Abstract
Rationale: Recent pediatric studies suggest a survival benefit exists for higher-volume extracorporeal membrane oxygenation (ECMO) centers.
Objectives: To determine if higher annual ECMO patient volume is associated with lower case-mix–adjusted hospital mortality rate.
Methods: We retrospectively analyzed an international registry of ECMO support from 1989 to 2013. Patients were separated into three age groups: neonatal (0–28 d), pediatric (29 d to <18 yr), and adult (≥18 yr). The measure of hospital ECMO volume was age group–specific and adjusted for patient-level case-mix and hospital-level variance using multivariable hierarchical logistic regression modeling. The primary outcome was death before hospital discharge. A subgroup analysis was conducted for 2008–2013.
Measurements and Main Results: From 1989 to 2013, a total of 290 centers provided ECMO support to 56,222 patients (30,909 neonates, 14,725 children, and 10,588 adults). Annual ECMO mortality rates varied widely across ECMO centers: the interquartile range was 18–50% for neonates, 25–66% for pediatrics, and 33–92% for adults. For 1989–2013, higher age group–specific ECMO volume was associated with lower odds of ECMO mortality for neonates and adults but not for pediatric cases. In 2008–2013, the volume–outcome association remained statistically significant only among adults. Patients receiving ECMO at hospitals with more than 30 adult annual ECMO cases had significantly lower odds of mortality (adjusted odds ratio, 0.61; 95% confidence interval, 0.46–0.80) compared with adults receiving ECMO at hospitals with less than six annual cases.
Conclusions: In this international, case-mix–adjusted analysis, higher annual hospital ECMO volume was associated with lower mortality in 1989–2013 for neonates and adults; the association among adults persisted in 2008–2013.
Keywords: extracorporeal membrane oxygenation, high-volume hospitals, low-volume hospitals, pediatric, adult
At a Glance Commentary
Scientific Knowledge on the Subject
The number of patients receiving extracorporeal membrane oxygenation (ECMO) support and the number of centers offering ECMO support is increasing rapidly, particularly for adults. Previous research suggests an association exists between higher ECMO hospital volume and lower ECMO mortality rates in pediatrics. Whether an ECMO volume–outcome association is present for adults is unknown.
What This Study Adds to the Field
Using hospital ECMO volumes from 290 international centers, this study is the first to find an association between higher adult ECMO hospital volume and lower adult ECMO mortality rates. This study also documents the significant variability in survival rates among centers.
Extracorporeal membrane oxygenation (ECMO) provides temporary pulmonary and/or cardiac support when medical management fails or when the degree of support required is considered injurious to the patient (1, 2). ECMO support can be provided in three medically refractory circumstances: (1) respiratory failure (3, 4), (2) cardiac failure (5, 6), and (3) when cardiopulmonary resuscitation (CPR) does not restore spontaneous circulation (7). These three different ECMO support types are named respiratory ECMO, cardiac ECMO, and ECMO during CPR (ECPR), respectively.
Neonatal and adult research suggests ECMO provides a survival benefit (1, 3, 4, 7), but experts debate the evidence regarding the benefit of ECMO support (2, 8, 9). Less subject to debate, however, is the observation that patients who require ECMO likely have substantially increased healthcare costs relative to conventional management (4). Importantly, the number of hospitals offering ECMO has grown rapidly, particularly for adult patients (10, 11), raising the concern that rapid diffusion of low-volume advanced care modalities across multiple institutions might compromise their efficiency and effectiveness (12, 13).
ECMO support requires an experienced and organized medical team to deliver technically sophisticated care (13). Optimal delivery of such complex care is often associated with a volume–outcome relationship in which higher case volumes are associated with better outcomes (14). An international group of physicians with ECMO expertise has recommended that ECMO centers providing ECMO for adult respiratory failure should perform at least 20 annual cases of total ECMO volume and at least 12 annual cases in the subset of adult respiratory ECMO (13). Two recent pediatric studies have demonstrated a relationship between higher annual hospital ECMO volume and lower patient mortality rates. These studies analyzed U.S. pediatric hospital administrative databases during study years 2000–2009 (15) and 2004–2011 (16). The studies did not include adult patients or pre-ECMO clinical data that could potentially confound the volume–outcome relationship and would improve case-mix adjustment.
This study analyzed data within an international ECMO registry to examine the relationship between age group–specific annual hospital ECMO volume and the case-mix–adjusted hospital mortality rate among neonatal, pediatric, and adult patients separately. The primary measure of outcome was death prior to discharge from the hospital that provided ECMO care. Some of the results of these studies have been previously reported in the form of abstracts (17–19).
Methods
After the study design and data protection methods were presented to the Extracorporeal Life Support (ELSO) steering committee, we were granted permission to conduct a retrospective analysis of the ELSO Registry. It is a voluntary international registry that has collected data on more than 56,000 patients treated at 290 centers. The database has been internally validated through an audit of clinical data submitted by participating institutions, with only 1% of 190 reported fields including any incorrect data (20).
Patient Selection
All cases in the ELSO Registry from January 1, 1989 to December 31, 2013 were included in the analysis. Records were divided into groups based on age: neonatal (0–28 d), pediatric (>28 d to <18 yr), and adults (≥18 yr). If a person received ECMO support on more than one occasion, only the first ECMO run was included in the analysis.
Selection of Variables and Risk Adjustment
Volume was defined as the total number of age group–specific annual ECMO cases performed at a hospital. Volume was modeled first as a continuous variable based on prior literature (21). To facilitate comparison between lower- and higher-volume centers, it was decided a priori to also analyze volume as a categorical variable with four categories: less than six, 6–14, 15–30, and more than 30 cases per year. This categorization was adopted from a recent publication in which centers were categorized into volume categories of less than 15, 15–30, and more than 30 based on hospitals’ total neonatal and pediatric ECMO volume (15), and on the ELSO recommendation that an ECMO center have an annual ECMO volume of at least six cases per year (22).
Case-mix adjustment variables were chosen a priori using two criteria: (1) the variable had been previously associated with mortality, and (2) the extent to which the variable was missing in the ELSO database. The following variables were chosen: calendar year in which ECMO was delivered (15, 23); patient age (23–25); primary diagnosis (23–27); ECMO support type (16, 27); presence of pre-ECMO acute renal failure (23, 26, 27); presence of comorbid conditions (23, 28); pre-ECMO cardiac arrest (25, 29); and pre-ECMO measures including duration of mechanical ventilation (23, 25, 30), arterial blood pH (23, 27, 31), PaCO2 (25), and the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PF ratio) (1, 23). In neonatal patients, case-mix was also adjusted for gestational age (24) and birth weight (24). See the Methods section in the online supplement and Tables E1–E3 in the online supplement for a further description of the primary diagnoses and comorbid conditions selection.
Since 2008, advances in technology have facilitated the administration of ECMO support (32). Therefore, it was decided a priori to conduct a subgroup analysis among patients treated from 2008 to 2013 to examine if the volume–outcome relationship persisted in the most recent period of ECMO care.
Statistical Analysis
We divided the ELSO Registry into three age group–specific datasets. For each dataset, separate hierarchical logistic regression models were fit with estimation of robust standard errors to examine possible association between age group–specific hospital volume and in-hospital mortality (hospital level) while case-mix adjusting for pre-ECMO patient characteristics (patient level). We modeled hospital as a random effect. We tested for significance of random effects with a likelihood ratio test.
To address missing values for variables, we used inverse probability weighting, which selectively weights patients with complete data to reflect similar patients with missing data. See the Methods section in the online supplement for a further description of inverse probability weighting and Table E4 for a report of missing data.
We tested the sensitivity of our findings by repeating the primary analyses under two separate conditions; in both cases we excluded those patients who received ECPR. First, we stratified our analyses by mode of cannulation: venovenous and venoarterial. Specifically, we considered only those patients who received venovenous ECMO support. Then we considered only those patients who received venoarterial ECMO support. Second, in a similar fashion we stratified our analyses by support type: respiratory and cardiac. We did this because patients who received venovenous ECMO definitively received respiratory ECMO support. Meanwhile, some patients who received venoarterial ECMO were placed on ECMO for respiratory support. See the Methods section in the online supplement for further description.
Given that the study was an analysis of deidentified data, it was determined to be exempt from human subjects review by the Institutional Review Board of the University of Michigan Medical School.
Results
Overall Patterns of ECMO Care: 1989–2013
Overall, 290 ELSO centers provided ECMO support to 56,222 individuals (30,909 neonates, 14,725 children, and 10,588 adults). ECMO mortality rates for neonatal respiratory, cardiac, and ECPR were 26%, 59%, and 59%, respectively, compared with 43%, 49%, and 59% among pediatric patients and 43%, 60%, and 71% among adults.
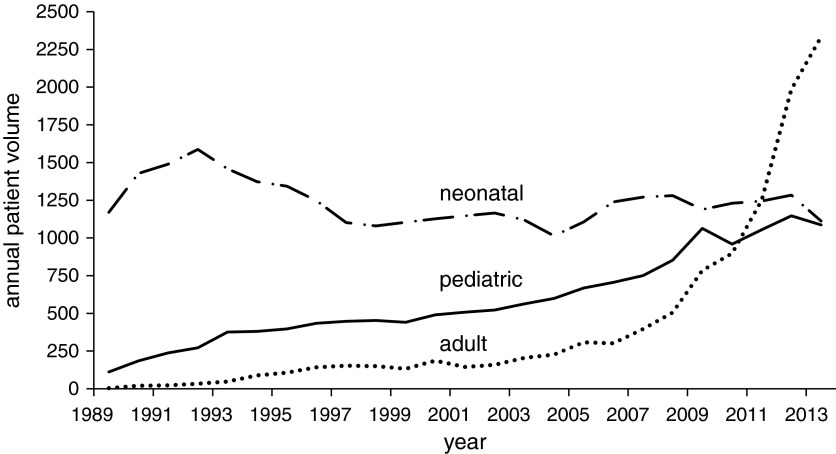
After declining in the 1990s, annual neonatal ECMO volumes remained generally stable over the study period, especially in the latter years. Conversely, annual pediatric ECMO volumes increased and adult ECMO volumes grew exponentially (Figure 1). This disproportionate growth is reflected in the proportion of ECMO cases performed at high-volume centers. Between 1989 and 2007, 18% of neonatal ECMO cases, 2% of pediatric ECMO cases, and 34% of adult ECMO cases were performed at centers with an age group–specific ECMO volume of more than 30 cases per year. Between 2008 and 2013, those numbers were 7% for neonatal ECMO, 7% for pediatric ECMO, and 49% of adult ECMO. This represents a decrease in the proportion of neonatal cases performed at high-volume centers and an upsurge in the proportion of adult cases performed at high-volume centers.
Figure 1.
Annual volume of patients receiving extracorporeal membrane oxygenation, 1989–2013.
Hospital ECMO Volume and Mortality: 1989–2013
Annual hospital ECMO case volumes for neonatal, pediatric, and adult ECMO ranged from 1 to 72, 1 to 40, and 1 to 129 cases, respectively. Characteristics of patients treated in each of the volume categories are presented in Table 1 and Table E5. In general, differences in patient characteristics were more evident across age groups than between volume categories within age groups.
Table 1.
Patient Characteristics Prior to ECMO Support by Age Group and Annual ECMO Volume Category, 1989–2013
| Neonatal (n = 30,909, N = 197) |
Pediatric (n = 14,725, N = 235) |
Adult (n = 10,588, N = 214) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual Hospital ECMO Volume Categories | 1–5 | 6–14 | 15–30 | >30 | 1–5 | 6–14 | 15–30 | >30 | 1–5 | 6–14 | 15–30 | >30 |
| Number of patients | 2,718 | 10,726 | 12,770 | 4,695 | 3,477 | 5,956 | 4,709 | 583 | 1,243 | 1,790 | 2,800 | 4,755 |
| Type of ECMO support, % |
||||||||||||
| Respiratory | 74 | 77 | 80 | 87 | 48 | 39 | 39 | 39 | 57 | 54 | 51 | 44 |
| Cardiac | 23 | 20 | 17 | 11 | 46 | 49 | 41 | 34 | 33 | 39 | 39 | 41 |
| ECPR | 3 | 3 | 4 | 2 | 7 | 12 | 20 | 26 | 10 | 7 | 10 | 16 |
| Pre-ECMO characteristics, % |
||||||||||||
| Female | 43 | 42 | 41 | 42 | 48 | 48 | 47 | 46 | 42 | 38 | 37 | 35 |
| Acute renal failure | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 4 | 6 | 4 | 4 |
| Comorbid condition* | 4 | 3 | 3 | 2 | 10 | 10 | 12 | 15 | 18 | 18 | 17 | 12 |
| Pre-ECMO characteristics, median |
||||||||||||
| Age, d | 2 | 2 | 1 | 1 | ||||||||
| Age, yr | 0 | 1 | 1 | 1 | 35 | 48 | 49 | 51 | ||||
| pH† | 7.28 | 7.29 | 7.32 | 7.38 | 7.30 | 7.28 | 7.27 | 7.24 | 7.25 | 7.25 | 7.26 | 7.29 |
| PF ratio† | 38 | 39 | 39 | 39 | 57 | 60 | 63 | 63 | 61 | 63 | 63 | 75 |
| Pre-ECMO hours of mechanical ventilation | 26 | 26 | 24 | 22 | 34 | 24 | 24 | 25 | 23 | 22 | 20 | 17 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; ECPR = ECMO during cardiopulmonary resuscitation; n = number of patients; N = number of centers; pH = arterial blood pH; PF ratio = the ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; pre-ECMO hours of mechanical ventilation = the number hours of mechanical ventilation a patient received prior to ECMO cannulation.
Comorbid conditions were defined as present or absent using definitions described in the literature for neonates (46), pediatrics (47), and adults (48).
Most abnormal value recorded within 6 h of receipt of ECMO support.
Annual ECMO mortality rates varied widely across ECMO centers: the interquartile range was 18–50% for neonates, 25–66% for pediatrics, and 33–92% for adults. Unadjusted all-ECMO mortality rates in neonate, pediatric, and adult patients were generally lower at institutions with higher hospital ECMO volume specific to the age groups (Table 2). When stratified by distinct ECMO support types, unadjusted mortality rates within each age group were generally lower at institutions with higher annual age group–specific ECMO volume. Exceptions to this pattern occurred within the ECPR ECMO category, which included the smallest proportions of cases in all age groups.
Table 2.
Mortality Rates by Age Group and Annual ECMO Volume Category for Patients Undergoing ECMO, Overall and by ECMO Support Type, 1989–2013
| Neonatal |
Pediatric |
Adult |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual Hospital ECMO Volume Categories | 1–5 | 6–14 | 15–30 | >30 | 1–5 | 6–14 | 15–30 | >30 | 1–5 | 6–14 | 15–30 | >30 |
| All ECMO support types | 41 | 37 | 32 | 25 | 51 | 49 | 45 | 41 | 59 | 54 | 53 | 51 |
| Respiratory ECMO | 33 | 29 | 25 | 20 | 45 | 45 | 39 | 30 | 51 | 45 | 44 | 40 |
| Cardiac ECMO | 60 | 60 | 59 | 55 | 54 | 51 | 45 | 42 | 67 | 65 | 59 | 57 |
| ECPR | 70 | 62 | 54 | 62 | 64 | 58 | 58 | 57 | 74 | 71 | 76 | 69 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; ECPR = ECMO during cardiopulmonary resuscitation.
Data are percentages.
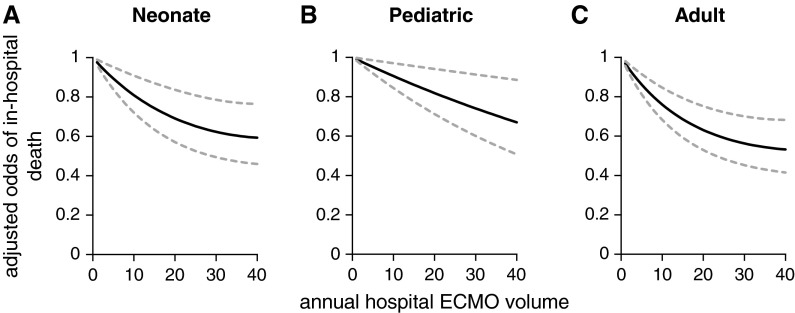
With volume analyzed as a continuous variable, higher age group–specific hospital ECMO volume was associated with progressively lower adjusted odds of in-hospital mortality in the neonatal, pediatric, and adult age groups for 1989–2013 (Figure 2). The significant volume–outcome relationship for neonates and adults receiving ECMO support was also evident when age group–specific volume was analyzed in categories (Table 3). For the pediatric age group, higher hospital volume was not associated with a statistically significant reduction in the adjusted odds of mortality (P = 0.07).
Figure 2.
Adjusted odds of in-hospital mortality among patients receiving ECMO support when volume is modeled continuously, 1989–2013. Hospital ECMO volume is defined as the age group–specific number of patients treated with ECMO per year. The adjusted odds of death are presented relative to the lowest-volume hospitals in each age group. The dashed lines represent the upper and lower bounds of the 95% confidence intervals for the estimated volume-specific point estimates. When volume is modeled as a continuous variable the P values are as follows: (A) neonatal, P < 0.001; (B) pediatric, P = 0.006; and (C) adult, P < 0.001. ECMO = extracorporeal membrane oxygenation.
Table 3.
Adjusted Odds of In-Hospital Mortality by Age Group and Annual ECMO Volume Category
| Adjusted Mortality Odds Ratio (95% CI) |
||||
|---|---|---|---|---|
| Period | Annual Hospital ECMO Volume | Neonate | Pediatric | Adult |
| 1989–2013 | 1–5 | Referent | Referent | Referent |
| 6–14 | 0.86 (0.75–0.98) | 0.99 (0.86–1.13) | 0.81 (0.66–0.995) | |
| 15–30 | 0.74 (0.63–0.88) | 0.86 (0.73–1.01) | 0.75 (0.59–0.94) | |
| >30 | 0.69 (0.56–0.84) | 0.89 (0.69–1.14) | 0.61 (0.48–0.79) | |
| 2008–2013 | 1–5 | Referent | Referent | Referent |
| 6–14 | 1.01 (0.79–1.28) | 1.03 (0.84–1.25) | 0.82 (0.64–1.05) | |
| 15–30 | 0.94 (0.70–1.25) | 0.92 (0.73–1.16) | 0.72 (0.55–0.96) | |
| >30 | 0.65 (0.42–1.01) | 0.85 (0.57–1.28) | 0.61 (0.46–0.80) | |
Definition of abbreviations: CI = confidence interval; ECMO = extracorporeal membrane oxygenation.
The adjusted odds ratio reflects findings from models that included hospital- and patient-level demographic and pre-ECMO clinical variables (see Methods); all analyses were performed with a hierarchical logistic regression model to account for patient-level and hospital-level variance.
In sensitivity analyses stratified by ECMO mode (venovenous and venoarterial) as well as by ECMO support type (respiratory and cardiac), the findings were not substantively different from the primary analysis (see Table E6).
Hospital ECMO Volume and Mortality: 2008–2013
In subgroup analyses of data from 2008 to 2013, ECMO volume was once again analyzed as a continuous and categorical variable. In both analyses, there was a significant association between higher age group–specific annual hospital ECMO volume and lower adjusted odds mortality for adults (Table 3; see Figure E1). In the neonatal and pediatric patients, however, these analyses did not demonstrate a statistically significant association between center volume and mortality rate.
In neonates and pediatrics, the stratified sensitivity analyses were not substantively different from the primary analyses (see Table E7). In adults, when we limited the analysis to those patients receiving venovenous ECMO, there was no statistically significant association between center ECMO volume and patient survival (see Table E7). Similarly, when we limited the analysis to those adult patients receiving respiratory ECMO support, there was no statistically significant association between center ECMO volume and patient survival. Among adult patients receiving venoarterial ECMO or cardiac ECMO support, there was a statistically significant association between higher hospital ECMO volume and lower ECMO mortality rate (see Table E7).
Discussion
This multilevel analysis of the international ELSO Registry (1989–2013) is the first known study to find an association between higher annual age group–specific hospital ECMO volume and lower case-mix–adjusted mortality for neonates and adult patients supported with ECMO. These findings are similar to, and expand on, results described in previous studies of ECMO for children in the United States (15, 16). When considering more recent data during a period of rapid expansion and innovation in available ECMO technology and expansion of ECMO provision for adults (2008–2013), the volume–outcome relationship persists in the analysis of adults but not in the neonatal and pediatric populations.
Our findings for pediatric ECMO diverge somewhat from other analyses, which found a statistically significant difference in neonatal and pediatric ECMO mortality for higher- versus lower-volume hospitals when considering cases from 2004 to 2011 (16). Our study may have yielded distinct findings because of differences in our analytic approach. We used a hierarchical logistic regression, which is the recommended model to test for volume–outcome relationships because it permits investigators to properly account for nonindependence of observations within hospitals in any given year and within hospitals over time (21, 33). On finding that our results differed from those published previously, we applied the same single-level (nonhierarchical) logistic regression model used in those studies (15, 16). Our single-level logistic regression analysis found a statistically significant difference between neonatal and pediatric ECMO mortality at higher- versus lower-volume hospitals. This reconciliation of the findings from different datasets illustrates the importance of methodologic considerations in analyses of data across multiple institutions and over multiple years. Based on recommendations in the literature, we believe that our hierarchical approach is more robust than a single-level model and also yields more conservative findings.
In the 1989–2013 period, stratified sensitivity analyses that limited the study population by ECMO cannulation mode and then ECMO support type were not substantively different from the primary analysis. In the 2008–2013 period, the sensitivity analysis diverged from the primary findings for adults. When we limited our analysis to adult patients receiving cardiac ECMO support, there was a statistically significant association between higher age group–specific hospital ECMO volume and lower mortality. When the analysis was limited to venovenous-respiratory ECMO support, there was no association between volume and outcome. This result is similar to the findings of a pediatric study on ECMO hospital volume and patient mortality (15), which suggested that the volume–outcome association was restricted to patients requiring cardiac ECMO.
Study Limitations
Registry-based studies have inherent limitations specific to the chosen registry. We designed this study to mitigate such limitations. The ELSO Registry does not contain a severity of illness score, but contains component data included in existing severity of illness measures, such as blood pH, PaCO2, and PF ratio (25). The registry also documents age and secondary diagnoses, such as acute renal failure and comorbid conditions, which are part of severity of illness measures (34–36).
These variables were included in the model to adjust for the case-mix at each hospital, but it is likely that there is some further case-mix variance unexplained by the available variables in the ELSO dataset. We adjusted for the presence or absence of specific comorbidities, but we acknowledge that using a comorbidity score may have improved the case-mix adjustment. It is possible that the ability to demonstrate an independent relationship between volume and outcome for patients who receive ECMO might be affected by residual confounding related to inadequate case-mix adjustment.
Another limitation in the ELSO Registry relates to missing data. There are complete data for the outcome variable of death and covariates of age, ECMO support type, hospital, and hospital volume, but the duration of mechanical ventilation preceding ECMO, blood pH, PF ratio, PaCO2, gestational age, and birth weight all had some missing data. The size of the database and our use of inverse probability weighting helped us to mitigate potential bias related to missing data. Nevertheless, it is possible that missing data might have biased the study findings.
Another potential limitation is that hospitals participating in the ELSO Registry are not a random sample of all hospitals performing ECMO. Members of ELSO voluntarily submit ECMO patient data to benchmark their hospital outcomes to peer institutions. Previous research using the Society of Thoracic Surgeons database suggested that such participant hospitals may be more likely to improve quality and outcomes, and therefore they may be systematically different from nonparticipant hospitals (37).
In this international database, we did not have access to the nation where a patient received ECMO support. This decision was made by ELSO to protect the identity of centers operating in nations with a limited number of ECMO centers. Consequently, we are unable to distinguish between U.S. hospitals and non-U.S. hospitals. However, we have no reason to suspect a priori that ECMO volume–outcome relationship would vary by state or national healthcare system.
Moreover, the database does not include measures of organizational structure, processes of care, or referral patterns that may be drivers of a volume–outcome relationship. The absence of these variables limits our ability to test a mechanism for a volume–outcome relationship.
Implications: Volume–Outcome Considerations for ECMO
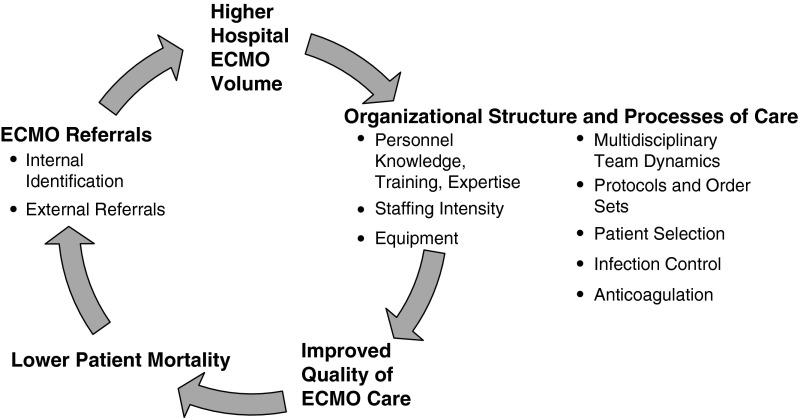
The volume–outcome relationship is hypothesized to be driven by one of two mechanisms: “selective referral” or “practice makes perfect” (38). Hospital ECMO volume may increase through selective referral to hospitals reputed to have favorable ECMO outcomes (1, 39). In “practice makes perfect,” increased experience improves performance and thereby outcomes. In ECMO, a multidisciplinary team provides care, so the entire team must evolve for this process to occur. We believed that both processes were at play in our hypothesized ECMO volume–outcome relationship (Figure 3).
Figure 3.
Conceptual model linking annual age group–specific hospital ECMO volume and outcome. ECMO = extracorporeal membrane oxygenation.
We hypothesized that increased volume would provide the experience and incentive to develop organizational structure and processes of care that would lead to improved quality (Figure 3). We believe that, without sufficient ECMO volume, some centers may not be able to justify the financial investment in training, staffing, and technology.
In 2008–2013, the volume–outcome relationship was absent for neonates and pediatric patients suggesting that ECMO volume was no longer a surrogate marker of ECMO quality. This could occur for three reasons. First, as the number of hospitals offering ECMO grew, referral patterns may have been be disrupted and referral may be more linked to patient proximity than center quality. Second, programs with best practice protocols may now have lower within-institution populations eligible for ECMO because they are able to avert ECMO by applying evidence-based approaches, such as inhaled nitric oxide (40), low tidal volume ventilation (41), open lung ventilation (42), and ventricular-assist devices (5). Third, ECMO circuits are now considered to be simpler and safer, require less anticoagulation, and are associated with fewer bleeding complications (32).
Despite improvements in care, variation in outcomes was constant across the 1989–2013 and 2008–2013 time periods across ECMO centers. Variations in practice likely underlie variations in outcomes. To date, there are no evidence-based protocols for patient selection or ECMO management. Without guiding evidence, there is variation in the case-mix (23, 25, 43), primary diagnosis (23, 25, 43), modes of cannulation (10), equipment used (44), and anticoagulation for patients receiving ECMO (45). Consequently, future research must focus on what enables one hospital to achieve better outcomes.
The existence of a volume–outcome relationship should inform policy, in conjunction with consensus opinions, such as described by Combes and colleagues (13). In making the decision to expand the capacity of existing centers versus developing ECMO capacity at new centers, we believe the potential benefits of treatment at a higher-volume center must be balanced with potential risks of transporting critically ill patients. If a current high-performing ECMO center meets community needs, then the volume–outcome association we found would support continuing to depend on the established center instead of starting ECMO at a new location.
Overall, on the basis of findings from 290 centers performing ECMO over the last 25 years, we would not recommend an absolute volume threshold to maintain a center. Volume is generally considered a surrogate marker for quality. Therefore, if a center has low ECMO volume, then we would suggest using a more proximate measure of quality, such as the risk-adjusted survival rate. An additional concern is that minimum volume thresholds may introduce a perverse incentive for centers to provide ECMO support to patients whose clinical circumstances may be marginally indicative for ECMO. When inappropriately or unnecessarily administered, ECMO support exposes patients to significant morbidity (13) and increases the costs of care.
Conclusions
In this international assessment of ECMO volume and patient mortality, we found strong associations of higher hospital-level ECMO volume and lower mortality for neonates and adults, but not for children. In the more recent era of ECMO care during 2008–2013, we found persistent volume–outcome associations for adults but not for neonates or children.
Acknowledgments
Acknowledgment
The authors thank the Extracorporeal Life Support Organization for the opportunity to conduct this research. They also thank Robert Gajarski Jr., M.D. (Division of Pediatric Cardiology, University of Michigan, Ann Arbor, MI) for his assistance in encoding the pediatric cardiac diagnoses, and Rachel Chapman, M.D. (Division of Neonatal Medicine, University of Southern California, Los Angeles, CA) for her assistance in encoding the neonatal diagnoses. Their assistance was provided without compensation.
Footnotes
Supported by a grant from the Extracorporeal Life Support Organization and the Charles Woodson Fund for Clinical Research. R.P.B. was supported by a T32 (F025206-064171) grant funded by the Eunice Kennedy Shriver National Institute for Child Health and Human Development, for which M.M.D. is the principal investigator.
Author Contributions: R.P.B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design, R.P.B., F.O.O., K.M.K., M.L.P., R.H.B., M.M.D., and G.M.A. Acquisition, analysis, or interpretation of data, R.P.B., F.O.O., K.M.K., M.L.P., R.H.B., M.M.D., and G.M.A. Drafting of the manuscript, R.P.B. Critical revision of the manuscript for important intellectual content, F.O.O., K.M.K., M.L.P., R.H.B., M.M.D., and G.M.A. Statistical analysis, K.M.K. and R.P.B. Obtained funding, R.P.B., F.O.O., G.M.A., and M.M.D. Administrative, technical, or material support, R.P.B. Study supervision, R.P.B.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201409-1634OC on February 19, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 2.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 3.UK Collaborative ECMO Trail Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [Google Scholar]
- 5.Fraser CD, Jr, Jaquiss RD, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, Naftel DC, Ichord RN, Bomgaars L, Tweddell JS, et al. Berlin Heart Study Investigators. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367:532–541. doi: 10.1056/NEJMoa1014164. [DOI] [PubMed] [Google Scholar]
- 6.Shekar K, Mullany DV, Thomson B, Ziegenfuss M, Platts DG, Fraser JF. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care. 2014;18:219. doi: 10.1186/cc13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 8.Checkley W. Extracorporeal membrane oxygenation as a first-line treatment strategy for ARDS: is the evidence sufficiently strong? JAMA. 2011;306:1703–1704. doi: 10.1001/jama.2011.1504. [DOI] [PubMed] [Google Scholar]
- 9.Hoglund P, Nilsson LA, Rehnqvist N.CPR with assisted extracorporeal life support Lancet 20083721878–1879.author reply 1879–1880 [DOI] [PubMed] [Google Scholar]
- 10.Paden ML, Rycus PT, Thiagarajan RR ELSO Registry. Update and outcomes in extracorporeal life support. Semin Perinatol. 2014;38:65–70. doi: 10.1053/j.semperi.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation (ECMO) use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO. 2015;61:31–36. doi: 10.1097/MAT.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 12.Lyman S, Sedrakyan A, Do H, Razzano R, Mushlin AI. Infrequent physician use of implantable cardioverter-defibrillators risks patient safety. Heart. 2011;97:1655–1660. doi: 10.1136/hrt.2011.226282. [DOI] [PubMed] [Google Scholar]
- 13.Combes A, Brodie D, Bartlett R, Brochard L, Brower R, Conrad S, De Backer D, Fan E, Ferguson N, Fortenberry J, et al. International ECMO Network (ECMONet) Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 14.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 15.Karamlou T, Vafaeezadeh M, Parrish AM, Cohen GA, Welke KF, Permut L, McMullan DM. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145:470–475. doi: 10.1016/j.jtcvs.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Freeman CL, Bennett TD, Casper TC, Larsen GY, Hubbard A, Wilkes J, Bratton SL. Pediatric and neonatal extracorporeal membrane oxygenation: does center volume impact mortality? Crit Care Med. 2014;42:512–519. doi: 10.1097/01.ccm.0000435674.83682.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbaro R, Odetola F, Kidwell K, Paden ML, Bartlett R, Davis M, Annich GM. Association between hospital extracorporeal membrane oxygenation (ECMO) volume and mortality [abstract]; Presented at the European Extracorporeal Life Support Organization. May 22–24, 2014, Paris, France. [Google Scholar]
- 18.Barbaro R, Odetola F, Kidwell K, Bartlett R, Annich GM, Davis M. Mortality association between hospital-level annual patient volume and respiratory extracorporeal membrane oxygenation cases [abstract]; Presented at the Pediatric Academic Societies Annual Meeting. April 25–28, 2014. Vancouver, British Columbia, Canada. [Google Scholar]
- 19.Barbaro R, Odetola F, Kidwell K, Paden ML, Bartlett R, Davis M, Annich GM. Association of mortality with hospital-level extracorporeal membrane oxygenation patient volume: abstracts of the 43rd Critical Care Congress. January 9–13, 2013. San Francisco, California, USA [abstract] Crit Care Med. 2013;41:A7. [Google Scholar]
- 20.Dalton HJ, Butt WW. Extracorporeal life support: an update of Rogers’ Textbook of Pediatric Intensive Care. Pediatr Crit Care Med. 2012;13:461–471. doi: 10.1097/PCC.0b013e318253ca17. [DOI] [PubMed] [Google Scholar]
- 21.Livingston EH, Cao J. Procedure volume as a predictor of surgical outcomes. JAMA. 2010;304:95–97. doi: 10.1001/jama.2010.905. [DOI] [PubMed] [Google Scholar]
- 22.Extracorporeal Life Support Organization (ELSO) Guidelines for ECMO CentersVersion 1.7. 2010. Feb [accessed 2012 Aug 23]. Available from: http://www.elsonet.org/resources/guidelines
- 23.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 24.Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J, Pandya H, Firmin R, Liddell M, Davis C, et al. Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK. Arch Dis Child Fetal Neonatal Ed. 2009;94:F129–F132. doi: 10.1136/adc.2008.141051. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, Pellegrino V, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure: the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 26.Kumar TK, Zurakowski D, Dalton H, Talwar S, Allard-Picou A, Duebener LF, Sinha P, Moulick A.Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome J Thorac Cardiovasc Surg 2010140330–336.e2 [DOI] [PubMed] [Google Scholar]
- 27.Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, Pavie A, Chastre J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36:1404–1411. doi: 10.1097/CCM.0b013e31816f7cf7. [DOI] [PubMed] [Google Scholar]
- 28.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, Mohr FW.Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock J Thorac Cardiovasc Surg 2010139302–311.e1 [DOI] [PubMed] [Google Scholar]
- 29.Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, Georgeson K, Barnhart DC, Dimmitt RA. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:e1–e6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin ED, Gauvreau K, Scheurer MA, Rycus PT, Salvin JW, Almodovar MC, Fynn-Thompson F, Thiagarajan RR. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2012;144:1337–1343. doi: 10.1016/j.jtcvs.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 32.Combes A, Bacchetta M, Brodie D, Müller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18:99–104. doi: 10.1097/MCC.0b013e32834ef412. [DOI] [PubMed] [Google Scholar]
- 33.Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139:658–665. doi: 10.7326/0003-4819-139-8-200310210-00009. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 35.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III—Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 36.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 37.Grover FL, Shroyer AL, Hammermeister K, Edwards FH, Ferguson TB, Jr, Dziuban SW, Jr, Cleveland JC, Jr, Clark RE, McDonald G. A decade's experience with quality improvement in cardiac surgery using the Veterans Affairs and Society of Thoracic Surgeons national databases. Ann Surg. 2001;234:464–472. doi: 10.1097/00000658-200110000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allareddy V, Ward MM, Wehby GL, Konety BR. The connection between selective referrals for radical cystectomy and radical prostatectomy and volume-outcome effects: an instrumental variables analysis. Am J Med Qual. 2012;27:434–440. doi: 10.1177/1062860611423728. [DOI] [PubMed] [Google Scholar]
- 39.Bryner B, Cooley E, Copenhaver W, Brierley K, Teman N, Landis D, Rycus P, Hemmila M, Napolitano LM, Haft J, et al. Two decades’ experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg. 2014;98:1363–1370. doi: 10.1016/j.athoracsur.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 40.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP Clinical Inhaled Nitric Oxide Research Group. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 41.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 42.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 43.Mascio CE, Austin EH, 3rd, Jacobs JP, Jacobs ML, Wallace AS, He X, Pasquali SK.Perioperative mechanical circulatory support in children: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database J Thorac Cardiovasc Surg 2014147658–664.discussion 664–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawson S, Ellis C, Butler K, McRobb C, Mejak B. Neonatal extracorporeal membrane oxygenation devices, techniques and team roles: 2011 survey results of the United States’ Extracorporeal Life Support Organization centers. J Extra Corpor Technol. 2011;43:236–244. [PMC free article] [PubMed] [Google Scholar]
- 45.Annich G, Adachi I. Anticoagulation for pediatric mechanical circulatory support. Pediatr Crit Care Med. 2013;14(5) Suppl 1:S37–S42. doi: 10.1097/PCC.0b013e318292dfa7. [DOI] [PubMed] [Google Scholar]
- 46.Agency for Healthcare Research and Quality (AHRQ) quality indicators. The pediatric quality indicators (PDI) risk adjustment coefficients for the PDI [version 4.4]. Rockville, MD: Agency for Healthcare Research and Quality; 2012 [accessed 2015 Mar 18]. pp. 65–68. Available from: http://www.qualityindicators.ahrq.gov/Downloads/Modules/PDI/V44/Risk_Adjustment_Tables_PDI_4.4.pdf
- 47.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106:205–209. [PubMed] [Google Scholar]
- 48.Johnston JA, Wagner DP, Timmons S, Welsh D, Tsevat J, Render ML. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care. 2002;40:929–940. doi: 10.1097/00005650-200210000-00010. [DOI] [PubMed] [Google Scholar]