Abstract
Neural stem cells (NSCs) can self-renew and differentiate into neurons and glia. Transplanted NSCs can replace lost neurons and glia after spinal cord injury (SCI), and can form functional relays to re-connect spinal cord segments above and below a lesion. Previous studies grafting neural stem cells have been limited by incomplete graft survival within the spinal cord lesion cavity. Further, tracking of graft cell survival, differentiation, and process extension had not been optimized. Finally, in previous studies, cultured rat NSCs were typically reported to differentiate into glia when grafted to the injured spinal cord, rather than neurons, unless fate was driven to a specific cell type. To address these issues, we developed new methods to improve the survival, integration and differentiation of NSCs to sites of even severe SCI. NSCs were freshly isolated from embryonic day 14 spinal cord (E14) from a stable transgenic Fischer 344 rat line expressing green fluorescent protein (GFP) and were embedded into a fibrin matrix containing growth factors; this formulation aimed to retain grafted cells in the lesion cavity and support cell survival. NSCs in the fibrin/growth factor cocktail were implanted two weeks after thoracic level-3 (T3) complete spinal cord transections, thereby avoiding peak periods of inflammation. Resulting grafts completely filled the lesion cavity and differentiated into both neurons, which extended axons into the host spinal cord over remarkably long distances, and glia. Grafts of cultured human NSCs expressing GFP resulted in similar findings. Thus, methods are defined for improving neural stem cell grafting, survival and analysis of in vivo findings.
Keywords: Neuroscience, Issue 89, nervous system diseases, wounds and injuries, biological factors, therapeutics, surgical procedures, neural stem cells, transplantation, spinal cord injury, fibrin, growth factors
Introduction
Spinal cord injury (SCI) often damages not only white matter tracts that carry signals to and from the brain, but also the central gray matter, causing segmental loss of interneurons and motor neurons. The consequence of SCI is loss of both motor and sensory function below the lesion. Unfortunately, the adult central nervous system (CNS) does not spontaneously regenerate, resulting in permanent functional deficits1. Therefore, reconstruction of the injured adult spinal cord and improvement in motor, sensory and autonomic function is an important goal of SCI research. Neural stem cells (NSCs), whether directly isolated from embryonic or adult CNS, are compelling candidate cells to replace lost neurons and glia. Moreover, these cells have the potential to form new functional relays to restore axonal conduction across a lesion site2,3.
To date, there has not been a detailed elucidation of the anatomical, electrophysiological and behavioral effects of neuronal relay formation by transplanted NSCs after severe SCI. There are several reasons for this: first, transplanted NSCs or fetal CNS tissue survive poorly when grafted into large lesion cavities. Previous studies show substantial early cell loss, leaving large empty cystic lesion cavities4,5. In some studies the grafted cells would subsequently divide and fill the lesion cavity4,5, but this might occur after a delay of days to weeks, and subsequent lesion site filling might not be complete or consistent. Second, an efficient tracking system that provides sound data on cellular survival, differentiation/maturation, and outgrowth of transplanted NSCs was lacking. Most early studies utilized antegrade and retrograde labeling to trace axonal projections from transplants2,3. However, these techniques only partially and often unclearly labeled axonal projections arising from grafted cells, and tracer methods are subject to artifacts caused by dye leakage beyond the implanted cells. Other groups used human specific neuronal markers to label axonal projections after transplantation of human fetal NSCs into injured rodent spinal cord5,6. However, in those studies, the xenografts did not consistently survive well. Recently, viral delivery of the GFP reporter gene was used to label cultured NSCs7,8. However, GFP expression was often inconsistent and can be down-regulated7. Recently, the use of transgenic donor mice or rats stably expressing the reporter gene, GFP, or the human placental alkaline phosphatase, has dramatically improved the tracking of transplanted neural stem cells/progenitors in vivo9,11. Third, several studies indicate that in vitro cultured rat NSCs derived from either embryonic or adult CNS exclusively differentiate into glial lineages when transplanted into the milieu of the intact or injured adult spinal cord7,12,13, despite the fact that these neural stem cells are capable of differentiating into both neurons and glia in vitro, indicating that local environments may dictate the fate of stem cells. Alternatively, cultured NSCs, especially those derived from adult CNS, may have intrinsic defaulty property to differentiate into glial lineages in vivo13.
Because of the limitations discussed above, our group recently developed a new protocol to improve embryonic NSC tracking, survival, and differentiation/maturation in the severely injured adult spinal cord. Briefly, we began with a stable transgenic Fischer 344 rat inbreed line expressing a GFP reporter gene that sustains GFP expression after in vivo transplantation14. Next, we used freshly isolated NSCs from embryonic day 14 Fischer 344 spinal cord, a stage of development that retains the potential to generate both neurons and glia. Finally, we embedded freshly dissociated NSCs into a fibrin matrix containing growth factors15-17 to retain the cells and evenly distribute them within a large lesion cavity, aiming to support graft cell survival, differentiation and integration. Grafts were placed into sites of T3 complete transection, two weeks after spinal cord injury. These grafted cells consistently filled complete transection sites and differentiated into abundant neurons that extended large numbers of axons into host spinal cord over long distances18. Similar results were obtained using cultured human neural stem cell grafts to immuno-deficient rats18.
Protocol
All animal protocols are approved by VA-San Diego Institutional Animal Care and Use Committee (IACUC). NIH guidelines for laboratory animal care and safety are strictly followed. Animals have free access to food and water throughout the study and are adequately treated for minimizing pain and discomfort.
1. Preparation of Fibrin Components Containing Growth Factor Cocktails
Dissolve 25 mg rat fibrinogen in 0.5 ml PBS to obtain 50 mg/ml 2x stock solution (see Materials table). Fibrinogen is difficult to dissolve and should be placed into a 37 °C incubator or waterbath and shaken every 5-10 min for 1-2 hr.
Dissolve 100 UN rat thrombin in 2 ml of 10 mM sterile CaCl2 to obtain 50 U/ml 2x stock solution.
Dissolve growth factors in PBS at the following concentrations to obtain 100 μl stock solution: 1 mg/ml: BDNF; NT-3 (high stock concentration as intrathecal infusion in vivo19); 200 μg/ml: GDNF; IGF; bFGF; EGF; PDGF; aFGF; HGF (about 1,000x higher than used for cell culture).
Dissolve 25 mg MDL28170 (calpain inhibitor) in 1.3 ml DMSO to obtain 50 mM DMSO stock solution, and then dilute 50x in PBS to obtain 1 mM stock solution.
Mix 100 μl of each growth factor component and 100 μl of MDL28170 (1 mM stock solution) to produce 1,000 μl growth factor cocktail solution (Figure 1).
Mix 500 μl fibrinogen 2x stock solution with 500 μl growth factor cocktail to obtain 25 mg/ml fibrinogen working solution containing growth factors and aliquot into 10 or 20 μl for storage at -70 °C. The solution is stable for up to 12 months at -70 °C.
Similarly, mix 500 μl thrombin 2x stock solution with 500 μl growth factor cocktail to obtain 25 U/ml thrombin working solution containing growth factors and aliquot into similar 10 or 20 μl volumes for storage at -70 °C for up to 12 months.
On the day of grafting, place fibrinogen- and thrombin-containing growth factor cocktails on ice until mixed with cells.
2. T3 Spinal Cord Transection
Anesthetize adult female Fischer 344 rats or athymic nude rats using acceptable methods. Subjects are deeply anesthetized, when responsiveness to tail and paw pinch are completely absent. Note: We typically use 150-200 g Fischer rats or 180-220 g athymic rats, and an anesthesia cocktail (2 ml/kg) of ketamine (25 mg/ml), xylazine (1.3 mg/ml) and acepromazine (0.25 mg/ml).
Apply eye ointment to prevent dryness or injury of eyes while the animals are under anesthesia.
Shave the upper thoracic area and clean the skin with Betadine.
Cut the skin and muscle using #15 blade, expose T3 vertebra using a surgical retractor, and perform a laminectomy at T3 vertebra to expose T3/4 spinal cord using a rongeur.
Make a longitudinal midline incision in the dura of approximately 2 mm length using a #11 blade.
Place iridectomy scissors underneath the dura to cut the right lateral entire spinal cord. Make another cut on the same side 1.5 mm more cadually.
Aspirate spinal cord tissue between the two cut segments with a blunt 23 G needle connected to moderate intensity vacuum. Use visual verification to ensure complete transection ventrally and laterally. This will complete a lateral hemisection.
To extend the lesion to a full-width spinal cord transection, place mirror incisions into the left hemicord. Attempt to leave the dura intact, other than the first cut, to retain the firm graft/fibrin matrix in the lesion site when grafted two weeks later.
Suture the muscle, apply antibiotic powder and staple the skin.
Inject lactated Ringer's (3 ml), Bannamine (2.5-5 mg/kg) and Ampicillin (80-100 mg/kg) (see Materials table) immediately after surgery and maintain rats in a warm incubator until thermoregulation is re-established.
Empty the bladder and inject the Ringer's and Ampicillin solution twice a day for about two weeks until the establishment of reflex bladder emptying (Figure 1B).
3. Preparation of Freshly Dissociated Embryonic Day 14 Spinal Cord Neural Stem Cells
Obtain inbreed transgenic GFP F344 rats (F344-Tg(UBC-EGFP)F455Rrrc) from Rat Resource and Research Center, University of Missouri.
Inject 40 μg des-Gly10, [D-Ala6]-Leuteinizing Hormone Releasing Hormone Ethylamide (LHRH) diluted in PBS at a concentration of 200 μg/ml intraperitoneally (IP) per mature female rat (8 weeks or older) after 2-3 hr light induction for synchronization of embryo collection.
Mate with a mature GFP homozygous or heterozygous male rat overnight 4 days after injection.
Collect embryos at days 13.5-14.5 postcoitus (p.c.) in cold HBSS buffer, examine GFP expression under a "NightSea" flashlight and filter glasses (BLS1 - BlueStar Flashlight and Model VG1 barrier filter) or a fluorescent microscope.
Dissect spinal cord out aseptically from each GFP-positive fetus and remove overlying meninges and attached spinal ganglia with iridectomy scissors and fine jewelers forceps.
Collect the dissected spinal cords into 2 ml cold HBSS buffer in a 15 ml cornical tube and keep on ice.
- Follow reference #20 to dissociate dissected spinal cords:
- Briefly, add 2 ml of 0.25% Trypsin-EDTA to the tube containing dissected spinal cords (several cords can be digested together, 1 cord can generate enough cells for graft of one subject) and digest at 37 °C for 10-12 min.
- Stop the trypsin reaction by adding 10 ml of DMEM containing 10% FBS to the tube and centrifuge the tissue at 2,500 rpm for 2 min.
- Resuspend tissue in 2 ml NeuroBasal medium containing 2% B27, and triturate the spinal cord tissue using progressively smaller fire-polished Pasteur pipets.
- Centrifuge the cells at 2,500 rpm for 2 min, resuspend in 2 ml of NeuroBasal medium containing B27, and filter cells with a 40 μm cell filter strainer.
Count the cells with a hemocytometer and divide the cells equally into two Eppendorf tubes.
Centrifuge the cells and completely remove supernatant (1-2 min are required to withdraw all supernatant with a low vacuum tip) so the growth factor cocktails will not be diluted by remaining supernatant after cell re-suspension.
Resuspend each half of the cells in the fibrinogen or thrombin working solution containing growth factors, respectively, at a concentration of 250,000 cells/μl (cell pellet counts for about ⅓ final volume) and place them on ice prior transplantation (Figure 1A). Note that fibrinogen may spontaneously gel when mixed with cells before combining with thrombin at the lesion/graft site; to avoid premature gellation, mix cells in fibrinogen immediately before cell grafting in vivo.
4. Transplantation
Reexpose the lesioned T3/4 spinal cord two weeks after spinal cord transection.
5 μl fibrinogen-cells and 5 μl thrombin-cells could be directly injected into nine points of the lesion site through sealed scar tissue and intact dura without re-opening the lesion site.
Use a 27 G needle to create 9 holes on the surface of the scar tissue and intact dura 1-2 weeks post lesion: 3 in center (one in middle point and two in laterals, and spaced 0.5 mm apart) and 3 in both rostral and 3 in caudal interface (about 0.5 mm rostral or caudal to the center of the lesion).
Then inject half volume of fibrinogen cell solution into three central points of the lesion site and a quarter volume into 3 points of the rostral interface and a quarter volume into 3 points of the caudal interface.
Then, immediately inject throbmbin cells into the same spots. The mixture of fibrinogen cells and thrombin cells inside the lesion site will form fibrin gel to hold cells into lesion site and the growth factor cocktails will support the survival of cells. Cells are injected using pulled glass pipettes connected to a PicoSpritzer (General Valve, Inc.).
Transplant cultured human NSCs21 in the same procedure described above, and use fibrin and growth factor cocktails made from human reagents.
Representative Results
GFP immunohistological labeling demonstrates that grafted rat NSCs resuspended in phosphate buffered saline (PBS) (lacking a fibrin matrix and growth factors) survived poorly in the T3 transection site, only attaching to the lesion/host margin and leaving most of the lesion site empty without grafted cells (Figure 2A). The survival of NSCs improved when co-grafted with fibrin matrices alone, but the graft did not completely fill up a large lesion cavity (data not shown). Therefore, we embedded NSCs into fibrin matrices containing growth factor cocktails to enhance their survival, filling of large lesion cavities and differentiation into neurons and glia after spinal cord transection15-17. Grafts of either rat or human NSCs consistently and completely fill the lesion cavity when assessed seven weeks post-grafting18 (Figures 2B-C).
We performed a time course study to examine early survival and filling of the lesion cavity after grafting of NSCs embedded in fibrin matrices with growth factor cocktails. Histological analysis revealed that GFP expressing NSCs survived well 24 hr after transplantation (Figures 3A-B). The grafted NSCs gradually became more dense over time to completely fill the transected lesion cavity by the first week after transplantation probably due to proliferation21 (Figures 3C-H). In addition, grafted NSCs integrated well at the host/lesion interface and appeared to reduce regions of GFAP immunoreactivity at the margins of the lesion cavity (Figure 3).
A portion of grafted NSCs differentiated into NeuN-expressing neurons seven weeks after transplantation (Figures 4A-B). In addition, graft-derived spinal cord neurons extended large numbers of axons into the host spinal cord in both rostral and caudal directions over long distances18 (Figure 4C). Grafted derived axons extended rostrally and caudally through both white matter and gray matter in the host spinal cord (Figures 4D-E).
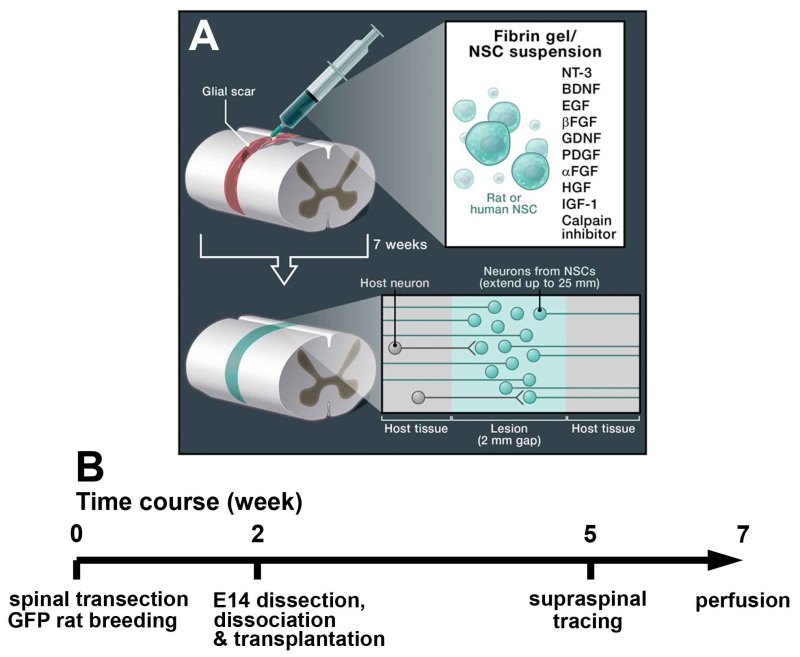 Figure 1. Experimental Schematic and Time Line. A) Schematic drawing adapted from Popovich22 (Cell, 2012) illustrates transplantation of neural stem cells with fibrin gel containing growth factor cocktail into completely transected spinal cord. B) Experimental time line. Behavioral and electriophysiological analyses can be performed after cell grafting and before perfusion. The survival time of subjects can be variable. Click here to view larger figure.
Figure 1. Experimental Schematic and Time Line. A) Schematic drawing adapted from Popovich22 (Cell, 2012) illustrates transplantation of neural stem cells with fibrin gel containing growth factor cocktail into completely transected spinal cord. B) Experimental time line. Behavioral and electriophysiological analyses can be performed after cell grafting and before perfusion. The survival time of subjects can be variable. Click here to view larger figure.
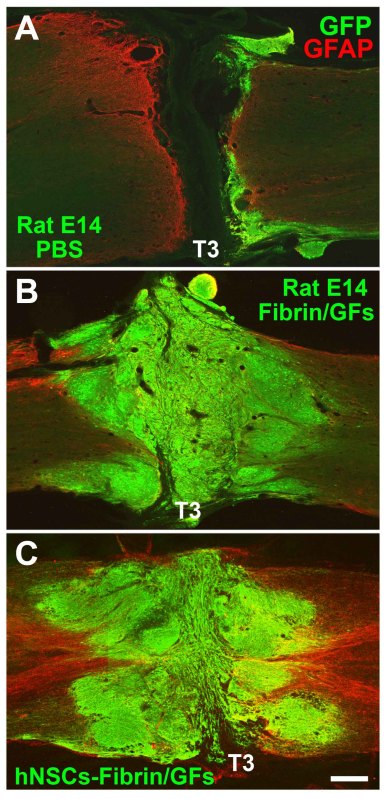 Figure 2. Survival and Filling of Neural Stem Cell Grafts in T3 Complete Transection Sites. GFP and GFAP fluorescent immunolabeling in horizontal sections demonstrates (A) poor survival of rat E14 NSCs (green) re-suspended in PBS lacking growth factors and fibrin matrix; B-C) Excellent survival and complete filling of lesion cavity when (B) rat or (C) human NSCs were embedded in fibrin matrices containing growth factor cocktails. Cell were grafted into T3 transection sites for 7 weeks. Scale bar, 310 μm. Click here to view larger figure.
Figure 2. Survival and Filling of Neural Stem Cell Grafts in T3 Complete Transection Sites. GFP and GFAP fluorescent immunolabeling in horizontal sections demonstrates (A) poor survival of rat E14 NSCs (green) re-suspended in PBS lacking growth factors and fibrin matrix; B-C) Excellent survival and complete filling of lesion cavity when (B) rat or (C) human NSCs were embedded in fibrin matrices containing growth factor cocktails. Cell were grafted into T3 transection sites for 7 weeks. Scale bar, 310 μm. Click here to view larger figure.
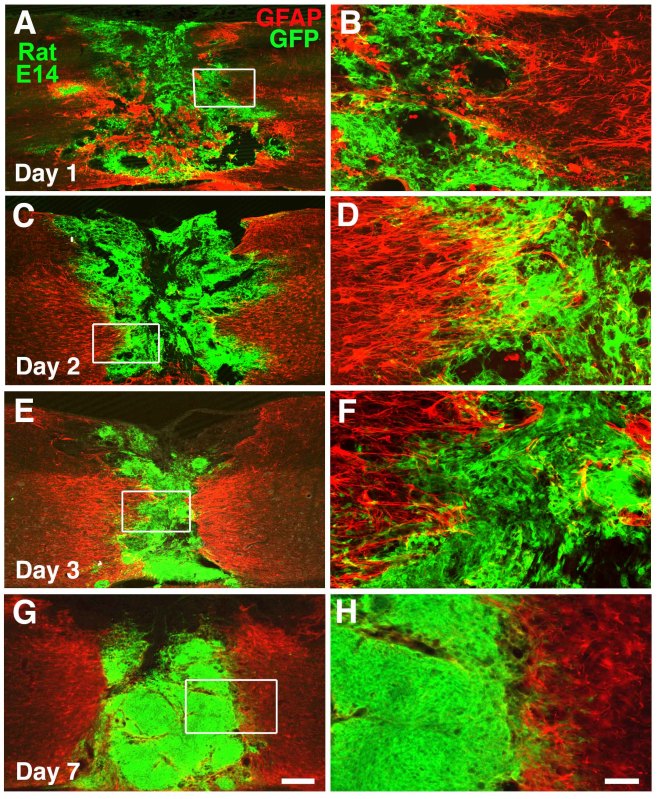 Figure 3. Graft Survival at Early Time Points. A-B) GFP and GFAP double immunolabeling show survival and distribution of grafted rat NSCs in the transection site, 1 day post-implantation in a fibrin matrix. Boxed area in A is shown at higher magnification in panel B. C-D) Cell survival and distribution is also evident at Day 2 and (E-F) Day 3 post-grafting. G-H) By 7 days, cells fill lesion site and are denser. Scale bar: A, C, E, G, 300 μm; B, D, F, H, 62 μm. Click here to view larger figure.
Figure 3. Graft Survival at Early Time Points. A-B) GFP and GFAP double immunolabeling show survival and distribution of grafted rat NSCs in the transection site, 1 day post-implantation in a fibrin matrix. Boxed area in A is shown at higher magnification in panel B. C-D) Cell survival and distribution is also evident at Day 2 and (E-F) Day 3 post-grafting. G-H) By 7 days, cells fill lesion site and are denser. Scale bar: A, C, E, G, 300 μm; B, D, F, H, 62 μm. Click here to view larger figure.
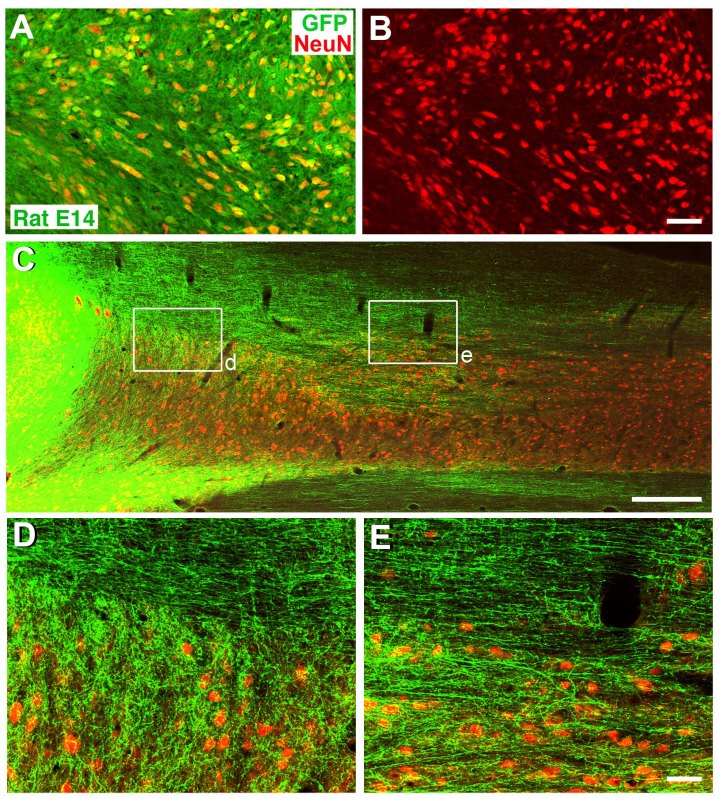 Figure 4. Differentiation and Outgrowth of Neural Stem Cell Grafts in T3 Complete Transection Site. A-B) GFP and NeuN labeling confirm extensive neuronal differentiation/maturation of grafted rat neural stem cells 7 weeks after grafting. C) GFP and NeuN immunolabeling reveals that GFP-expressing neural stem cell grafts extend large numbers of axons into the host spinal cord rostral and caudal to the transection site (caudal shown), two weeks post-grafting. D-E) Higher magnification from boxed areas of panel C show that extensive regions of the host spinal cord contain graft-derived axonal projections in both white matter and gray matter. Scale bar: A-B, 62 μm; C, 700 μm; D-E, 62 μm. Click here to view larger figure.
Figure 4. Differentiation and Outgrowth of Neural Stem Cell Grafts in T3 Complete Transection Site. A-B) GFP and NeuN labeling confirm extensive neuronal differentiation/maturation of grafted rat neural stem cells 7 weeks after grafting. C) GFP and NeuN immunolabeling reveals that GFP-expressing neural stem cell grafts extend large numbers of axons into the host spinal cord rostral and caudal to the transection site (caudal shown), two weeks post-grafting. D-E) Higher magnification from boxed areas of panel C show that extensive regions of the host spinal cord contain graft-derived axonal projections in both white matter and gray matter. Scale bar: A-B, 62 μm; C, 700 μm; D-E, 62 μm. Click here to view larger figure.
Discussion
One of the major hurdles for NSC transplantation in the injured spinal cord is poor survival in the lesion center. Any gaps or cavities in the lesion site could potentially reduce or weaken the formation of functional neuronal relays between supraspinal axons and separated spinal cord segments below the injury. In addition, poor survival of grafted NSCs may affect their integration with host tissue, and therefore reduce connectivity of grafted neurons with host neurons. Potential mechanisms to explain cell loss include the cytotoxic environment created by acute SCI, secondary degeneration of the host spinal cord, and insufficient vascularization of the injured region4.
Fibrin has been used to deliver cells, drugs and growth factors in various experimental models and clinical applications, since it can retain cells and molecules when it becomes polymerized23. A previous study demonstrated that human fibrin sealant can serve as a biomatrix to enhance axonal regeneration in the subtotally transected spinal cord without eliciting detectable adverse effects24. We previously used fibrin matrices to embed bone marrow stromal cells for transplantation into the chronically injured spinal cord17. Grafted cells survived well in the chronic lesion site and integrated well with host spinal cord parenchyma17. Therefore, we extended the use of fibrin to embed NSCs for transplantation into large open lesion sites in the transected spinal cord. Although the survival of grafted NSCs improved with fibrin, the survival of grafted NSCs remained somewhat variable and grafted NSCs seldom completely filled large lesion cavity.
We therefore added a cocktail of growth factors in an effort to enhance survival and proliferation of NSCs in the lesion site. Growth factors have been shown in numerous studies to support NSCs in vitro25. While the addition of growth factors to cultures of NSCs can change their fate, we did not qualitatively detect apparent change in neuronal density when growth factor cocktails added to the graft comparing to those partially survived graft without growth factors. A more detailed analysis is necessary before we can appreciate with certainty the influence of growth factor cocktails on cell fate. During normal neural development, the survival and growth of neurons depend on neurotrophic factors that are often released by target tissue guide axonal growth and, in some cases, influence neuronal survival26.
Similar but simpler growth factor cocktails have been utilized by other groups to enhance survival and differentiation of NSCs both in vitro and in vivo15,16. These efforts have generally resulted in less extensive NSC survival than the multiple factor approach that we have adopted. An essential next step in this development effort is to narrow down the list of growth factors in the cocktail that are essential to support NSC survival, an effort that is underway. It is entirely possible that a more limited number of growth factors will support equal degrees of graft survival, a finding that would simplify this experimental approach and its potential translation to human studies.
In addition to the use of fibrin matrices and growth factor cocktails, the success of grafting in vivo to the severe lesion site may depend on mechanical factors in the lesion site. We make every effort to maintain the integrity of the extremely thin dural membrane that overlies the rodent spinal cord after lesions and grafting, in an effort to mechanically retain grafted cells in the lesion site. Thus, we make careful and diligent efforts to optimize consistent cell filling of the lesion site. Without these multiple efforts, graft filling is incomplete. These techniques require extensive practice.
In attempting to teach this technique to others, we have identified some challenges. If cells are injected into the host spinal cord instead of the lesion cavity, then cells may preferentially differentiate into glia. In this case, the number of emerging axons is greatly reduced. If great care and diligence are not made to fill the lesion cavity using the multiple techniques and approaches described in the preceding paragraphs, cell survival is less consistent and biological responses, including axon outgrowth, are limited. Thus, it is essential to devote considerable time, diligence and meticulous technique to successfully use these methods. Once the techniques are acquired, the results are highly consistent from animal to animal, yielding the ability to study the remarkable biological properties of these NSCs in central nervous system lesion sites.
Disclosures
We have nothing to disclose.
Acknowledgments
We thank the Rat Resource and Research Center, University of Missouri, Columbia, Missouri, for providing GFP rats; Neuralstem Inc. for providing human neural stem cells. This work was supported by the Veterans Administration, NIH (NS09881), Canadian Spinal Research Organization, The Craig H. Neilsen Foundation, and the Bernard and Anne Spitzer Charitable Trust.
References
- Ramon y Cajal S. New York: Hafner: 1991. In Degeneration and regeneration of the nerve system. [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Bamber NI, Li H, Aebischer P, Xu XM. Fetal spinal cord tissue in mini-guidance channels promotes longitudinal axonal growth after grafting into hemisected adult rat spinal cords. Neural Plast. 1999;6:103–121. doi: 10.1155/NP.1999.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Exp Neurol. 1996;142:128–143. doi: 10.1006/exnr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Giovanini MA, Reier PJ, Eskin TA, Wirth E, Anderson DK. Characteristics of human fetal spinal cord grafts in the adult rat spinal cord: influences of lesion and grafting conditions. Exp Neurol. 1997;148:523–543. doi: 10.1006/exnr.1997.6703. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Björklund A. Axon outgrowth from grafts of human embryonic spinal cord in the lesioned adult rat spinal cord. Neuroreport. 1992;3:1045–1048. doi: 10.1097/00001756-199212000-00003. [DOI] [PubMed] [Google Scholar]
- Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- Blits B, et al. Lentiviral vector-mediated transduction of neural progenitor cells before implantation into injured spinal cord and brain to detect their migration, deliver neurotrophic factors and repair tissue. Restor Neurol Neurosci. 2005;23:313–324. [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Gaillard A, et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- Remy S, et al. New lines of GFP transgenic rats relevant for regenerative medicine and gene therapy. Transgenic Res. 2010;19:745–763. doi: 10.1007/s11248-009-9352-2. [DOI] [PubMed] [Google Scholar]
- Cao QL, et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Kulbatski I, Parr A, Mohareb M, Tator CH. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplant. 2008;17:735–751. doi: 10.3727/096368908786516756. [DOI] [PubMed] [Google Scholar]
- Bryda EC, Pearson M, Agca Y, Bauer BA. Method for detection and identification of multiple chromosomal integration sites in transgenic animals created with lentivirus. Biotechniques. 2006;41:715–719. doi: 10.2144/000112289. [DOI] [PubMed] [Google Scholar]
- Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007;25:2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbles RM, Sesodia S, Wood PM, Thomas CK. Neurotrophic factors improve motoneuron survival and function of muscle reinnervated by embryonic neurons. J Neuropathol Exp Neurol. 2009;68:736–746. doi: 10.1097/NEN.0b013e3181a9360f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumans JV, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21(23):9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Lee H, Tu CT, Cribbs D, Cotman C, Jeon NL. Preparing e18 cortical rat neurons for compartmentalization in a microfluidic device. J Vis Exp. 2007. [DOI] [PMC free article] [PubMed]
- Yan J, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4(2):e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG. Building bridges for spinal cord repair. Cell. 2012;150(6):1105–1106. doi: 10.1016/j.cell.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Catelas I. Fibrin. Comprehensive Biomaterials. 2011;2:303–328. [Google Scholar]
- Petter-Puchner AH, et al. The long-term neurocompatibility of human fibrin sealant and equine collagen as biomatrices in experimental spinal cord injury. Exp Toxicol Pathol. 2007;58:237–245. doi: 10.1016/j.etp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. Journal of Neural Engineering. 2006;3:172–179. doi: 10.1088/1741-2560/3/2/011. [DOI] [PubMed] [Google Scholar]


