branched sialylated lactosamine were able to exclude propidium iodide, and thus were viable (data not shown). As a control, the disaccharide lactosamine was linked to polyacrylamide and fluoresceinated. Less than 5% of sperm bound lactosamine, and the fluorescence intensity was very low (Fig. 5, G–J). Thus, the receptors for 6-sialylated biantennary glycan were found on the sperm head.
FIG. 5.
The 6-sialylated biantennary glycans bound to the sperm head, but binding was reduced after capacitation. Sperm were either capacitated or not and incubated with fluorescein-labeled, branched, 6-sialylated lactosamine on a mannose core or simply fluorescein-labeled lactosamine. The photomicrographs show representative fields of differential interference contrast (DIC), fluorescence, and merged images. DIC, fluorescence, and merged images of uncapacitated sperm incubated with fluoresceinated, branched, sialylated lactosamine are in A–C, respectively. DIC, fluorescence, and merged images of capacitated sperm incubated with fluoresceinated, branched, sialylated lactosamine are in D–F, respectively. DIC, fluorescence, and merged images of uncapacitated sperm incubated with the fluoresceinated lactosamine disaccharide are in G–I, respectively. Bars = 50 μm. The percentage of either uncapacitated sperm or sperm incubated under capacitating conditions that bound branched, 6-sialylated lactosamine or the disaccharide lactosamine is shown in J. This is the average of three replicates.
Release of sperm from the oviduct has been proposed to occur after sperm are capacitated [9, 30]. To investigate this model, we tested whether capacitated sperm retained affinity for glycans. We incubated capacitated sperm with fluorescein-labeled 6-sialylated lactosamine biantennary glycan. About 40% of sperm in these preparations bound branched sialylated lactosamine, much less than the number of uncapacitated sperm that bound branched sialylated lactosamine (Fig. 5, D–F and J). Because capacitation does not occur synchronously, it is likely that not all sperm in the sample that was prepared under conditions that promote capacitation were actually capacitated. Controls using fluoresceinated lactosamine bound to less than 5% of sperm (Fig. 5J). Therefore, during capacitation, most sperm lost their ability to initiate binding to branched glycans containing sialylated lactosamine.
Normal Sperm Binding to Oviduct Cells Requires Glycans with 6-Sialylated Lactosamine
Results in Figures 1 and 2 demonstrate that branched sialylated lactosamine from oviduct cells was sufficient to bind sperm. In the subsequent experiments, we examined whether branched sialylated lactosamine was necessary for oviduct cells to bind sperm. Epithelial sheets were stripped from the isthmus, the cells dissociated, and then allowed to reaggregate to form roughly spherical aggregates with diameters from 75 to 150 μm. First, isthmic epithelial cell aggregates were incubated with SNA to block sialylated lactosamine, and were then challenged with sperm. SNA reduced sperm binding in a dose-dependent fashion up to 62% at a concentration of 250 μg/ml (Fig. 6B). This inhibition was specific to SNA, because a control using the MAL II, a monovalent lectin that binds structures containing sialic acid in an α2,3 linkage [57], did not inhibit binding (Fig. 6B). To confirm that sialylated lactosamine was required for binding, we used GL7, a monoclonal antibody that recognizes sialylated lactosamine, the α2,6-linked sialic acid on a lactosamine glycan chain [58]. GL7 was incubated with isthmic epithelial cell aggregates prior to incubation with sperm. GL7 reduced sperm binding to aggregates by up to 63% at a concentration of 100 μg/ml (Fig. 6C). The conclusion from both experiments is that sialylated lactosamine is required for normal sperm binding to isthmic epithelial cells. In summation, these results indicate that normal binding of sperm to isthmic epithelial cells requires branched sialylated lactosamine with termini presented as Neu5Acα2-6Galβ1-4GlcNAc.
FIG. 6.
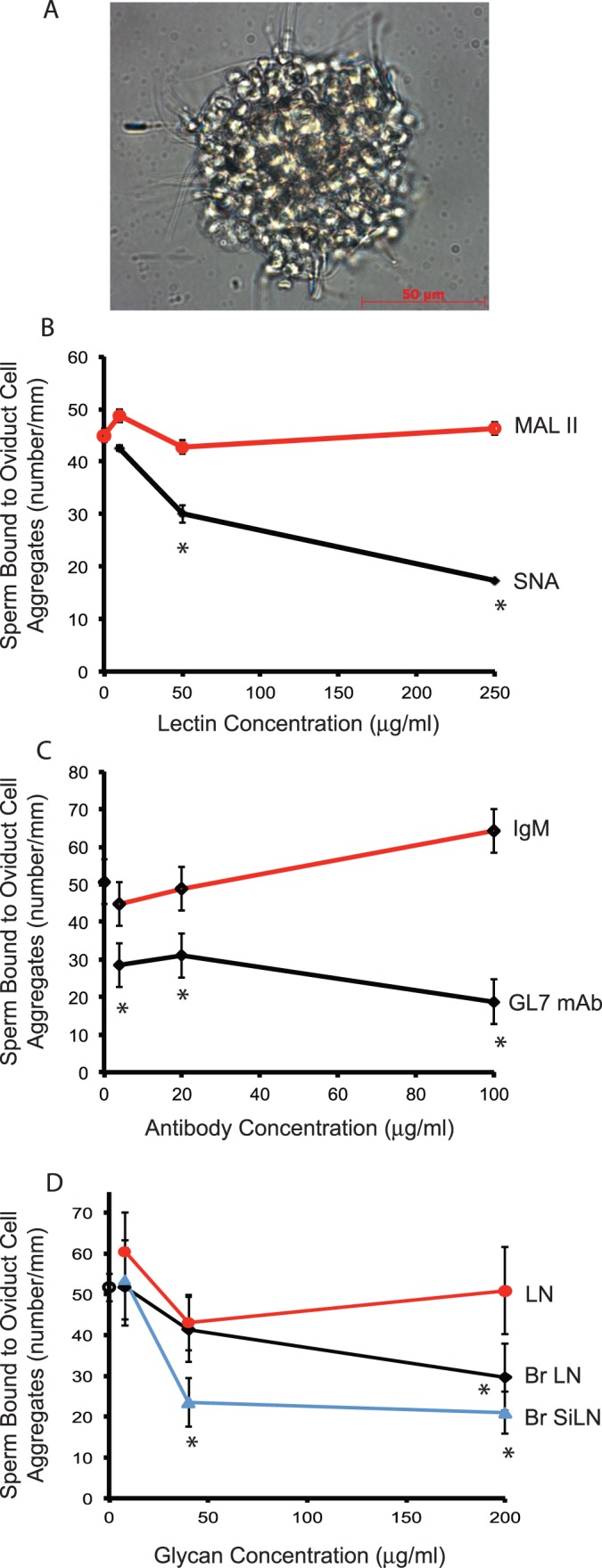
Blocking oviduct sialylated lactosamine or its putative receptor on sperm reduces sperm binding to oviduct cells. Oviduct cells were collected from the isthmus and allowed to form spherical aggregates. The aggregates were incubated with varying concentrations of either medium, showing (A) sperm bound to an oviduct aggregate, (B) SNA, a lectin that binds structures containing sialic acid in an α2,6 linkage, or M. amurensis lectin II (MAL II), a lectin that binds structures containing sialic acid in an α2,3 linkage, or (C) a monoclonal antibody (GL7 mAb) to sialylated lactosamine or IgM, and allowed to bind sperm. In D, sperm were preincubated with varying concentrations of branched, 6-sialylated lactosamine glycans (Br SiLN), branched lactosamine glycans (Br LN), or simply the disaccharide lactosamine (LN), and then challenged with oviduct cell aggregates. Binding was expressed as the number of sperm bound per millimeter of aggregate circumference. Data are the least square means of at least three experiments. Asterisks indicate concentrations at which SNA, GL7, or glycans were different from controls that used medium only.
To determine if the putative sialyl lactosamine receptors on sperm are required for sperm to bind isthmic epithelial cells, these receptors were occupied with branched sialylated lactosamine on an acrylamide core [54, 55]. Either 40 or 200 μg/ml of 6-sialylated lactosamine on a biantennary mannose core inhibited sperm-isthmic cell binding by up to 60% (Fig. 6D). Branched lactosamine without any sialyl residues reduced sperm binding to oviduct cells, but required a higher concentration; a concentration of 200 μg/ml reduced sperm-isthmic cell binding by 43%. A control using simply the lactosamine disaccharide did not affect sperm-isthmic cell binding. Therefore, blocking either the oviduct glycan or its putative receptor(s) on sperm reduced sperm binding to isthmic epithelial cells by about 60%.
DISCUSSION
This article reports the first large-scale screen of the ability of glycans to bind mammalian cells. Using an array of 377 glycans, we found that all glycans that bound porcine sperm contained either of two glycan motifs, LeX structures or 6-sialylated lactosamine on one or more termini of a biantennary mannose core. Binding to either motif on a microarray slide was sufficient to tether a motile sperm against the force of gravity and washing, suggesting that the affinity of this interaction was relatively high. Either glycan could bind sperm independently of any other structures. The ability of fluoresceinated 6-sialylated biantennary glycans to bind the sperm head corroborated the results with the glycan array. In addition to these sufficiency experiments, 6-sialylated lactosamine on oviduct cells was also necessary for normal sperm binding. Blocking this glycan reduced sperm binding to oviduct cells by up to 60%. It is not clear if this maximum binding inhibition was because the remaining adhesion was accounted for by sperm binding to nonsialylated biantennary lactosamine structures, LeX, or other adhesive molecules or simply reflected a technical inability to block sialylated lactosamine completely.
Sperm binding to glycans was highly specific. Sperm bound abundantly to sialylated lactosamine termini in which sialic acid was linked to the 6 position of galactose, but no detectable binding was observed to sialic acid linked to the 3 position of galactose. Furthermore, although sperm bound to oligosaccharides with a LeX motif, no binding was observed to Lewis A-derived structures, a trisaccharide that is a positional isomer of LeX.
Glycan multivalency was important in binding sperm. Sialylated lactosamine was present on two antennae of a biantennary glycan for maximum sperm binding; the sialylated lactosamine trisaccharide, by itself, did not bind sperm (Figs. 1 and 2). We tested neoglycoconjugates that had multiple copies of the glycans presented on the same molecule by linking them to a polyacrylamide core. The glycan inhibition experiments demonstrated that these multivalent glycans bound to sperm and inhibited sperm from binding to oviduct epithelial cells.
Previous reports have concluded that porcine sperm bind to oligomannose structures and oligosaccharides with nonreducing galactose [36]. Neither of these structures on the glycan array bound sperm. It may be that the affinity of these glycans for sperm is lower and inadequate to tether a motile sperm against gravity and the washing conditions.
The glycan profile of oviduct cells revealed that sialylated lactosamine was abundant in the N-linked and O-linked glycan fractions. Among the N-glycans, some had two termini with sialyl residues, and some had one sialyl residue while the second terminus contained galactose. Interestingly, two “hybrid” glycans were detected: one terminus had sialylated lactosamine and the second had a Lewis structure, with and without core fucosylation. Whether these hybrid glycans have greater affinity for sperm than either biantennary sialylated lactosamine or LeX glycans is an interesting question.
Sialylated lactosamine was detected throughout the ampulla and isthmus. This suggests that the reason that more sperm bind to the isthmus than ampulla is that sperm, in their movement through the genital tract, encounter 6-sialylated biantennary glycans in the isthmus first and are retained in this region of the oviduct. In swine, prior to reaching the isthmus, sperm also bind in large numbers to the utero-tubal junction [7, 8, 11]. It is unclear if 6-sialylated glycans are also present in this region although other glycans, such as hyaluronan and sulfated glycosaminoglycans, are present in both the isthmus and utero-tubal junction [11]. We did not observe differences in the abundance of 6-sialylated lactosamine in oviducts collected from sows at different stages of the estrus cycle. This is consistent with observations in cows [16, 42] and mares [60] that the density of sperm binding sites in the oviduct does not change during the estrous cycle, even though oviduct fluid constituents are variable [61].
The fertilizing sperm must be released from the storage site to move into the ampulla and fertilize the eggs. Recent evidence suggests that the development of hyperactivated motility may be sufficient to detach a sperm from the oviduct epithelium [62]. Furthermore, mouse sperm deficient in CatSper that cannot hyperactivate do not detach from the oviduct [63]. In addition to hyperactivation, a loss or modification in putative oviduct receptors on sperm during capacitation may also contribute to sperm release. Consistent with this hypothesis, our results indicate that a population of sperm incubated under capacitating conditions had reduced binding to 6-sialylated lactosamine. This suggests that, as sperm are capacitated, detachment may be partially mediated by a loss or modification of putative receptors for oviduct glycans. However, release may also be controlled by components from the oviduct, including the cumulus-oocyte complex, progesterone, or disulfide reducants [64, 65], altered behavior of sperm-bound oviduct cells [66], oviduct smooth muscle contractions [67], or the effects of locally produced anandamide and nitric oxide on sperm [68, 69]. The dynamic nature of sperm interaction with the oviduct epithelium suggests that a variety of factors may regulate sperm release.
The effect of binding to specific oviduct glycans on sperm function is not clear. There is evidence that binding to oviduct cells affects sperm intracellular calcium, capacitation, and lifespan [17, 18, 70, 71]. Learning how binding to the oviduct epithelium affects sperm behavior may lead to methods for lengthening sperm lifespan in the oviduct or improving sperm storage outside the oviduct. A recent report concluded that an oviduct heat shock protein, when added to semen extender, improved the survival of ram sperm [72, 73]. However, how any putative ligand-receptor pair influences sperm behavior has yet to be established.
These results are the first report of mammalian cells binding to a glycan array. Sperm were useful test cells, because they survive well in suspension and their motility provides greater probability of colliding with glycans on the array. However, it is possible that the ability of other cells to bind many glycans could also be investigated by use of a glycan array. This approach may provide insight in to how a variety of cells can interact with glycans in a dynamic extracellular matrix.
ACKNOWLEDGMENT
Semen was a gift from Prairie State Semen, Inc. (Champaign, IL) and tissues were gifts from Meadowbrooke Farms (Rantoul, IL), Calihan Pork Producers (Peoria, IL), and Rantoul Foods (Rantoul, IL).
Footnotes
Current address: Division of Animal Production, ICAR Research Complex, Meghalaya-793103, India.
This project was supported by Agriculture and Food Research Initiative Competitive grant 2011-67015-20099 from the United States Department of Agriculture National Institute of Food and Agriculture to D.J.M., by a Better Opportunities for Young Scientists in Chosen Areas of Science and Technology (BOYSCAST) award from the Department of Science and Technology, India to G.K., by the grant Molecular and Cell Biology of RAS Presidium to N.B., and by NIHGMS–the Consortium for Functional Glycomics GM62116.
These authors contributed equally to this work.
REFERENCES
- Holt WV. Mechanisms of sperm storage in the female reproductive tract: an interspecies comparison. Reprod Domest Anim 2011; 46 (Suppl 2): 68 74. [DOI] [PubMed] [Google Scholar]
- Sever DM, Brizzi R. Comparative biology of sperm storage in female salamanders. J Exp Zool 1998; 282: 460 476. [PubMed] [Google Scholar]
- Gist DH, Jones JM. Storage of sperm in the reptilian oviduct. Scanning Microsc 1987; 1: 1839 1849. [PubMed] [Google Scholar]
- Holt WV, Elliott RM, Fazeli A, Sostaric E, Georgiou AS, Satake N, Prathalingam N, Watson PF. Harnessing the biology of the oviduct for the benefit of artificial insemination. Soc Reprod Fertil Suppl 2006; 62: 247 259. [PubMed] [Google Scholar]
- Crichton EG, Krutzsch PH. Reproductive biology of the female little mastiff bat, Mormopterus planiceps (Chiroptera: Molossidae) in southeast Australia. Am J Anat 1987; 178: 369 386. [DOI] [PubMed] [Google Scholar]
- Hunter RH. Oviduct function in pigs, with particular reference to the pathological condition of polyspermy. Mol Reprod Dev 1991; 29: 385 391. [DOI] [PubMed] [Google Scholar]
- Mburu JN, Einarsson S, Lundeheim N, Rodriguez-Martinez H. Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci 1996; 45: 109 121. [DOI] [PubMed] [Google Scholar]
- Mburu JN, Rodriguez-Martinez H, Einarsson S. Changes in sperm ultrastructure and localisation in the porcine oviduct around ovulation. Anim Reprod Sci 1997; 47: 137 148. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 2008; 52: 455 462. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update 2006; 12: 23 37. [DOI] [PubMed] [Google Scholar]
- Tienthai P, Kjellen L, Pertoft H, Suzuki K, Rodriguez-Martinez H. Localization and quantitation of hyaluronan and sulfated glycosaminoglycans in the tissues and intraluminal fluid of the pig oviduct. Reprod Fertil Dev 2000; 12: 173 182. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Isotani A, Yamaguchi R, Ikawa M, Baba T, Suarez SS, Okabe M. Selective passage through the uterotubal junction of sperm from a mixed population produced by chimeras of calmegin-knockout and wild-type male mice. Biol Reprod 2004; 71: 959 965. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol Biol Cell 2012; 23: 2671 2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RH, Flechon B, Flechon JE. Distribution, morphology and epithelial interactions of bovine spermatozoa in the oviduct before and after ovulation: a scanning electron microscope study. Tissue Cell 1991; 23: 641 656. [DOI] [PubMed] [Google Scholar]
- Kawakami E, Kashiwagi C, Hori T, Tsutsui T. Effects of canine oviduct epithelial cells on movement and capacitation of homologous spermatozoa in vitro. Anim Reprod Sci 2001; 68: 121 131. [DOI] [PubMed] [Google Scholar]
- Pollard JW, Plante C, King WA, Hansen PJ, Betteridge KJ, Suarez SS. Fertilizing capacity of bovine sperm may be maintained by binding of oviductal epithelial cells. Biol Reprod 1991; 44: 102 107. [DOI] [PubMed] [Google Scholar]
- Boilard M, Bailey J, Collin S, Dufour M, Sirard MA. Effect of bovine oviduct epithelial cell apical plasma membranes on sperm function assessed by a novel flow cytometric approach. Biol Reprod 2002; 67: 1125 1132. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Smith TT, Suarez SS, Ball BA. Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol Reprod 1997; 56: 861 869. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Elliott RM, Duncan AE, Moore A, Watson PF, Holt WV. In vitro maintenance of boar sperm viability by a soluble fraction obtained from oviductal apical plasma membrane preparations. Reproduction 2003; 125: 509 517. [PubMed] [Google Scholar]
- Tienthai P, Johannisson A, Rodriguez-Martinez H. Sperm capacitation in the porcine oviduct. Anim Reprod Sci 2004; 80: 131 146. [DOI] [PubMed] [Google Scholar]
- Coy P, Canovas S, Mondejar I, Saavedra MD, Romar R, Grullon L, Matas C, Aviles M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc Natl Acad Sci U S A 2008; 105: 15809 15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeiro JM, Dapino DG, Marini PE. Porcine oviduct sperm binding glycoprotein and its deleterious effect on sperm: a mechanism for negative selection of sperm? Biol Res 2011; 44: 329 337. [PubMed] [Google Scholar]
- Teijeiro JM, Marini PE. The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro-AKAP4 phosphorylation. Reproduction 2012; 143: 773 785. [DOI] [PubMed] [Google Scholar]
- Coy P, Lloyd R, Romar R, Satake N, Matas C, Gadea J, Holt WV. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 2010; 74: 632 642. [DOI] [PubMed] [Google Scholar]
- Killian G. Physiology and endocrinology symposium: evidence that oviduct secretions influence sperm function: a retrospective view for livestock. J Anim Sci 2011; 89: 1315 1322. [DOI] [PubMed] [Google Scholar]
- Pacey AA, Davies N, Warren MA, Barratt CL, Cooke ID. Hyperactivation may assist human spermatozoa to detach from intimate association with the endosalpinx. Hum Reprod 1995; 10: 2603 2609. [DOI] [PubMed] [Google Scholar]
- Pacey AA, Hill CJ, Scudamore IW, Warren MA, Barratt CL, Cooke ID. The interaction in vitro of human spermatozoa with epithelial cells from the human uterine (fallopian) tube. Hum Reprod 1995; 10: 360 366. [DOI] [PubMed] [Google Scholar]
- Kervancioglu ME, Saridogan E, Aitken RJ, Djahanbakhch O. Importance of sperm-to-epithelial cell contact for the capacitation of human spermatozoa in fallopian tube epithelial cell cocultures. Fertil Steril 2000; 74: 780 784. [DOI] [PubMed] [Google Scholar]
- Hung PH, Suarez SS. Regulation of sperm storage and movement in the ruminant oviduct. Soc Reprod Fertil Suppl 2010; 67: 257 266. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez H. Role of the oviduct in sperm capacitation. Theriogenology 2007; 68 (Suppl 1): S138 S146. [DOI] [PubMed] [Google Scholar]
- Murray SC, Smith TT. Sperm interaction with fallopian tube apical membrane enhances sperm motility and delays capacitation. Fertil Steril 1997; 68: 351 357. [DOI] [PubMed] [Google Scholar]
- Smith TT, Nothnick WB. Role of direct contact between spermatozoa and oviductal epithelial cells in maintaining rabbit sperm viability. Biol Reprod 1997; 56: 83 89. [DOI] [PubMed] [Google Scholar]
- Boilard M, Reyes-Moreno C, Lachance C, Massicotte L, Bailey JL, Sirard MA, Leclerc P. Localization of the chaperone proteins GRP78 and HSP60 on the luminal surface of bovine oviduct epithelial cells and their association with spermatozoa. Biol Reprod 2004; 71: 1879 1889. [DOI] [PubMed] [Google Scholar]
- Ignotz GG, Cho MY, Suarez SS. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol Reprod 2007; 77: 906 913. [DOI] [PubMed] [Google Scholar]
- Ekhlasi-Hundrieser M, Gohr K, Wagner A, Tsolova M, Petrunkina A, Topfer-Petersen E. Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol Reprod 2005; 73: 536 545. [DOI] [PubMed] [Google Scholar]
- Topfer-Petersen E, Ekhlasi-Hundrieser M, Tsolova M. Glycobiology of fertilization in the pig. Int J Dev Biol 2008; 52: 717 736. [DOI] [PubMed] [Google Scholar]
- Amann RP, Griel LC., Jr. Fertility of bovine spermatozoa from rete testis, cauda epididymidis, and ejaculated semen. J Dairy Sci 1974; 57: 212 219. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Carbohydrate-mediated formation of the oviductal sperm reservoir in mammals. Cells Tissues Organs 2001; 168: 105 112. [DOI] [PubMed] [Google Scholar]
- Wagner A, Ekhlasi-Hundrieser M, Hettel C, Petrunkina A, Waberski D, Nimtz M, Topfer-Petersen E. Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol Reprod Dev 2002; 61: 249 257. [DOI] [PubMed] [Google Scholar]
- Demott RP, Lefebvre R, Suarez SS. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 1995; 52: 1395 1403. [DOI] [PubMed] [Google Scholar]
- Green CE, Bredl J, Holt WV, Watson PF, Fazeli A. Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro. Reproduction 2001; 122: 305 315. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Lo MC, Suarez SS. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol Reprod 1997; 56: 1198 1204. [DOI] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A 2004; 101: 17033 17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol 2010; 480: 417 444. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem 2007; 282: 9127 9142. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M, Konstantopoulos K, Schnaar RL. E-selectin receptors on human leukocytes. Blood 2008; 112: 3744 3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill ER, Aoki K, Lopez PH, Colacurcio D, Vajn K, Lorenzini I, Majic S, Yang WH, Heffer M, Tiemeyer M, Marth JD, Schnaar RL. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 2012; 22 10: 1289 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumula KR, Taylor PB. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal Biochem 1992; 203: 101 108. [DOI] [PubMed] [Google Scholar]
- Aoki K, Porterfield M, Lee SS, Dong B, Nguyen K, Mcglamry KH, Tiemeyer M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J Biol Chem 2008; 283: 30385 30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SY, Wu SW, Hsiao HH, Khoo KH. Enabling techniques and strategic workflow for sulfoglycomics based on mass spectrometry mapping and sequencing of permethylated sulfated glycans. Glycobiology 2009; 19: 1136 1149. [DOI] [PubMed] [Google Scholar]
- Vukelić Z, Zamfir AD, Bindila L, Froesch M, Peter-Katalinić J, Usuki S, Yu RK. Screening and sequencing of complex sialylated and sulfated glycosphingolipid mixtures by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom 2005; 16: 571 580. [DOI] [PubMed] [Google Scholar]
- Awda BJ, Buhr MM. Extracellular signal-regulated kinases (ERKs) pathway and reactive oxygen species regulate tyrosine phosphorylation in capacitating boar spermatozoa. Biol Reprod 2010; 83: 750 758. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dube C, Bailey JL. Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol Reprod 2003; 68: 207 213. [DOI] [PubMed] [Google Scholar]
- Galanina O, Feofanov A, Tuzikov AB, Rapoport E, Crocker PR, Grichine A, Egret-Charlier M, Vigny P, Le Pendu J, Bovin NV. Fluorescent carbohydrate probes for cell lectins. Spectrochim Acta A Mol Biomol Spectrosc 2001; 57: 2285 2296. [DOI] [PubMed] [Google Scholar]
- Galanina OE, Tuzikov AB, Rapoport E, Le Pendu J, Bovin NV. Carbohydrate-based probes for detection of cellular lectins. Anal Biochem 1998; 265: 282 289. [DOI] [PubMed] [Google Scholar]
- Knox RV. Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig. Domest Anim Endocrinol 2005; 29: 385 397. [DOI] [PubMed] [Google Scholar]
- Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, Tiwari VK, Cao H, Chokhawala HA, Zheng H, Cummings RD, Smith DF. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J Biol Chem 2011; 286: 31610 31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, Sugai M, Okuno Y, Tsujimoto G, Yamaji T, Hashimoto Y, Itohara S. et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol 2007; 27: 3008 3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S, Redfern K, Raynor P, Martin F, Phillips DM. Attachment of boar sperm to mucosal explants of oviduct in vitro: possible role in formation of a sperm reservoir. Biol Reprod 1991; 44: 998 1004. [DOI] [PubMed] [Google Scholar]
- Thomas PG, Ball BA, Brinsko SP. Interaction of equine spermatozoa with oviduct epithelial cell explants is affected by estrous cycle and anatomic origin of explant. Biol Reprod 1994; 51: 222 228. [DOI] [PubMed] [Google Scholar]
- Killian GJ, Chapman DA, Kavanaugh JF, Deaver DR, Wiggin HB. Changes in phospholipids, cholesterol and protein content of oviduct fluid of cows during the oestrous cycle. J Reprod Fertil 1989; 86: 419 426. [DOI] [PubMed] [Google Scholar]
- Curtis MP, Kirkman-Brown JC, Connolly TJ, Gaffney EA. Modelling a tethered mammalian sperm cell undergoing hyperactivation. J Theor Biol 2012; 309: 1 10. [DOI] [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez SS. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Fertil Dev 2009; 21: 345 350. [DOI] [PubMed] [Google Scholar]
- Brussow KP, Ratky J, Rodriguez-Martinez H. Fertilization and early embryonic development in the porcine fallopian tube. Reprod Domest Anim 2008; 43 (Suppl 2): 245 251. [DOI] [PubMed] [Google Scholar]
- Talevi R, Zagami M, Castaldo M, Gualtieri R. Redox regulation of sperm surface thiols modulates adhesion to the fallopian tube epithelium. Biol Reprod 2007; 76: 728 735. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Affara NA, Hubank M, Holt WV. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol Reprod 2004; 71: 60 65. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol Reprod 2012; 86: 140, 1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi MG, Osycka-Salut C, Caballero J, Vazquez-Levin M, Pereyra E, Billi S, Franchi A, Perez-Martinez S. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS ONE 2011; 6: e16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osycka-Salut C, Gervasi MG, Pereyra E, Cella M, Ribeiro ML, Franchi AM, Perez-Martinez S. Anandamide induces sperm release from oviductal epithelia through nitric oxide pathway in bovines. PLoS ONE 2012; 7: e30671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Suarez SS, Ball BA. Intracellular calcium concentration in equine spermatozoa attached to oviductal epithelial cells in vitro. Biol Reprod 1996; 54: 783 788. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Friedrich J, Drommer W, Bicker G, Waberski D, Topfer-Petersen E. Kinetic characterization of the changes in protein tyrosine phosphorylation of membranes, cytosolic Ca2+ concentration and viability in boar sperm populations selected by binding to oviductal epithelial cells. Reproduction 2001; 122: 469 480. [DOI] [PubMed] [Google Scholar]
- Lloyd RE, Elliott RM, Fazeli A, Watson PF, Holt WV. Effects of oviductal proteins, including heat shock 70 kDa protein 8, on survival of ram spermatozoa over 48 h in vitro. Reprod Fertil Dev 2009; 21: 408 418. [DOI] [PubMed] [Google Scholar]
- Lloyd RE, Fazeli A, Watson PF, Holt WV. The oviducal protein, heat-shock 70-kDa protein 8, improves the long-term survival of ram spermatozoa during storage at 17 degrees C in a commercial extender. Reprod Fertil Dev 2012; 24: 543 549. [DOI] [PubMed] [Google Scholar]