Abstract
Rationale: Airway smooth muscle (ASM) plays a key role in airway hyperresponsiveness (AHR) but it is unclear whether its contractility is intrinsically changed in asthma.
Objectives: To investigate whether key parameters of ASM contractility are altered in subjects with asthma.
Methods: Human trachea and main bronchi were dissected free of epithelium and connective tissues and suspended in a force–length measurement set-up. After equilibration each tissue underwent a series of protocols to assess its methacholine dose–response relationship, shortening velocity, and response to length oscillations equivalent to tidal breathing and deep inspirations.
Measurements and Main Results: Main bronchi and tracheal ASM were significantly hyposensitive in subjects with asthma compared with control subjects. Trachea and main bronchi did not show significant differences in reactivity to methacholine and unloaded tissue shortening velocity (Vmax) compared with control subjects. There were no significant differences in responses to deep inspiration, with or without superimposed tidal breathing oscillations. No significant correlations were found between age, body mass index, or sex and sensitivity, reactivity, or Vmax.
Conclusions: Our data show that, in contrast to some animal models of AHR, human tracheal and main bronchial smooth muscle contractility is not increased in asthma. Specifically, our results indicate that it is highly unlikely that ASM half-maximum effective concentration (EC50) or Vmax contribute to AHR in asthma, but, because of high variability, we cannot conclude whether or not asthmatic ASM is hyperreactive.
Keywords: airway smooth muscle mechanics, airway hyperresponsiveness, shortening velocity, asthma, smooth muscle
At a Glance Commentary
Scientific Knowledge on the Subject
Contraction of airway smooth muscle is directly responsible for acute airway constriction in asthmatic attacks. However, evidence on whether airway smooth muscle contractility is altered in asthma is contradictory, incomplete, and often derived from problematic tissue sources.
What This Study Adds to the Field
We have measured a range of parameters of airway smooth muscle contractility that have never been tested on reliable human airway smooth muscle tissues. Our study found, at least in trachea and main bronchi, no changes in contractility that could contribute to airway hyperresponsiveness in subjects with asthma.
It is well established that airway smooth muscle (ASM) contraction leads to the airway constriction typical of airway hyperresponsiveness (AHR) in asthma. Nonetheless, it is unclear whether AHR is the result of altered ASM contractility, or even whether altered ASM function is required at all for AHR. For example, the inflammatory mediators typically present in asthma could be responsible for triggering abnormal airway narrowing even when the ASM itself is entirely normal. However, maximal concentrations of histamine in healthy subjects do not reduce FEV1 to asthmatic levels (1), so an excess of other contractile agonists is unlikely to cause excessive ASM contraction either. It thus remains a plausible hypothesis that ASM contractility is intrinsically altered in asthma. Nevertheless, the veracity of this seemingly straightforward idea has so far proved very difficult to establish or refute.
In the 1980s and early 1990s many studies were conducted on human ASM tissue (2–7), including some that compared asthmatic with control ASM. These studies were mainly focused on the isometric active tension generated in response to a variety of agonists (2, 3, 5, 7) with mixed results. Some studies found asthmatic ASM to be hyperresponsive (3), but most found either no change or that the asthmatic ASM was actually hyporesponsive (3, 5, 7). Nevertheless, these studies all had a major drawback in that they studied tissues procured either from patients with lung cancer (4, 6), many of whom were current smokers, or from cadavers many hours post-mortem (2, 3, 5, 7). It is likely that the different tissue conditions used in these studies, none of which were representative of typical asthma, contributed at least in part to the disagreement between results.
Recently, lungs donated for transplantation that, for one reason or another, do not meet transplantation criteria have become available for use in medical research. These organs are shipped according to stringent transplantation protocols that minimize decline in function, so one would expect them to provide more valid data about the properties of ASM in either people with asthma or healthy individuals compared with ASM from surgically resected lungs that are usually severely diseased. To date, however, the only published study comparing asthmatic and control ASM from this tissue source is that of Chin and colleagues (8). Those investigators found that human trachealis exhibited no difference in either tension or shortening velocity between control subjects and subjects with asthma in response to electrical field stimulation (EFS). They did find small differences in force recovery following 30 seconds of large-amplitude oscillations and in length-tension relationships, but the study did not address ASM sensitivity to agonists or EFS. Also, some of the results may have been affected by the large difference in average age of the subjects between the asthmatic and control groups (15.0 ± 5.9 vs. 31.7 ± 17.5, respectively), because shortening velocity has been shown in animal models to decrease with age (9), and the shortening velocity data themselves were highly variable.
There thus remains much that can be learned about the nature of ASM in asthma from further study of tissue from lungs originally destined for transplantation. This applies particularly to the question of whether intrinsic ASM contractility is altered in asthma. Accordingly, addressing this question was the goal of the present study. We expanded on the work of Chin and colleagues (8) by assessing ASM responsiveness to methacholine (MCh), the response to a large stretch equivalent to a single deep inspiration (DI) and unloaded tissue shortening velocity, while using more stringent subject selection criteria and a higher-resolution force–length apparatus. Most importantly, we made these measurements not only in the trachealis but also in ASM from the main bronchi to establish whether our findings may be generalized to more than a single generation of the airway tree.
Some of the results of these studies have been previously reported in the form of an abstract (10).
Methods
Procurement and Dissection
Asthmatic and control transplant-grade lungs were procured by the International Institute for the Advancement of Medicine. The demographics and clinical details of the donors are shown in Table 1. The tissues were stored in Custodial histidine-tryptophan-ketoglutarate (HTK) or University of Wisconsin (UW) solution during shipment, with trachea and main bronchi separately packed from the lungs, which were used for a different study. On arrival the trachea and main bronchi were placed in oxygenated Hanks' balanced salt solution (composition in mM: 5.3 KCl, 0.44 KH2PO4, 137.9 NaCl, 0.336 Na2PO4, 2.33 CaCl2, 0.79 MgSO4, 10 glucose, 10 HEPES buffer, pH adjusted to 7.4 with NaOH) at 4°C and used within 12 hours. Smooth muscle (SM) bundles were dissected from epithelium and connective tissue in calcium-free Krebs solution (composition in mM: 110 NaCl, 0.82 MgSO4, 1.2 KH2PO4, 3.4 KCl, 25.7 NaHCO3, 5.6 glucose, pH at 7.4, bubbled with 95/5% O2/CO2 gas mixture) on ice and aluminum foil clips were attached on either end of the tissue.
Table 1.
Subject Medical and Demographic Data
| Subject | Sex | Age | Body Mass Index | Ethnicity | Cause of Death | Asthma History | Other | Medication(s) | Medication in Hospital | |
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with asthma |
||||||||||
| 1 | M | 72 | 44.2 | W | CVA secondary to ICH | Age of diagnosis unknown | Smoking 16 pack-years, quit 50 YA | Unknown | Unknown | |
| 2 | F | 34 | 31.93 | W | Anoxia secondary to drug intoxication | Diagnosed 20 YA, hospitalized twice with exacerbations | Inhaler–prednisone | Solu-Medrol, Levophed | ||
| 3 | M | 29 | 30.8 | W | Anoxia secondary to cardiovascular | Diagnosed 7 YA | Chewing tobacco for 1 yr | Albuterol inhaler | Esmolol, Levophed | |
| 4 | F | 60 | 25.59 | W | HT secondary to blunt injury |
Diagnosed 10 YA | Complete hysterectomy 40 YA, hypertension | Infrequent inhaler use, hypertension medication | Levophed, Solu-Medrol, phentolamine | |
| 5 | M | 38 | 29.49 | W | CVA secondary to ICH | Diagnosed at 4 mo | Allergy medications | Norepinephrine, Levophed, phenylephrine, dobutamine | ||
| 6 | M | 35 | 29.4 | W | Anoxia (asthma) secondary to cardiovascular | Asthma since childhood | Albuterol, Pulmicort | Levophed, Solu-Medrol, albuterol | ||
| 7 | M | 40 | 26.95 | A | Anoxia secondary to cardiovascular | Asthma diagnosed 5 YA | Dulera | Levophed, Solu-Medrol | ||
| 8 | F | 38 | 35.78 | W | Anoxia secondary to cardiovascular | Asthma diagnosed at 8 | Singulair | Levophed, epinephrine, dopamine | ||
| Control subjects |
||||||||||
| 9 | M | 22 | 23 | W | HT secondary to SIGSW | Asthma as child, not taken medication in 7 yr | Smoked hookah past year | Albuterol as child and Pepcid | Levophed, Neo-Synephrine, Atrovent, albuterol, Solu-Medrol | |
| 10 | F | 61 | 35.1 | H | CVA secondary to ICH | Tobacco product 20 YA, diabetes, hypertension | Levophed, albuterol-ipratropium | |||
| 11 | M | 47 | 26.2 | W | CVA secondary to ICH | Neosynephrine | ||||
| 12 | F | 55 | 26 | W | CVA secondary to ICH | Alcohol abuse, marijuana and cocaine use | Neosynephrine, dopamine, Levophed, Solu-Medrol, dobutamine | |||
| 13 | F | 35 | 21.87 | W | Anoxia secondary to ICH | Teenage marijuana use | Levophed | |||
| 14 | M | 30 | 21.85 | W | Anoxia secondary to asphyxiation | Marijuana, cocaine use | Solu-Medrol | |||
| 15 | F | 54 | 36.13 | W | CVA secondary to ICH | Smoking 10 pack-years, quit 8 YA | Epinephrine | |||
| 16 | F | 62 | 30.86 | W | CVA secondary to natural causes | Hypertension, basal cell carcinoma in nose 3 YA | Dopamine | |||
| 17 | M | 55 | 26.79 | W | HT secondary to blunt injury |
Marijuana occasionally, alcohol abuse | Neosynephrine, Solu-Medrol | |||
| 18 | F | 58 | 33.87 | W | CVA secondary to ICH | Neosynephrine, Solu-Medrol | ||||
| 19 | F | 54 | 24.97 | W | HT secondary to blunt injury |
Smoking 30 pack-years, quit 16 YA, hypertension | Levophed, albuterol | |||
Definition of abbreviations: A = African American; CVA = cerebrovascular accident; H = Hispanic; HT = head trauma; ICH = intracerebral hemorrhage; SIGSW = self-inflicted gunshot wound; W = white; YA = years ago.
Tissue Mechanics
Equilibration
The tissue was attached horizontally with foil clips to a length controller (model 322C-I; Aurora Scientific, Aurora, ON, Canada) and a force transducer (model 400A; Aurora Scientific) controlled by Aurora Scientific 600A software at a reference length equal to the in situ length in a relaxed state (intact trachea or main bronchi ring in calcium-free Krebs). The tissue was continuously flushed with Krebs solution (as previously with 2.4 mM CaCl2) at a rate of approximately 1 ml/min in a 1-ml tissue bath. The tissue was equilibrated for at least 30 minutes with EFS (10-s duration 25 V/cm, 50 Hz, 2-ms pulse width) every 5 minutes, followed by at least five contractions with MCh 10−6 M. These EFS settings were used in all protocols. Both equilibration protocols were continued until a stable baseline (without spontaneous contractions) and contractile force were achieved.
Dose response
The tissue was exposed to increasing concentrations of MCh every minute, from a concentration of 10−7 M up to 10−4 M (Figure 1A). The peak force reached (relative to baseline force and corrected for measurement noise) after each administered dose was used as the force representative of that dose. Maximum stress was calculated from the maximum force, extrapolated from the dose–response curve fit divided by the SM cross-sectional area. To determine the SM cross-sectional area, the tissues were fixed in 10% formalin for 12–24 hours, and embedded in paraffin for histology. Five-micrometer-thick slices were stained with Masson's trichrome, which provided the best contrast between nonmuscle and SM tissue. The average ratio of SM to total tissue area for each tissue was calculated (average for all tissues, 0.49 ± 0.02) and multiplied by the tissue cross-sectional area (average for all tissues, 0.166 ± 0.005 mm2).
Figure 1.
Traces from all airway smooth muscle tissue mechanics experiments, and a histology sample. Traces are the average of all tissues from subjects with asthma. (A) Trace of methacholine (MCh) dose–response protocol. (B) Trace of force–velocity protocol. (C and D) Traces of deep inspiration (DI) protocols. (E) Sample of a histology image of smooth muscle cross-section with Masson's trichrome staining. EFS = electrical field stimulation.
EFS force–velocity
The tissue was contracted using EFS for 10 seconds every 5 minutes immediately followed by a measurement of the force (Fref) and a rapid force clamp of 5, 10, 20, 40, or 80% of Fref for 120 milliseconds (Figure 1B). The shortening velocity was determined from the rate of length change during the last 60 milliseconds of the force clamp, when the force had stabilized. The data were rejected if any of the force clamps had not stabilized before this 60-millisecond period. To compensate for force transducer drift, true zero force was measured at the start of the protocol by rapidly shortening the muscle to 75% of L0 followed by relengthening.
Unloaded tissue shortening velocity (Vmax) was calculated by extrapolation using a perpendicular least-squares fitting method to a classic Hill curve of the form V = b(F0 − F)/(a + F). For details, see Reference 11.
Deep inspiration
Two protocols for measuring DI effects were performed (Figures 1C and 1D). For each protocol the tissue was first exposed to three consecutive EFS contractions 5 minutes apart to establish a stable reference contractile force. Subsequently, a length change equivalent to a DI was applied to the tissue (half sinusoidal wave, 0.2 Hz, 0.3 Lref amplitude) followed by five EFS contractions, 5 minutes apart. The tissue was then contracted with 10−6 M MCh and after 2 minutes the tissue was again exposed to a DI equivalent length change (half sinusoidal wave, 0.2 Hz, 0.2 Lref amplitude to adjust for increased stiffness of the tissue), followed by flushing with Krebs solution for 5 minutes and five EFS contractions, each 5 minutes apart. In both protocols a continuous sinusoidal length oscillation was superimposed, with the only difference being the amplitude and frequency of this oscillation. One protocol had a 30-Hz 0.0125 Lref oscillation applied to measure stiffness throughout the protocol; the other had a 0.2-Hz 0.04 Lref oscillation applied to simulate the effect of continuous breathing oscillations. The order of the two protocols was randomized for each tissue.
Rejection Criteria
Tissues were rejected if control EFS and MCh 10−6 M contractions did not achieve a maximal force level within 30% of the reference contractions at the end of the equilibration phase. Tissues were also rejected if they failed to relax fully after a contraction with either EFS or MCh 10−6 M or if spontaneous contractions failed to subside before the end of the equilibration phase. Rejection rates were similar between subjects with asthma (10%) and control subjects (15%).
Data Analysis and Statistics
Linear mixed models were used to estimate the expected difference in maximum contractile force (Fmax), the half-maximum effective concentration (EC50), and Vmax between subjects with asthma and control subjects, and site, adjusted for one another. We included a random intercept to account for correlation between measures on the same subject. Two-way repeated measures analysis of variance was used for the DI protocols. Error bars are standard errors. All protocols have been applied to tissues from all the lungs described in Table 1. For each trachea and main bronchi (unless they were not provided, damaged, or rejected) two tissues were tested and the results averaged (n = number of subjects). The 95% confidence intervals of the difference of the mean of the control subjects compared with the subjects with asthma was calculated by averaging all available tissues for each subject. The confidence intervals were expressed as a percentage of the mean of the control subjects.
Results
Dose–Response Curves
Dose–response curves are shown in Figure 2 as absolute stress (Figure 2A) and stress normalized to maximum contractile stress (Figure 2B). Absolute stress was not significantly different with location (P = 0.60) or disease (P = 0.66) (Figures 2A and 2C). EC50, the dose at which 50% of the maximum stress is generated, was significantly reduced (hyposensitive) in subjects with asthma (P = 0.050), with no significant difference with location (P = 0.1718) (Figures 2B and 2D). The confidence interval of the difference of the means of subjects with asthma versus control subjects (Figure 2E) shows the large variability in the absolute stress and a less than 2.5% chance that asthmatic ASM (n = 8) is hypersensitive to MCh compared with control subjects (n = 11).
Figure 2.

Methacholine (MCh) dose–response curves. Triangles represent trachea and circles main bronchi tissues; solid symbols are control subjects and open symbols are subjects with asthma. (A) Absolute stress dose–response of main bronchi (MB) and trachea (T) in subjects with asthma and control subjects. (B) Dose–response curves normalized to maximum stress (σmax). (C) σmax derived from curve fits of the dose response. No significant differences were found. (D) EC50 derived from dose–response curves. EC50 showed significant differences with disease state (*P = 0.05) but not location. (E) Confidence interval of the difference of the means of pooled trachea and main bronchi data for EC50 and σmax in control subjects versus subjects with asthma. EC50 = half-maximum effective concentration.
Shortening Velocity
To assess whether Vmax is changed in subjects with asthma, it was calculated from force–velocity curves of five force clamps during five separate, consecutive EFS contractions. Figure 3 shows the average data for the individual force clamps as well as the Hill-curve–extrapolated Vmax. No significant difference was found with disease state (P = 0.38) or location(P = 0.42). The 95% confidence interval of the difference of the means (Figure 3C) showed a less than 2.5% chance that the Vmax is more than 9.8% increased in subjects with asthma (n = 8) compared with control subjects (n = 11).
Figure 3.
(A) Electrical field stimulation force–velocity curves. Shortening velocity was measured at five force clamps, and Vmax was calculated using extrapolation of a Hill-curve curve fit. (B) Vmax for main bronchi (MB) and trachea (T) in asthma and control. Triangles represent trachea and circles main bronchi tissues; solid symbols are control subjects and open symbols are subjects with asthma. No significant differences were found. (C) Confidence interval of the difference of the means of pooled trachea and main bronchi data of control subjects versus subjects with asthma
Deep Inspiration
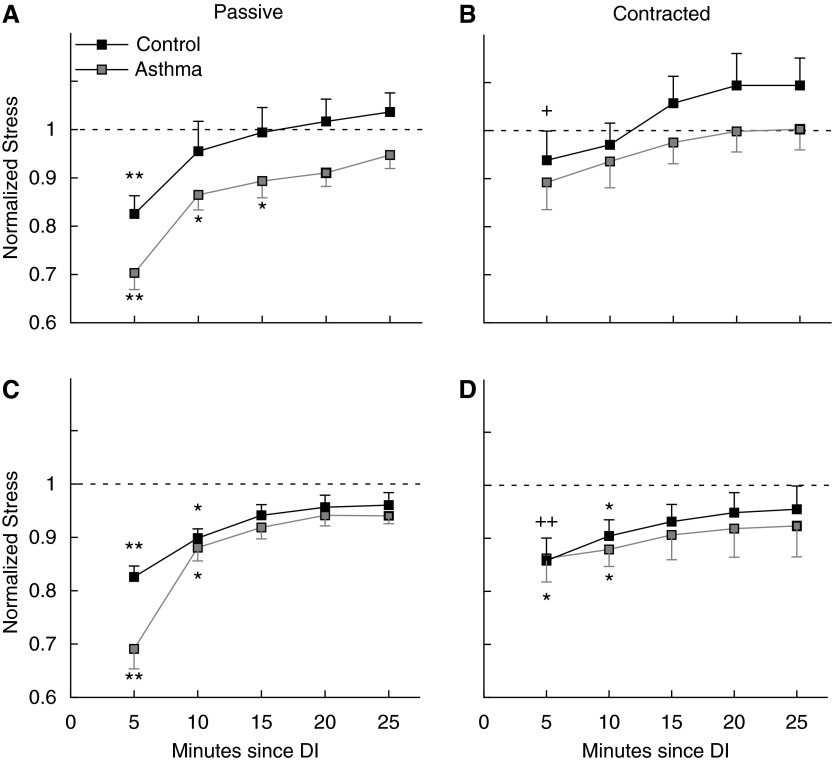
The effect of DI on the contractile force in successive contractions is shown in Figure 4. Because no significant differences between main bronchi and trachea were found, only the pooled data for all tissues per subject are shown. No significant differences were found between subjects with asthma and control subjects. The force of the first contraction after a DI in relaxed muscle was less than the force in all subsequent contractions for control subjects and subjects with asthma both with and without superimposed breathing oscillations, but not when the DI was applied to contracted muscle. Furthermore, a significant difference was found between the force prior to DI and the second and third contraction in subjects with asthma and the second contraction only in control subjects and subjects with asthma after a DI in both relaxed and contracted ASM when breathing oscillations were superimposed. Although subjects with asthma showed a trend toward less contractile force after a DI, particularly in the first contraction after the DI in relaxed muscle, none of the differences between subjects with asthma and control subjects were statistically significant. The superimposed breathing oscillation did not have a significant effect on the response to DIs.
Figure 4.
Deep inspiration (DI) response. All stresses are normalized to the average contractile stress over three electrical field stimulation (EFS) contractions prior to the first DI. (A and C) EFS contractile stress after a DI in passive, relaxed airway smooth muscle. (B and D) EFS contractile stress after a DI in methacholine 10−6 M contracted airway smooth muscle. C and D follow the same protocol as A and B but with a continuous superimposed length oscillation equivalent in amplitude and frequency to tidal breathing. Black squares are control subjects (n = 6) and gray squares subjects with asthma (n = 6). Two-way repeated measures analysis of variance showed statistically significant differences within the same group of subjects, but not between subjects with asthma and control subjects. Markers indicate significant differences: *different from force prior to DI; **same as * but also different from all subsequent EFS contractions; +different from force at 20 and 25 minutes; ++different from force at 25 minutes.
Body Mass Index, Age, and Sex Effects
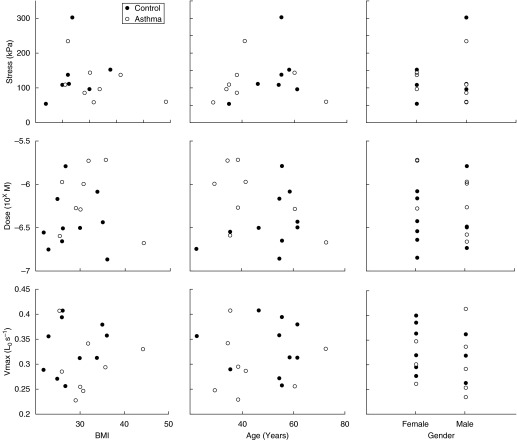
The asthmatic and control groups were not significantly different in age (43.3 ± 2.2 vs. 48.5 ± 4.0) and body mass index (BMI) (31.8 ± 1.0 vs. 27.9 ± 1.6), with a small difference in sex distribution (62% male vs. 36% male). Three main parameters from the protocols (EC50 and maximal stress from the MCh dose–response and Vmax) were tested for correlation with age, sex, and BMI (Figure 5). Only the pooled data for all tissues per subject are shown. None of the parameters showed any significant correlation.
Figure 5.
Body mass index (BMI), age, and sex correlations for three contractility parameters. None of the parameters showed a significant correlation with BMI, age, or sex.
Discussion
We examined several key indicators of human ASM contractility in subjects with asthma and control subjects at two sites in the bronchial tree. We found strong evidence that contractility as expressed by EC50 and Vmax is unlikely to be altered to favor AHR in asthma. Furthermore, no differences in reactivity or DI response were found. Although peripheral ASM may show increased contractility in asthma, our results indicate that such differences are not intrinsic to ASM. In addition, our study did not find any evidence of age, sex, or BMI effects on tracheal and main bronchial ASM contractility.
Asthmatic ASM Is neither Intrinsically Hyperreactive nor Hypersensitive
We studied tissues from both main bronchi and trachea, because previous animal studies have shown that ASM contractility is not uniform throughout the lung (12), which may be more so in subjects with asthma. Our MCh dose–response data indicate that tracheal and bronchial ASM are not intrinsically hyperresponsive in asthma. In fact, our data show that ASM EC50 is slightly, but significantly, hyposensitive. Large variability in our maximal stress data leaves some uncertainty to the contribution of ASM reactivity to AHR. Furthermore, we cannot exclude the possibility that ASM is hyperresponsive in the asthmatic intrapulmonary airways. However, studies on human intrapulmonary bronchial SM from cadavers with fatal asthma mostly showed hyposensitivity to a range of agonists (2, 5, 7). One of those studies showed hyperresponsiveness to histamine, but hyporesponsiveness to acetylcholine and in the EFS frequency response (2). However, in these studies the connective tissue and epithelium were not removed (usual practice for the spiral strip dissection technique) and the tissues were dissected up to 14 hours post-mortem. Also, forces were normalized by tissue weight.
Because the remodeling in the airway wall may have changed the quantity of ASM relative to that of the connective tissues and epithelium, the results on reactivity may have been misinterpreted (13). Whole-airway comparisons in MCh dose–response between control subjects and subjects with asthma have also been done recently, and showed a clear decrease in airway diameter at every dose in subjects with asthma, but no changes in sensitivity of the airways to MCh (14). However, it is unknown to what extent those dose–responses were affected by the epithelium and other airway wall tissues. Consequently, our data should be more representative of the actual intrinsic ASM contractility.
The hyposensitivity observed in our data may be the result of desensitization from prolonged agonist exposure, which would be expected to occur in asthma. This has previously been shown in cultured vascular SM cells (15) and more recently in rabbit tracheal SM cells (16). Furthermore, the increase in ASM mass found in asthma is caused by hypertrophy and hyperplasia, and both have been shown to reduce the contractility of ASM. Our laboratory has shown that rat tracheal ASM responds to repeated allergen challenge with a reduced contractility of SM cells and a commensurate increase in SM cell number (17). Hypertrophy of ASM has also been shown to result in reduced contractility in some studies (18), although not consistently (19). Although detailed medication intake of the donors in the last weeks of life is difficult to get, it is possible that end-of-life drugs or asthma medication, particularly long-acting β-agonists, may have reduced contractility. However, it is unlikely that pharmaceutical agents remain in effective concentrations after dissection and equilibration protocols, and prolonged exposure to β-agonists has been shown to lead to aggravated AHR (20, 21).
Shortening Velocity
The force–velocity data shown in Figure 3 directly contradict a range of findings in both human and animal studies. Several animal models of AHR have shown an increase in Vmax in MCh contracted trachea (22–24). In humans, asthmatic bronchial ASM cells harvested by endobronchial biopsies were shown to have an increased Vmax and total shortening when exposed to contractile agonists compared with control subjects (25). Our laboratory has also shown in a mathematical model that an increase in shortening velocity could, in principle, account for the differential response to DI in subjects with asthma because increased shortening velocity may lead to a faster return to a prestretch length (26). Furthermore, Jackson and colleagues (27) showed that immediately after a DI the rate of increase of airway resistance is much higher in subjects with asthma compared with control subjects, which may indeed be caused by an increase in shortening velocity.
Nonetheless our current study showed no change in Vmax, with high confidence that Vmax in subjects with asthma is not considerably increased compared with control subjects. One possible explanation may be that, because Vmax is not uniform throughout the lung (12), ASM shortening velocity may also not be changed uniformly throughout the lung in asthma. Trachea and main bronchi are likely exposed to a different inflammatory and mechanical environment than peripheral bronchi and consequently the trend of decreased Vmax in asthmatic main bronchi may not extend into the periphery. The only other study of EFS shortening velocity in human trachealis SM also found no differences between subjects with asthma and control subjects (8), in agreement with our data.
DI Response
A defining feature of asthma is the lack of response to DIs, whereas in healthy subjects DI bronchodilating and bronchoprotective abilities surpass any currently available medication (28, 29). Several studies have shown that DIs can reduce subsequent contractile force generation in animal ASM and this effect has been hypothesized to be reduced in asthma (30, 31). Our results do indicate that the contractile force is reduced after a DI, but subjects with asthma only show a nonsignificantly greater force reduction following a DI in relaxed muscle compared with control subjects. Even length oscillations equivalent to continuous breathing did not have much effect on the contractile force in subjects with asthma or control subjects. The subject with severe asthma showed contractile force potentiation after a DI in contracted muscle, but this was not seen in any of the other subjects with asthma, including the subject with fatal asthma.
The DI response in relaxed muscle in subjects with asthma versus control subjects has previously been assessed by Chin and colleagues (8), showing a reduced effect on subsequent EFS contractions in subjects with asthma. However, their protocol used 10-minute 30% Lref length oscillations, whereas we simulated a physiologic single DI with a single half-sinusoidal stretch of 30% of Lref in relaxed muscle and a similar 20% Lref stretch in 10−6 M MCh contracted muscle. Although there are some differences in the type of subjects (age and asthma severity), the difference likely lies in the applied protocols. Perhaps stretch-activated mechanisms in ASM respond differently to a single stretch than to a long duration of repeated stretches. Another study, on whole airway segments taken from lung resections of control subjects and mild to moderate subjects with asthma, showed an immediate effect of DI similar to ours on the dose–response to MCh in control subjects and subjects with asthma, but over time, they observed a greater narrowing in the subjects with asthma (31). While this narrowing did not surpass the narrowing prior to the DI, these results were different from ours, which showed no significant difference between the equilibrium contractile force after the DI and the contractile force prior to the DI.
Age, Sex, and BMI Effects
Age effects on ASM response have been found in animal studies (9) and both weight (32) and sex (33) have been implicated in human asthma. Although not enough subjects could be tested for conclusive answers regarding correlations, no obvious trends were apparent for any of the three tested parameters. The lack of sex effects may be attributed to age, because sex effects are more obvious in teenagers (33). Alternatively, any short-term hormonal effects may not be present because the hormones would be washed out during equilibration or degraded during transport. Maturational studies in sheep (34) and guinea pigs (9) show changes in both contractile force (increase with age) and shortening velocity (strong decrease with age). However, these studies focused on very young animals and little is known about changes in adulthood.
Conclusions
Our data suggest that, in contrast to many animal models of AHR, human tracheal and main bronchi SM does not contribute to AHR through persistent changes in contractile properties. Our results indicate that it is highly unlikely that ASM EC50 or Vmax contribute to AHR in asthma, but, because of high variability, we cannot conclude whether asthmatic ASM is hyperreactive. We conclude that ASM is probably not intrinsically and homogenously altered in asthma. Further research will have to address whether transient or more peripheral ASM changes do occur.
Footnotes
Supported by National Heart, Lung, and Blood Institute grant R01-HL 103405-02 and the Costello Fund. The Meakins-Christie Laboratories (McGill University Health Centre Research Institute) are supported in part by a center grant from Le Fonds de la Recherche en Santé du Québec (FRSQ).
Author Contributions: G.I., acquisition of data, analysis and interpretation of data, drafting of manuscript. L.K. and O.S.M., acquisition of data and article review. J.H.T.B. and J.G.M., analysis and interpretation of data and article review. A.B., statistical analysis and article review. A.-M.L., conception and design, analysis and interpretation of data, and drafting and review of manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201407-1296OC on February 19, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977;7:235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 2.Bai TR. Abnormalities in airway smooth muscle in fatal asthma: a comparison between trachea and bronchus. Am Rev Respir Dis. 1991;143:441–443. doi: 10.1164/ajrccm/143.2.441. [DOI] [PubMed] [Google Scholar]
- 3.Bai TR. Abnormalities in airway smooth muscle in fatal asthma. Am Rev Respir Dis. 1990;141:552–557. doi: 10.1164/ajrccm/141.3.552. [DOI] [PubMed] [Google Scholar]
- 4.de Jongste JC, van Strik R, Bonta IL, Kerrebijn KF. Measurement of human small airway smooth muscle function in vitro with the bronchiolar strip preparation. J Pharmacol Methods. 1985;14:111–118. doi: 10.1016/0160-5402(85)90048-8. [DOI] [PubMed] [Google Scholar]
- 5.Goldie RG, Spina D, Henry PJ, Lulich KM, Paterson JW. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, beta-adrenoceptor agonists and theophylline. Br J Clin Pharmacol. 1986;22:669–676. doi: 10.1111/j.1365-2125.1986.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida K, Paré PD, Hards J, Schellenberg RR. Mechanical properties of human bronchial smooth muscle in vitro. J Appl Physiol (1985) 1992;73:1481–1485. doi: 10.1152/jappl.1992.73.4.1481. [DOI] [PubMed] [Google Scholar]
- 7.Whicker SD, Armour CL, Black JL. Responsiveness of bronchial smooth muscle from asthmatic patients to relaxant and contractile agonists. Pulm Pharmacol. 1988;1:25–31. doi: 10.1016/0952-0600(88)90007-5. [DOI] [PubMed] [Google Scholar]
- 8.Chin LYM, Bossé Y, Pascoe C, Hackett TL, Seow CY, Paré PD. Mechanical properties of asthmatic airway smooth muscle. Eur Respir J. 2012;40:45–54. doi: 10.1183/09031936.00065411. [DOI] [PubMed] [Google Scholar]
- 9.Chitano P, Wang L, Murphy TM. Three paradigms of airway smooth muscle hyperresponsiveness in young guinea pigs. Can J Physiol Pharmacol. 2007;85:715–726. doi: 10.1139/y07-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ijpma G, Kachmar L, Zitouni N, Bates G, Lauzon A-M. Shortening velocity and in vitro motility of human airway smooth muscle in atopic or obese asthmatics and control subjects [abstract] Am J Respir Crit Care Med. 2013;187:A1997. [Google Scholar]
- 11.Bullimore SR, Saunders TJ, Herzog W, MacIntosh BR. Calculation of muscle maximal shortening velocity by extrapolation of the force-velocity relationship: afterloaded versus isotonic release contractions. Can J Physiol Pharmacol. 2010;88:937–948. doi: 10.1139/y10-068. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Li W, Stephens NL. Detection of two clusters of mechanical properties of smooth muscle along the airway tree. J Appl Physiol (1985) 1996;80:857–861. doi: 10.1152/jappl.1996.80.3.857. [DOI] [PubMed] [Google Scholar]
- 13.Armour CL, Black JL, Berend N, Woolcock AJ. The relationship between bronchial hyperresponsiveness to methacholine and airway smooth muscle structure and reactivity. Respir Physiol. 1984;58:223–233. doi: 10.1016/0034-5687(84)90150-6. [DOI] [PubMed] [Google Scholar]
- 14.Noble PB, Jones RL, Cairncross A, Elliot JG, Mitchell HW, James AL, McFawn PK. Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J Appl Physiol (1985) 2013;114:1460–1471. doi: 10.1152/japplphysiol.01489.2012. [DOI] [PubMed] [Google Scholar]
- 15.Kai H, Fukui T, Lassègue B, Shah A, Minieri CA, Griendling KK. Prolonged exposure to agonist results in a reduction in the levels of the Gq/G11 alpha subunits in cultured vascular smooth muscle cells. Mol Pharmacol. 1996;49:96–104. [PubMed] [Google Scholar]
- 16.Stamatiou R, Paraskeva E, Vasilaki A, Mylonis I, Molyvdas PA, Gourgoulianis K, Hatziefthimiou A. Long-term exposure to muscarinic agonists decreases expression of contractile proteins and responsiveness of rabbit tracheal smooth muscle cells. BMC Pulm Med. 2014;14:39. doi: 10.1186/1471-2466-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labonté I, Hassan M, Risse P-A, Tsuchiya K, Laviolette M, Lauzon A-M, Martin JG. The effects of repeated allergen challenge on airway smooth muscle structural and molecular remodeling in a rat model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:L698–L705. doi: 10.1152/ajplung.00142.2009. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Zhou D, Seow CY, Bai TR. Cardiotrophin-1 alters airway smooth muscle structure and mechanical properties in airway explants. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1165–L1171. doi: 10.1152/ajplung.00171.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Brown M, Kogut P, Serban K, Li X, McConville J, Chen B, Bentley JK, Hershenson MB, Dulin N, et al. Akt activation induces hypertrophy without contractile phenotypic maturation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;300:L701–L709. doi: 10.1152/ajplung.00119.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galland BC, Blackman JG. Enhancement of airway reactivity to histamine by isoprenaline and related beta-adrenoceptor agonists in the guinea-pig. Br J Pharmacol. 1993;108:1016–1023. doi: 10.1111/j.1476-5381.1993.tb13499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sears MR. Adverse effects of β-agonists. J Allergy Clin Immunol. 2002;110(Suppl 6):S322–S328. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]
- 22.Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JHT, Eidelman DH. Bronchial responsiveness among inbred mouse strains. Role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med. 2000;161:839–848. doi: 10.1164/ajrccm.161.3.9906054. [DOI] [PubMed] [Google Scholar]
- 23.Fan T, Yang M, Halayko A, Mohapatra SS, Stephens NL. Airway responsiveness in two inbred strains of mouse disparate in IgE and IL-4 production. Am J Respir Cell Mol Biol. 1997;17:156–163. doi: 10.1165/ajrcmb.17.2.2628. [DOI] [PubMed] [Google Scholar]
- 24.Wang CG, Almirall JJ, Dolman CS, Dandurand RJ, Eidelman DH. In vitro bronchial responsiveness in two highly inbred rat strains. J Appl Physiol (1985) 1997;82:1445–1452. doi: 10.1152/jappl.1997.82.5.1445. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 26.Bullimore SR, Siddiqui S, Donovan GM, Martin JG, Sneyd J, Bates JHT, Lauzon A-M. Could an increase in airway smooth muscle shortening velocity cause airway hyperresponsiveness? Am J Physiol Lung Cell Mol Physiol. 2011;300:L121–L131. doi: 10.1152/ajplung.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson AC, Murphy MM, Rassulo J, Celli BR, Ingram RH., Jr Deep breath reversal and exponential return of methacholine-induced obstruction in asthmatic and nonasthmatic subjects. J Appl Physiol (1985) 2004;96:137–142. doi: 10.1152/japplphysiol.00504.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol (1985) 2000;89:711–720. doi: 10.1152/jappl.2000.89.2.711. [DOI] [PubMed] [Google Scholar]
- 29.Nadel JA, Tierney DF. Effect of a previous deep inspiration on airway resistance in man. J Appl Physiol. 1961;16:717–719. doi: 10.1152/jappl.1961.16.4.717. [DOI] [PubMed] [Google Scholar]
- 30.Raqeeb A, Solomon D, Paré PD, Seow CY. Length oscillation mimicking periodic individual deep inspirations during tidal breathing attenuates force recovery and adaptation in airway smooth muscle. J Appl Physiol (1985) 2010;109:1476–1482. doi: 10.1152/japplphysiol.00676.2010. [DOI] [PubMed] [Google Scholar]
- 31.LaPrad AS, West AR, Noble PB, Lutchen KR, Mitchell HW. Maintenance of airway caliber in isolated airways by deep inspiration and tidal strains. J Appl Physiol (1985) 2008;105:479–485. doi: 10.1152/japplphysiol.01220.2007. [DOI] [PubMed] [Google Scholar]
- 32.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093, quiz 1094–1095. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4:S133–S146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 34.Panitch HB, Deoras KS, Wolfson MR, Shaffer TH. Maturational changes in airway smooth muscle structure-function relationships. Pediatr Res. 1992;31:151–156. doi: 10.1203/00006450-199202000-00012. [DOI] [PubMed] [Google Scholar]