ABSTRACT
Humans are exposed daily to di(2-ethylhexyl) phthalate (DEHP), a plasticizer found in many consumer, medical, and building products containing polyvinyl chloride. Large doses of DEHP disrupt normal ovarian function; however, the effects of DEHP at environmentally relevant levels, the effects of DEHP on folliculogenesis, and the mechanisms by which DEHP disrupts ovarian function are unclear. The present study tested the hypothesis that relatively low levels of DEHP disrupt estrous cyclicity as well as accelerate primordial follicle recruitment by dysregulating phosphatidylinositol 3-kinase (PI3K) signaling. Adult CD-1 mice were orally dosed with DEHP (20 μg/kg/day–750 mg/kg/day) daily for 10 and 30 days. Following dosing, the effects on estrous cyclicity were examined, and follicle numbers were histologically quantified. Further, the ovarian mRNA and protein levels of PI3K signaling factors that are associated with early folliculogenesis were quantified. The data indicate that 10- and 30-day exposure to DEHP prolonged the duration of estrus and accelerated primordial follicle recruitment. Specifically, DEHP exposure decreased the percentage of primordial follicles and increased the percentage of primary follicles counted following 10-day exposure and increased the percentage of primary follicles counted following 30-day exposure. DEHP exposure, at doses that accelerate folliculogenesis, increased the levels of 3-phosphoinositide-dependent protein kinase-1, mammalian target of rapamycin complex 1, and protein kinase B and decreased the levels of phosphatase and tensin homolog, potentially driving PI3K signaling. Collectively, relatively low levels of DEHP disrupt estrous cyclicity and accelerate primordial follicle recruitment potentially via a mechanism involving dysregulation of PI3K signaling.
Keywords: di(2-ethylhexyl) phthalate, endocrine disruptors, environmental contaminants and toxicants, follicular development, folliculogenesis, ovary, phosphatidylinositol 3-kinase signaling pathway, toxicology
INTRODUCTION
Phthalate esters are synthetic plasticizers added to plastics to impart flexibility. The most widely used phthalate ester is di(2-ethylhexyl) phthalate (DEHP), which is used in the manufacturing of common consumer, medical, and building products containing polyvinyl chloride. Products that contain DEHP include flooring, roofing, carpeting, food and beverage packaging, automobile upholstery, shower curtains, packaging equipment, medical bags and tubing, and personal care products. Although production volumes of DEHP alone are not quantified, DEHP belongs to a group of phthalates known as dioctyl phthalates. Domestic production of these phthalates reaches up to 300 million pounds per year [1]. DEHP is noncovalently bound to plastics, and therefore leaching can occur after repeated use, heating, and/or cleaning of the products, allowing the toxicant to be ingested on a daily basis via oral ingestion, inhalation, and dermal contact [2]. In fact, the Agency for Toxic Substances and Disease Registry estimates that the range of daily human exposure to DEHP is 3–30 μg/kg/day, but measurements of DEHP in household dust can reach as high as 700 mg/kg [1, 3].
Exposure to DEHP represents a public health concern, partially because it has been identified as a top contaminant present in human tissues. DEHP and its metabolites are present in over 95% of human blood samples and nearly 100% of human urine samples tested [2–6]. Especially in terms of reproduction, DEHP and its metabolites are present in 90%–100% of amniotic fluid samples from second-trimester fetuses, cord blood samples from newborns, breast milk from nursing mothers, and even human ovarian follicular fluid, indicating its ability to reach the ovary [2, 4, 5, 7].
Continuous, daily exposure to DEHP, along with its prevalence in human tissues, is of major concern because DEHP has been designated as an endocrine-disrupting chemical and reproductive toxicant for its ability to interfere with reproductive and hormone-regulated processes [8–10]. In males, DEHP exhibits antiandrogenic effects by inducing tubular atrophy and testicular degeneration and by inhibiting testicular steroidogenesis [11, 12]. To date, the majority of studies examining the effects of DEHP focus on male reproduction and development; however, in females, chronic occupational exposure to phthalates has been associated with decreased rates of pregnancy and high rates of miscarriage [2]. In laboratory animals, DEHP reduces implantations, increases resorptions, and decreases fetal weights of offspring [9, 13]. Surprisingly, much less is known about the effects of DEHP on the adult ovary, but recent work suggests that the ovary may be a target of DEHP toxicity. Specifically, DEHP exposure has been shown to decrease serum and antral follicle-produced 17β-estradiol levels, decrease aromatase levels, cause anovulation, and disrupt estrous cyclicity [14–19]. Some of the doses of DEHP used in these previous studies, however, are not environmentally relevant; thus, the effects of DEHP at environmentally relevant levels on ovarian function remain unclear. Further, the mechanisms by which DEHP disrupts ovarian function remain unclear; however, DEHP is hypothesized to cause toxicity independent of steroid receptor signaling, as both DEHP and its metabolite, mono(2-ethylhexyl) phthalate (MEHP), have weak to no binding to the androgen and estrogen receptor [20–25].
Understanding the effects of DEHP on ovarian function is vital because the ovary is an integral regulator of reproductive and nonreproductive health, and one of the most essential ovarian processes is folliculogenesis. The ovary is a heterogeneous endocrine organ containing ovarian follicles, which are the functional units of the ovary. In mammals, the female is born with a finite number of ovarian follicles; thus, the follicular reserve is set at birth and represents a female's reproductive potential and reproductive life span [26]. Within the ovarian unit, follicles undergo several irreversible developmental transitions, starting from the primordial stage, in which the follicles are immature, dormant, and constitute the ovarian reserve, to the antral stage, in which the follicles are mature and capable of ovulation [26, 27]. This process of follicular development is known as ovarian folliculogenesis. Because the ovarian reserve is established at birth and folliculogenesis is an irreversible process, aberrant regulation of folliculogenesis can have adverse reproductive implications. In particular, the accelerated depletion of primordial follicles, through either apoptosis or irregular activation of development, can result in infertility and/or premature ovarian failure or early onset of menopause. Although DEHP has been shown to disrupt normal ovarian function, its effects on folliculogenesis remain unclear.
Because ovarian folliculogenesis is essential for reproductive and nonreproductive health and increasing evidence indicates that the ovary is a potential target of DEHP toxicity, the present study was designed to investigate the effects of in vivo DEHP exposure on normal ovarian function as well as the role and mechanisms by which DEHP disrupts folliculogenesis. Specifically, the present study was designed to test the hypothesis that relatively low levels of DEHP disrupt folliculogenesis by dysregulating the phosphatidylinositol 3-kinase (PI3K) signaling pathway, potentially leading to the acceleration of primordial follicle recruitment. We decided to investigate the effects of DEHP on the PI3K signaling pathway because PI3K signaling has recently been shown to be a critical regulator of early folliculogenesis, specifically in primordial follicle survival, quiescence, and recruitment [28–33]. In further detail, increased PI3K signaling is associated with the promotion of primordial follicle recruitment. To test our hypothesis, we orally dosed adult mice with DEHP daily for 10 and 30 days. Following dosing, we investigated reproductive end points and histologically examined the effect of DEHP on follicular dynamics. To provide insight into the mechanism of DEHP-induced defects in folliculogenesis, we measured the levels of key PI3K signaling factors that are associated with early folliculogenesis. Specifically, we examined the effects of DEHP on 3-phosphoinositide-dependent protein kinase-1 (PDPK1), mammalian target of rapamycin complex 1 (mTORC1), mast/stem cell growth factor receptor (KIT), protein kinase B (AKT), and ribosomal protein s6 (rpS6), all of which are factors that drive PI3K signaling. Additionally, we examined the effects of DEHP on forkhead box O3A (FOXO3A), phosphatase and tensin homolog (PTEN), and tuberous sclerosis 1 (TSC1), which negatively regulate PI3K signaling.
MATERIALS AND METHODS
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Five stock solutions of DEHP were prepared using tocopherol-stripped corn oil (MP Biomedicals, Solon, OH) as the vehicle. The doses used for these experiments were 20 μg/kg/day, 200 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day, and 750 mg/kg/day. To achieve these doses, the stock concentrations were 0.0125, 0.125, 12.5, 125, and 468.8 mg/ml for each respective dose.
The doses were chosen because they are much more environmentally relevant than doses used in previous DEHP ovotoxicity studies (2 g/kg/day) [15, 17, 34]. In particular, the Agency for Toxic Substances and Disease Registry estimates that the range of daily human exposure to DEHP is 3–30 μg/kg/day [1]. Additionally, they note that the no-observed-adverse-effect level for DEHP is 5.8 mg/kg/day and that the lowest-observed-adverse-effect level is 140 mg/kg/day; however, potential reproductive effects of DEHP occur at lower levels ranging from 1 to 30 μg/kg/day [1, 2]. Further, the Environmental Protection Agency (EPA) reference dose for DEHP is 20 μg/kg/day, but it is possible that certain populations can be exposed to much higher levels than this EPA reference dose [3].
Animals
Cycling, adult CD-1 female mice at 39 days of age were obtained from Charles River Laboratories (Wilmington, MA) and were allowed to acclimate to the facility prior to dosing. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. The mice were provided food and water ad libitum. Housing in a controlled animal room environment was established by the maintenance of temperature at 22 ± 1°C and 12L:12D cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In Vivo Dosing Regimen
The mice were orally dosed with vehicle or DEHP daily for 10 and 30 days. For the 10-day dosing study, the mice received vehicle or DEHP at 20 μg/kg/day, 200 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day, and 750 mg/kg/day (n = 8/group). For the 30-day dosing study, the mice received vehicle or DEHP at 20 μg/kg/day, 200 μg/kg/day, 20 mg/kg/day, and 200 mg/kg/day (n = 8/group). Dosing volumes were determined daily by corresponding mouse weight, and the dose was given orally by inserting a pipette tip beyond the incisors toward the cheek pouch. Estrous cyclicity was monitored daily, and the mice were euthanized in estrus following the 10- and 30-day dosing period.
Body and Organ Weight Analysis
The mice were weighed daily to both calculate a dosing volume and monitor the effect of DEHP on body weight. Following 10 and 30 days of dosing, the mice were weighed and euthanized in estrus (n = 8/group). Organs were aseptically removed, cleaned of interstitial tissue, and weighed. Percent body weight change was calculated by dividing the weight at the time of euthanasia by the weight at the start of dosing and multiplying that value by 100. Organ weights were recorded as whole organ weights or relative organ weights. Relative organ weights were calculated by dividing the weight of the organ by the body weight at the time of euthanasia and multiplying that value by 100.
Estrous Cyclicity Analysis
During dosing, the mice were subjected to daily vaginal smears to monitor estrous cyclicity (n = 8/group). Stage of estrus was determined by vaginal cytology using a light microscope and was recorded based on previously defined and well-documented criteria [35–37]. Percentage of days in estrus was calculated by dividing the number of days in estrus by the number of days in the study and multiplying that value by 100. Percentage of days in metestrus/diestrus was calculated by dividing the number of days in either metestrus or diestrus by the number of days in the study and multiplying that value by 100.
Histological Evaluation of Follicle Numbers
Following dosing, mice were euthanized in estrus, and some ovaries were aseptically collected and fixed in Dietrich fixative for at least 24 h for histological evaluation of follicle numbers (n = 3–8 ovaries/group). The ovaries were then transferred to 70% ethanol, embedded in paraffin wax, and serial sectioned (8 μM) using a microtome. The serial sections were mounted on a glass slide and stained with hematoxylin and eosin. The numbers of oocyte containing follicles were counted in every 10th serial section using a light microscope, and the percentage of each follicle type was calculated by dividing the number of follicles of each specific type by the total number of follicles counted in every 10th serial section and multiplying that value by 100. Stage of follicular development was assessed using previously defined criteria [38, 39]. Briefly, primordial follicles contained the oocyte surrounded by a single layer of squamous granulosa cells, primary follicles contained the oocyte surrounded by a single layer of cuboidal granulosa cells, preantral follicles contained the oocyte surrounded by at least two layers of cuboidal granulosa cells and theca cell layers, and antral follicles contained the oocyte surrounded by multiple layers of cuboidal granulosa cells with a fluid-filled antral space and theca cell layers. All primordial and primary follicles with oocytes, regardless of nuclear material in the oocytes, were counted, whereas only preantral and antral follicles with visible nuclear material in the oocyte were counted to avoid the risk of double counting follicles large enough to span serial sections.
Gene Expression Analysis
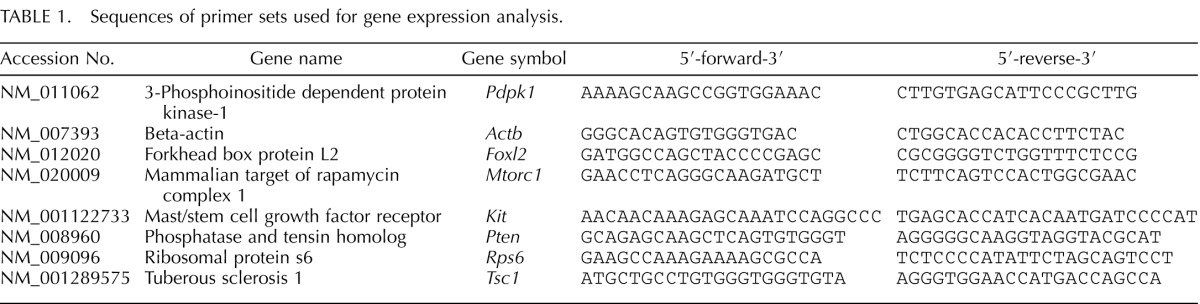
Following dosing, mice were sacrificed in estrus, and some ovaries were aseptically collected, snap frozen in liquid nitrogen, and stored at −80°C for quantitative real-time polymerase chain reaction (qPCR) analysis (n = 3–5 ovaries/group). Total RNA was extracted from the ovaries using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. Total RNA (1000 ng) was then reverse transcribed to cDNA using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer's protocol. Each cDNA sample was diluted 1:4 with nuclease-free water prior to analysis. Analysis of qPCR was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories) and accompanying CFX Manager Software according to the manufacturer's protocol. The machine quantifies the amount of PCR product generated by measuring SsoFastEvaGreen dye (Bio-Rad Laboratories) that fluoresces when bound to double-stranded DNA. A standard curve was generated from six serial dilutions of three samples spanning different treatment groups to calculate the amplification efficiencies of each primer. Specific qPCR primers (Integrated DNA Technologies, Inc., Coralville, IA) for the genes of interest as well as the reference gene, beta-actin (Actb), which did not differ across treatment groups, can be found in Table 1. All qPCR reactions were done in triplicate using 2 μl cDNA, forward and reverse primers (5 pmol) for Mtorc1, Pdpk1, Pten, Kit, Rps6, Tsc1, Foxl2, or Actb, in addition to a SsoFastEvaGreen Supermix qPCR kit for a final reaction volume of 10 μl. The qPCR program consisted of an enzyme activation step (95°C for 1 min), an amplification and quantification program (40 cycles of 95°C for 10 sec, 60°C for 10 sec, single fluorescence reading), a step of 72°C for 5 min, a melt curve (65°C–95°C heating, 0.5°C/sec with continuous fluorescence readings), and a final step at 72°C for 5 min as per the manufacturer's protocol. Expression data were generated using the mathematical model for relative quantification of real-time PCR data developed by Pfaffl [40]. The genes tested were chosen because they are associated with the regulation of early folliculogenesis, in particular, primordial follicle recruitment [28, 29, 31, 41–43]. Additionally, each gene tested, except Foxl2, is involved in PI3K signaling [28–33, 44].
TABLE 1.
Sequences of primer sets used for gene expression analysis.
Immunohistochemistry
Following 10 days of dosing, mice were sacrificed in estrus, and some ovaries were aseptically collected, fixed in 4% paraformaldehyde overnight, and transferred to 70% ethanol for immunohistochemical analysis (n = 3/group). The ovaries were then embedded in paraffin wax and serial sectioned (5 μM), and five representative sections that span the entire length of the ovary were mounted on a glass slide. Following deparaffinization, the tissues were subjected to heat-induced antigen retrieval (10 mM sodium citrate buffer at pH 6.0) for 60 min followed by endogenous peroxide quenching in 3% hydrogen peroxide for 20 min. The tissues were then blocked in 5% goat serum and avidin for 30 min. In some experiments, the tissues were incubated with a rabbit anti-mouse phosphorylated-AKT (Ser473; the active form of the protein) antibody (1:50; Cell Signaling Technology, Inc., Boston, MA) with biotin for 60 min, while in other experiments, the tissues were incubated with a rabbit anti-mouse PTEN antibody (1:200; Cell Signaling Technology) with biotin for 60 min. The tissues were then incubated with a secondary biotinylated goat anti-rabbit antibody (Vector Laboratories, Inc., Burlingame, CA) for 20 min, followed by an incubation with an avidin biotin complex solution (Vector Laboratories) for 20 min. ImmPACT NovaRED peroxidase substrate solution (Vector Laboratories) was then applied until color optimally developed. Each sample was exposed to the chromogen for equal amounts of time. The slides were then rinsed, counterstained with a 1:10 dilution of hematoxylin, and coverslipped. A negative control was used in each experiment and was subjected to the same methods listed above, except instead of primary antibody, the negative control tissues were incubated with a negative control rabbit immunoglobulin fraction (Dako, Carpinteria, CA) at the same concentration of each primary antibody. Negative controls were confirmed to have no positive staining after exposure to the chromogen for equal amounts of time as the samples. Both pAKT and PTEN were chosen because they are integral regulators of the PI3K signaling pathway and have been associated with the regulation of primordial follicle recruitment [31, 43–46]. Specifically, pAKT drives PI3K signaling and ultimately promotes primordial follicle recruitment [31, 43–46]. Additionally, PTEN inhibits PI3K signaling and ultimately maintains primordial follicle quiescence [31, 43–46].
Analysis of the Levels of Protein Staining
Following immunohistochemistry, the levels of pAKT and PTEN staining in the ovaries were quantified using published methods [47–49]. Briefly, images of whole ovaries and primordial and primary follicles were digitally captured using a Leica DFC 290 camera and analyzed using the ImageJ software (http://rsb.info.nih.gov/nih-image). For whole ovarian analysis, five representative sections from each ovary were assessed from three separate animals per treatment group. For follicular analysis, two to three follicles of each type from the five representative ovarian sections were assessed from three separate animals per treatment group. Digital images were converted to eight-bit grayscale images and then converted to pseudocolored images. Colors were based on relative stain intensity, as defined digitally. Areas with no staining appeared black and blue, while areas with the most intense staining appeared deep yellow to orange. The pAKT and PTEN labeling index was defined as the percentage of positively stained area per whole ovary, primordial follicles, and primary follicles. The pixels of positively stained areas were divided by the total amount of pixels in the whole ovary, primordial follicles, and primary follicles, and that value was multiplied by 100.
Statistical Analysis
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± SEM. Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance (ANOVA) followed by Tukey post hoc comparison. Multiple comparisons between nonnormally distributed experimental groups were made using Mann-Whitney and Kruskal Wallis tests when appropriate. Statistical significance was assigned at P ≤ 0.05
RESULTS
Effect of DEHP Exposure on Body and Organ Weights
To observe if DEHP is overtly toxic to the body and/or specific organs, we recorded body and organ weight changes in response to DEHP treatment. Importantly, DEHP exposure for 10 and 30 days had no effect on mortality of the mice and had no statistically significant effect on percent body weight change when compared to the vehicle control group (Table 2). However, DEHP exposure for 10 days significantly increased the whole and relative weight of the liver at the 750-mg/kg/day dose when compared to the vehicle control group (n = 8/group, P ≤ 0.05). Additionally, DEHP exposure for 10 days significantly increased the whole and relative weight of the spleen at the 200-μg/kg/day and 20-mg/kg/day doses, respectively, when compared to the vehicle control group (n = 8/group, P ≤ 0.05). In the 30-day dosing study, DEHP significantly increased the whole and relative weight of the uterus at the 20-μg/kg/day dose when compared to the vehicle control group (n = 8/group, P ≤ 0.05). Again, these changes in organ weights had no effect on body weight change and mortality. Thus, the doses chosen for these studies were not overtly toxic to the body.
TABLE 2.
Effect of DEHP exposure on body and organ weights.
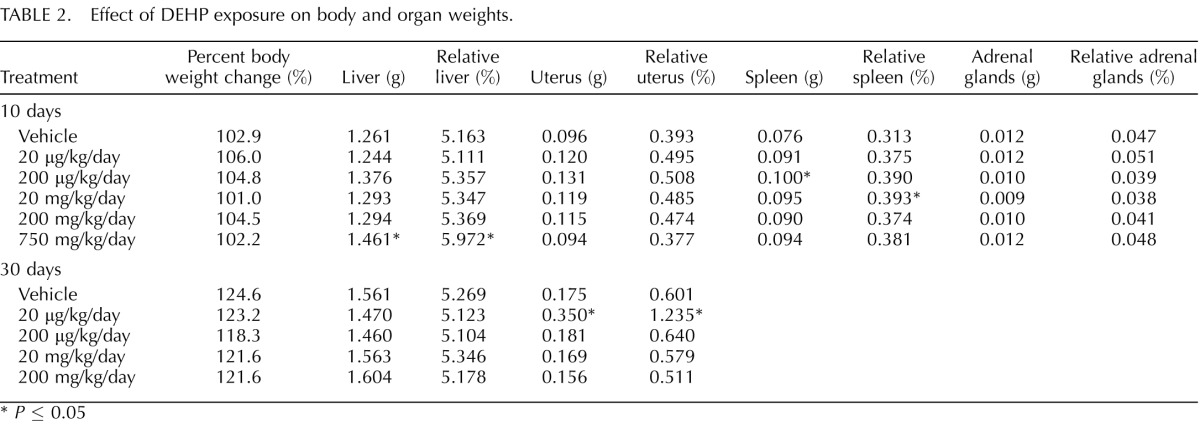
P ≤ 0.05
Effect of DEHP Exposure on Estrous Cyclicity
DEHP exposure at high doses (2 g/kg/day) in adult rats previously has been shown to prolong estrous cycles, and DEHP-treated antral follicles exhibit inhibition of estradiol production in vitro [15, 19]; thus, we decided to investigate if DEHP treatment altered estrous cyclicity in our dosing studies. In the 10-day dosing study, DEHP exposure significantly increased the percentage of days the mice were in estrus at the 20-μg/kg/day and the 20-, 200-, and 750-mg/kg/day doses when compared to the vehicle control group (Fig. 1A, n = 8/group, P ≤ 0.05). Similarly, DEHP exposure for 30 days significantly increased the percentage of days the mice were in estrus at the 200-mg/kg/day dose when compared to the vehicle control group (Fig. 1B, n = 8/group, P ≤ 0.05). In both studies, DEHP exposure had no effect on the percentage of days the mice were in metestrus and diestrus when compared to the vehicle control group (Fig. 1, A and B).
FIG. 1.
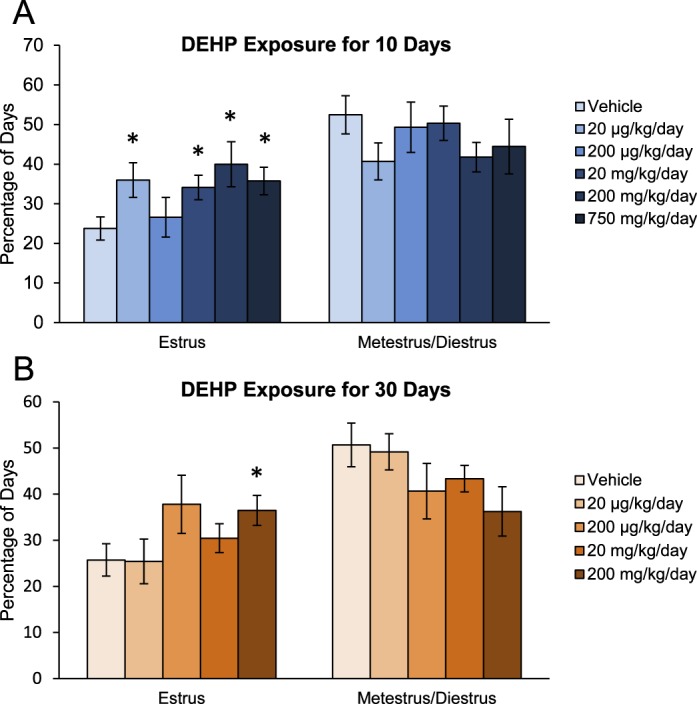
Effect of DEHP exposure on estrous cyclicity. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 μg/kg/day–750 mg/kg/day) daily for 10 (A) and 30 (B) days. Vaginal swabs were taken daily using PBS administered from a pipette tip. Stage of estrus was recorded based on previously defined criteria using a light microscope. Percentage of days in estrus and metestrus/diestrus was calculated and compared in each treatment group. Graph represents means ± SEM (n = 8 mice/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of DEHP Exposure on Folliculogenesis
Because the ovary is a potential target of DEHP toxicity and ovarian folliculogenesis is essential for fertility and the maintenance of appropriately timed reproductive senescence, we histologically evaluated the effects of DEHP on follicular dynamics. In the 10-day dosing study, DEHP exposure significantly decreased the percentage of primordial follicles counted at the 20- and 200-mg/kg/day doses when compared to the vehicle control group (Fig. 2A, n = 3–4/group, P ≤ 0.05). Likewise, DEHP exposure significantly increased the percentage of primary follicles counted at all selected doses of DEHP when compared to the vehicle control group (n = 3–4/group, P ≤ 0.05). In accordance, DEHP exposure for 30 days significantly increased the percentage of primary follicles counted at the 200-μg/kg/day and 20-mg/kg/day doses when compared to the vehicle control group (Fig. 2B, n = 8/group, P ≤ 0.05). These data suggest that DEHP exposure may affect early folliculogenesis in that it may accelerate primordial follicle recruitment to the primary stage of development.
FIG. 2.
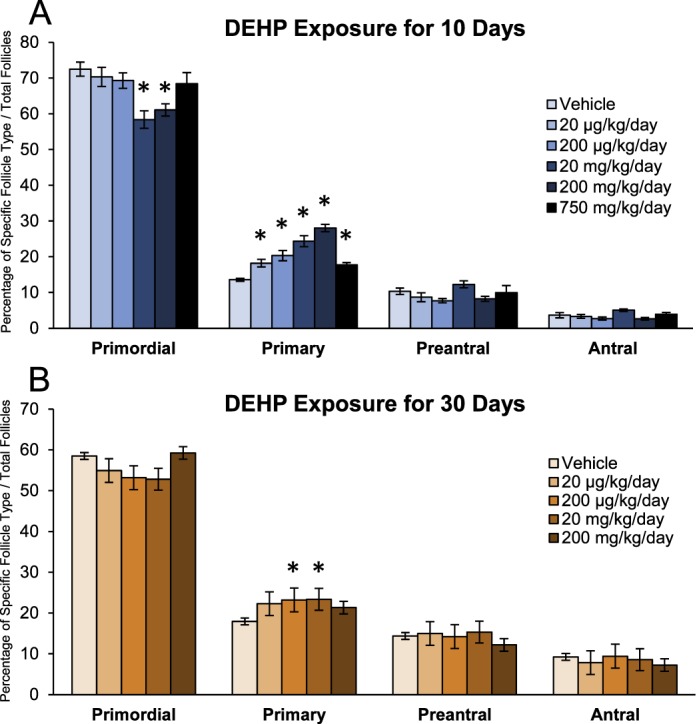
Effect of DEHP exposure on folliculogenesis. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 μg/kg/day–750 mg/kg/day) daily for 10 (A) and 30 (B) days. Following dosing, ovaries were processed for histological evaluation of follicle counts. Percentages of each follicle type were calculated and compared in each treatment group. Graph represents means ± SEM (n = 3–8 ovaries/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of DEHP Exposure on the mRNA Levels of Factors Within the PI3K Signaling Pathway
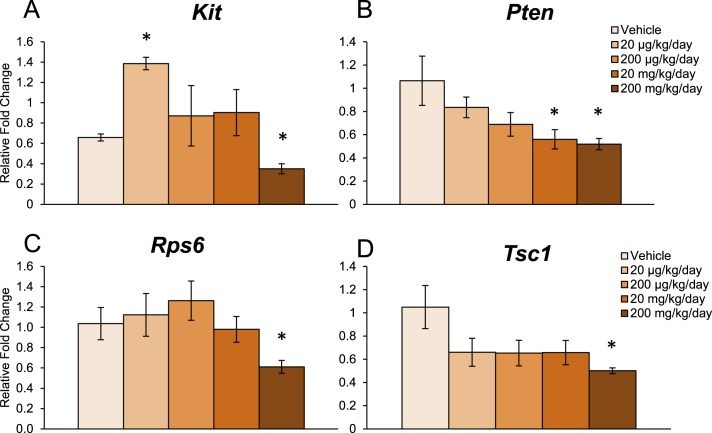
Early folliculogenesis, specifically primordial follicle recruitment, relies on paracrine and autocrine regulation by intrinsic ovarian growth factors, and recent work has established the PI3K signaling pathway as an integral regulator of this process [26–33]. Thus, we measured the ovarian mRNA levels of several PI3K signaling factors that are associated with primordial follicle recruitment. In the 10-day dosing study, DEHP exposure significantly increased the mRNA levels of Mtorc1, a stimulator of the PI3K signaling pathway, at the 20- and 200-μg/kg/day doses and at the 200- and 750-mg/kg/day doses when compared to the vehicle control group (Fig. 3A, n = 4–5/group, P ≤ 0.05). Additionally, 10-day exposure to DEHP significantly increased the mRNA levels of Pdpk1, another stimulatory PI3K signaling factor, at the 200- and 750-mg/kg/day doses when compared to the vehicle control group (Fig. 3B, n = 4–5/group, P ≤ 0.05). In the same study, DEHP exposure significantly decreased the mRNA levels of Pten, an inhibitory PI3K signaling factor, at the 200-μg/kg/day and 20-mg/kg/day doses when compared to the vehicle control group (Fig. 3C, n = 4–5/group, P ≤ 0.05). However, 10-day exposure to DEHP had no effect on the mRNA levels of Kit, Rps6, Tsc1, or Foxl2 when compared to the vehicle control group.
FIG. 3.
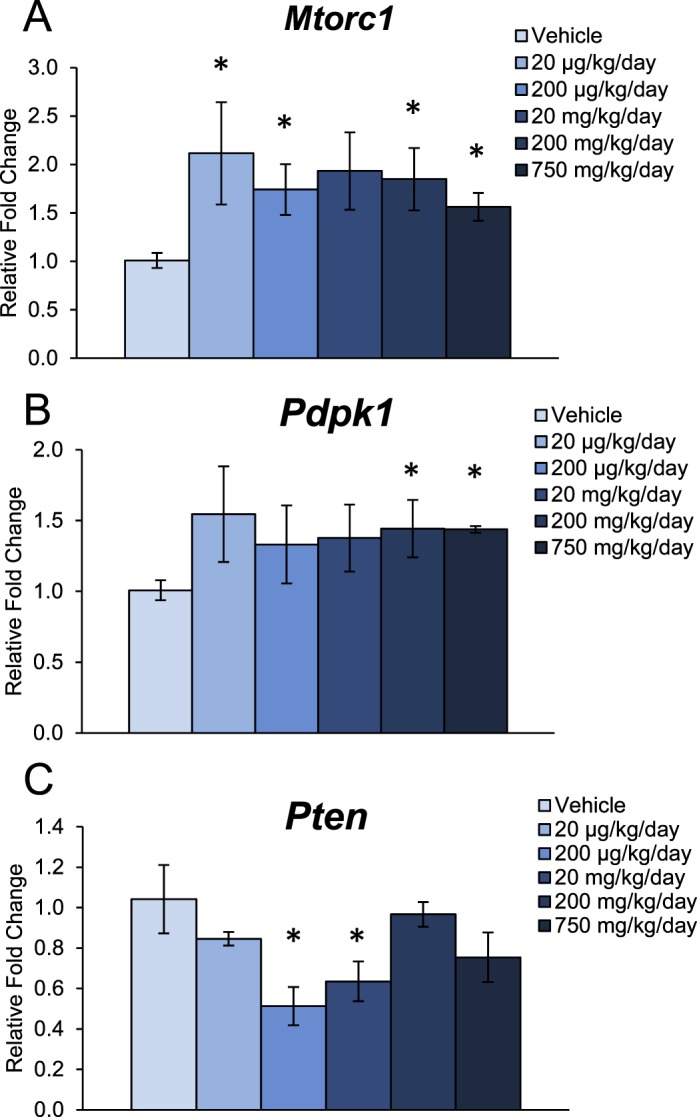
Effect of DEHP exposure for 10 days on the mRNA expression of key factors in the PI3K signaling pathway. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 μg/kg/day–750 mg/kg/day) daily for 10 days. Following dosing, whole ovaries were subjected to qPCR for Mtorc1 (A), Pdpk1 (B), and Pten (C). All values were normalized to Actb. Graph represents means ± SEM (n = 4–5 ovaries/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
In the 30-day dosing study, DEHP exposure significantly increased the mRNA levels of Kit, a stimulatory PI3K signaling factor, at the 20-μg/kg/day dose but decreased its expression at the 200-mg/kg/day dose when compared to the vehicle control group (Fig. 4A, n = 3–4/group, P ≤ 0.05). Similar to the 10-day dosing study, DEHP exposure for 30 days significantly decreased the mRNA levels of Pten at the 20- and 200-mg/kg/day doses when compared to the vehicle control group (Fig. 4B, n = 3–4/group, P ≤ 0.05). DEHP exposure for 30 days also significantly decreased the mRNA levels of Rps6, a stimulatory PI3K signaling factor, at the 200-mg/kg/day dose when compared to the vehicle control group (Fig. 4C, n = 3–4/group, P ≤ 0.05). Additionally, DEHP exposure significantly decreased the mRNA levels of Tsc1, another inhibitory PI3K signaling factor, at the 200-mg/kg/day dose when compared to the vehicle control group (Fig. 4D, n = 3–4/group, P ≤ 0.05). Interestingly, DEHP exposure for 30 days altered factors outside of the PI3K signaling pathway that are also associated with primordial follicle recruitment. Specifically, DEHP significantly decreased the mRNA levels of Foxl2, an inhibitor of recruitment, at the 20-mg/kg/day dose when compared to the vehicle control group (vehicle: 1.1 ± 0.3; 20 mg/kg/day: 0.5 ± 0.1; n = 4/group, P ≤ 0.05). However, DEHP exposure for 30 days had no effect on the mRNA levels of Pdpk1 and Mtorc1 when compared to the vehicle control group.
FIG. 4.
Effect of DEHP exposure for 30 days on the mRNA expression of key factors in the PI3K signaling pathway. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 μg/kg/day–200 mg/kg/day) daily for 30 days. Following dosing, whole ovaries were subjected to qPCR for Kit (A), Pten (B), Rps6 (C), and Tsc1 (D). All values were normalized to Actb. Graph represents means ± SEM (n = 3–4 ovaries/treatment group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
Effect of DEHP Exposure on Protein Levels of PI3K Signaling Factors
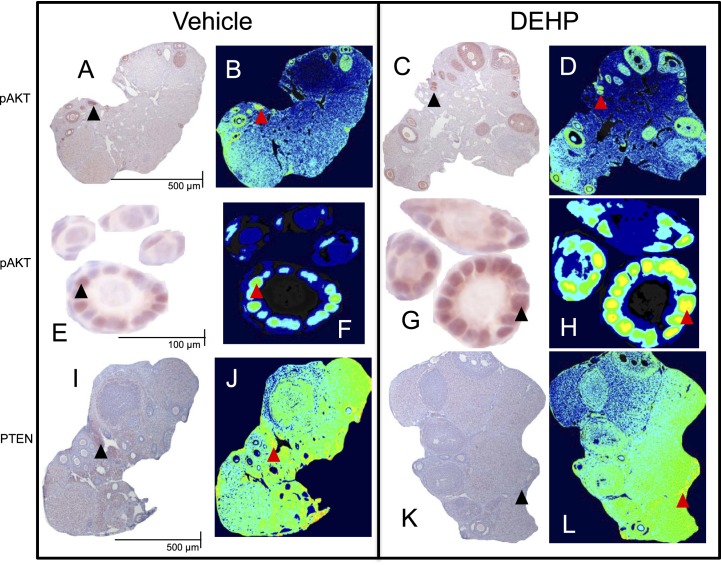
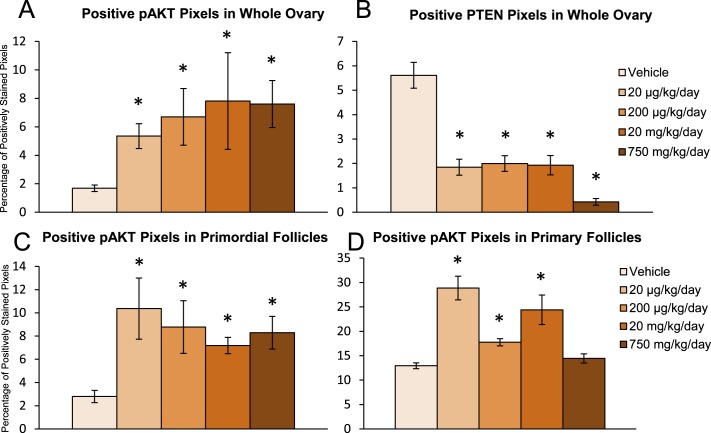
DEHP exposure in the 10- and 30-day dosing studies selectively altered the mRNA levels of PI3K signaling factors that are associated with early folliculogenesis; however, some doses that exhibited phenotypic changes in folliculogenesis had unaltered mRNA expression. Additionally, certain factors within the PI3K signaling pathway are translationally and posttranslationally regulated; thus, the mRNA levels may not be indicative of the ovarian phenotypic changes observed in our dosing studies. Therefore, we measured protein levels of two key PI3K signaling factors that regulate primordial follicle recruitment in the 10-day dosing study. Representative images of pAKT, a stimulatory PI3K signaling factor involved in the promotion of primordial follicle recruitment, and PTEN, an inhibitory PI3K signaling factor involved in maintaining primordial follicle quiescence, staining in the ovary are found in Figure 5. Qualitatively, it appears that DEHP exposure increased the staining of pAKT (Fig. 5, A–D) and decreased the staining of PTEN (Fig. 5, I–L) in the whole ovary and in immature follicle types (Fig. 5, E–H). When quantified, DEHP exposure significantly increased pAKT and significantly decreased PTEN staining in the whole ovary at all selected doses of DEHP when compared to the vehicle control group (Fig. 6, A and B, n = 3/group, P ≤ 0.05). To confirm that these effects were evident in the follicles of interest, we measured protein staining within the primordial and primary follicles themselves. DEHP exposure significantly increased pAKT staining in primordial follicles at all selected doses of DEHP and in primary follicles at the 20- and 200-μg/kg/day doses and the 20-mg/kg/day dose when compared to the vehicle control group (Fig. 6, C and D, n = 3/group, P ≤ 0.05).
FIG. 5.
Representative images of immunohistochemistry and pseudocolored protein quantification. Panels A–H exhibit pAKT staining in the whole ovary (A–D) and in selected primordial and primary follicles (E–H). Panels I–L exhibit PTEN staining in the whole ovary. Panels A, B, E, F, I, and J were from vehicle control treated ovaries and follicles. Panels C, D, G, H, K, and L were from 20-μg/kg/day DEHP-treated ovaries and follicles. Black arrowheads represent positive staining in the immunohistochemistry images, which translate to the yellow/orange pseudocolored pixels represented by red arrowheads. Scale bars are set as 500 μm for whole ovary images (A–D and I–L) and 100 μm for follicle images (E–H) and the same scale bars should be used across each row.
FIG. 6.
Effect of DEHP exposure on protein levels of PI3K signaling factors associated with early folliculogenesis. Adult CD-1 mice were orally dosed with vehicle (tocopherol-stripped corn oil) or DEHP (20 μg/kg/day–750 mg/kg/day) daily for 10 days. Following dosing, ovaries were subjected to immunohistochemistry for quantification of pAKT (A, C, and D) and PTEN (B) staining. Digital images were converted to eight-bit grayscale images and then converted to pseudocolored images. Colors were based on relative stain intensity. Percentage of positively stained pixels in the whole ovary (A and B) and in follicles (C and D) were calculated and compared in each treatment group. Graph represents means ± SEM (n = 3/group). Asterisks (*) represent significant difference from vehicle control (P ≤ 0.05).
DISCUSSION
We have shown that 10- and 30-day oral exposure to DEHP disrupts normal reproductive and ovarian function. Specifically, we provide evidence that relatively low levels of DEHP, including a dose within the estimated range of daily human exposure, alter estrous cyclicity and accelerate primordial follicle recruitment. Further, we are the first, to our knowledge, to provide mechanistic evidence by which adult exposure to DEHP accelerates early folliculogenesis. Our data show that DEHP exposure may interfere with normal PI3K signaling by altering the expression of key genes and/or the levels of key proteins.
An important note is that the selected doses of DEHP had no effect on mortality and very minor effects on organ weights. Thus, the adverse reproductive outcomes in response to DEHP exposure are most likely not due to overt toxicity. Additionally, the doses used in these studies are more environmentally relevant than doses used in several previous studies found within the literature [15, 17, 34]. Therefore, these findings provide some insight into the effects of DEHP on ovarian function at human exposure levels, though we acknowledge that investigating the effects at even lower levels would be beneficial.
Our data provide evidence that relatively low levels of DEHP disrupt estrous cyclicity, which may cause complications in reproductive function. DEHP exposure for 10 days increased the percentage of days that the mice were in estrus at the 20-μg/kg/day and the 20-, 200-, and 750-mg/kg/day doses when compared to the vehicle control group. Likewise, but to a lesser degree, DEHP exposure for 30 days increased the percentage of days the mice were estrus at the 200-mg/kg/day dose when compared to the vehicle control group. These results correlate to previous reports suggesting that higher doses of DEHP (2 g/kg/day) disrupt estrous cyclicity, but also expand on those findings in that the lower levels of DEHP used in this study also exhibit effects on estrous cyclicity [15]. Interestingly, the effects of DEHP on estrous cyclicity exhibit a nonmonotonic dose response and appear to be greater following 10 days of exposure than what is observed following 30 days of exposure. A nonmonotonic dose response is typical among common endocrine-disrupting chemicals, suggesting that different effects and mechanisms may exist at different doses. Further, we hypothesize that the ovary and/or the hypothalamus-pituitary-ovarian axis is able to compensate for the toxicity of DEHP between Days 10 and 30. Additionally, it is possible that the biotransformation of DEHP is altered following a longer period of dosing so that altered metabolism of the chemical helps protect the ovary from toxicity, but further work investigating this matter would need to be conducted to verify this statement.
Although DEHP has been shown to disrupt reproductive function in males and, to lesser extent, in females, the effect of DEHP on ovarian folliculogenesis, especially in the adult, is relatively unknown. For normal fertility and the appropriate timing of reproductive senescence, primordial follicles must undergo a strict and coordinated process known as primordial follicle recruitment. Under normal conditions, the primordial follicle is essentially destined for three fates: to survive in dormancy for varying lengths of time throughout reproductive life, to be recruited into the growing population of follicles, or to undergo death in the dormant state [50–52]. Our data show that 10- and 30-day exposure to DEHP accelerates this essential stage of early folliculogenesis. Specifically, 10-day exposure to DEHP decreased the percentage of primordial follicles counted at the 20- and 200-mg/kg/day doses and increased the percentage of primary follicles counted at all selected doses (20 μg/kg/day–750 mg/kg/day) when compared to the vehicle control group. A similar effect is observed following a longer period of dosing where 30-day exposure to DEHP increased the percentage of primary follicles counted at the 200-μg/kg/day and 20-mg/kg/day doses when compared to the vehicle control group. Thus, DEHP exposure at these two time points accelerates primordial follicle recruitment to the primary stage of development in the adult murine model. These results are consistent with those from other studies using different methods. Specifically, DEHP in vitro and in vivo alters early folliculogenesis and primordial follicle assembly in mice; however, the exposure periods were limited to the neonate [53, 54]. Additionally, in utero exposure to high levels of MEHP, the metabolite of DEHP, accelerates early folliculogenesis in the F1 generation of mice [55]. Our data add to these existing studies by showing that adult exposure to relatively low levels of DEHP accelerates early folliculogenesis in the mouse.
Interestingly, in our study, the effects on early folliculogenesis appear to be more profound following 10 days of exposure than what is observed following 30 days of exposure. We attribute this observation to the ovary being able to compensate for the toxicity of DEHP between Days 10 and 30 by potentially altering the biotransformation of DEHP. Although the defects in follicle numbers appear to be undergoing compensation, it is possible that the oocytes in follicles that undergo accelerated folliculogenesis may not be of fertilizable quality. In fact, previous work has determined that accelerated early folliculogenesis results in poor-quality oocytes and compromised fertility [56]. Further, it is possible that our follicle count data represent a single snapshot following 10 and 30 days of DEHP exposure and that changes in follicle numbers may be observed at later time points. Additionally, it is possible that the excess primary follicles may undergo atresia, which may be why we do not see changes in the later stages of folliculogenesis. In accordance with the estrous cyclicity results, we also observe a nonmonotonic dose response for the effects of DEHP on early folliculogenesis. Future studies should examine the mechanisms underlying compensation and the nonmonotonic dose response. Further, these studies should determine if accelerated primordial follicle recruitment has long-term implications for fertility.
Primordial follicle recruitment is a gonadotropin-independent process that relies on paracrine and autocrine regulation by intrinsic ovarian growth factors [26, 27]. These factors either suppress activation and thus maintain primordial follicle dormancy or activate primordial follicles and thus promote recruitment. A balance exists between these inhibitory and stimulatory factors to maintain primordial follicle survival so that downregulation of inhibitory factors and/or overactivation of stimulatory factors favor an environment conducive for primordial follicle recruitment [43]. Although the exact mechanism by which follicles are recruited to the growing pool is unknown, recent work has established the existence of several key intrinsic ovarian factors that belong to multiple signaling pathways [41, 42, 50, 57]. Interestingly, several of these factors are involved in the PI3K signaling pathway, indicating this pathway as a critical regulator of primordial follicle survival, quiescence, and recruitment to the primary stage of development [28–33].
The PI3K signaling pathway is involved in cell proliferation, survival, migration, metabolism, and, most recently, early ovarian folliculogenesis [43, 45, 46]. Components of this signaling pathway that are also associated with primordial follicle recruitment and have been tested in these studies include PDPK1, mTORC1, KIT, pAKT, and rpS6, all of which are factors that drive PI3K signaling. Additional components of this pathway that are associated with primordial follicle recruitment and have been tested in these studies include FOXO3A, PTEN, and TSC1, which negatively regulate PI3K signaling. Conditional deletion of Foxo3a, Pten, and Tsc1 from mouse oocytes results in global activation of all primordial follicles, leading to premature ovarian failure [31, 44, 58–61]. Thus, these inhibitors of PI3K signaling maintain primordial follicle dormancy. Acceleration of primordial follicle recruitment occurs because of overactivation of the PI3K signaling pathway brought about by the unrestricted regulation of stimulatory PI3K factors, such as pAKT, mTORC1, and PDPK1 [44, 59, 62, 63]. Therefore, proper regulation of PI3K signaling is needed for appropriate primordial follicle survival, quiescence, and recruitment, and dysregulation of this pathway may lead to the acceleration of primordial follicle recruitment and ultimately premature ovarian failure and infertility.
Our data provide evidence that 10- and 30-day DEHP exposure dysregulates the ovarian mRNA and protein levels of key PI3K signaling factors that are associated with primordial follicle recruitment. These findings are critical because PI3K signaling is an integral regulator of primordial follicle recruitment and because they provide mechanistic evidence by which DEHP disrupts folliculogenesis in an adult model. Specifically, in the 10-day dosing study, DEHP selectively increased the mRNA levels of the stimulatory PI3K signaling factors Mtorc1 and Pdpk1 at doses that correspond to the acceleration of early folliculogenesis when compared to the vehicle control group. Additionally, DEHP exposure for 10 days selectively decreased the mRNA levels of the inhibitory PI3K signaling factor Pten at doses that correspond to the acceleration of early folliculogenesis when compared to the vehicle control group. Importantly, DEHP exposure for 30 days also decreased the mRNA levels of Pten at a dose that corresponds to the acceleration of early folliculogenesis when compared to the vehicle control group. Interestingly, DEHP exposure for 30 days also selectively altered the mRNA levels of stimulatory Kit and Rps6 and inhibitory Tsc1 PI3K signaling factors, but DEHP did so at doses in which early folliculogenesis was unaffected. Further, DEHP exposure altered the mRNA levels of factors outside the PI3K signaling pathway that also are involved in primordial follicle recruitment. In the 30-day dosing study, DEHP exposure decreased the mRNA levels of Foxl2 when compared to the vehicle control group. Thus, although we provide evidence that DEHP disrupts folliculogenesis by dysregulating the PI3K signaling pathway, the additive effects of other factors/signaling pathways in contribution to PI3K signaling cannot be discounted and should be examined in future studies.
Based on the selective ability of DEHP to aberrantly alter the mRNA levels of PI3K signaling factors that are associated with early folliculogenesis and that several PI3K signaling factors are translationally and posttranslationally regulated, we decided to expand on the gene expression findings by measuring protein levels of pAKT and PTEN. These proteins were chosen because they serve as integral regulators of PI3K signaling and are associated with primordial follicle recruitment. Specifically, the phosphatase PTEN negatively regulates the PI3K-induced activation of the secondary messenger AKT; thus, PTEN lies upstream of AKT [45, 46]. Further, the loss of Pten in oocytes leads to global acceleration of all primordial follicles in the ovary via increased AKT-regulated PI3K signaling [44]. In the 10-day dosing study, DEHP exposure increased the staining of pAKT and decreased the staining of PTEN in the whole ovary at all selected doses of DEHP when compared to the vehicle control group. In confirmation that these effects were evident in the follicles of interest, DEHP exposure increased pAKT staining in primordial follicles at all selected doses of DEHP and in primary follicles at the 20- and 200-μg/kg/day doses and the 20-mg/kg/day dose when compared to the vehicle control group. Thus, the mRNA and protein data provide evidence that DEHP may first downregulate PTEN leading to the overactivation of the PI3K/pAKT signaling cascade. Similar effects of DEHP-induced dysregulation of PI3K signaling have been noted in other tissues [64–66]. Our data expand on these previous findings by showing DEHP-induced PI3K dysregulation in the ovary. Additionally, our data are consistent with our follicle count data in that increased pAKT and decreased PTEN would favor an environment conducive for primordial follicle recruitment. Furthermore, these data are fairly consistent with the mRNA data; however, not all of the gene expression changes correlate with the phenotypic changes in folliculogenesis. Additionally, the effects of DEHP exposure on gene expression exhibit a nonmonotonic dose response. Thus, the protein data are more definitive than the mRNA data in that all doses that cause the acceleration of primordial follicle recruitment contain significant protein data.
Collectively, these data provide evidence that relatively low levels of DEHP alter estrous cyclicity and accelerate primordial follicle recruitment, which potentially can interfere with normal reproductive function and appropriate timing of reproductive senescence. Further, our data show that the DEHP-induced defects in early folliculogenesis may be mediated by dysregulation of the PI3K signaling pathway. This study is novel because we provide mechanistic evidence by which a ubiquitous endocrine-disrupting chemical disrupts folliculogenesis, an essential ovarian process necessary for normal reproductive and nonreproductive health. However, further work is needed to completely elucidate the mechanism of DEHP-induced acceleration of primordial follicle recruitment. Additionally, although early folliculogenesis is aberrantly regulated in our dosing windows, it is of great interest to investigate if the primordial follicle pool is depleted at an accelerated rate in response to DEHP exposure over an extended period of time or if DEHP exposure compromises oocyte quality in accelerated follicles, thus leading to the potential of premature ovarian failure and infertility.
ACKNOWLEDGMENT
The authors thank Dr. Arnon Gal for assistance with immunohistochemistry, Dr. Liying Gao, and all members of Dr. Flaws' laboratory for dosing and technical assistance.
Footnotes
Supported by the National Institute on Environmental Health grant R01ES019178 and an Interdisciplinary Environmental Toxicology Program Fellowship (P.R.H.).
REFERENCES
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for di(2-ethylhexyl)phthalate (DEHP). In: Production, Import, Use, and Disposal Potential for Human Exposure. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2002:176–213. [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet. 2012;29:773–777. doi: 10.1007/s10815-012-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev. 2009;12:225–249. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Shaffer CBCC, Smyth HF., Jr. Acute and subacute toxicity of di(2-ethylhexyl)phthalate with note upon its metabolism J Ind Hyg Toxicol 1945. 27. [Google Scholar]
- Kaul AF, Souney PF, Osathanondh R. A review of possible toxicity of di-2-ethylhexylphthalate (DEHP) in plastic intravenous containers: effects on reproduction. Drug Intell Clin Pharm. 1982;16:689–692. doi: 10.1177/106002808201600908. [DOI] [PubMed] [Google Scholar]
- Laskey JW, Berman E. Steroidogenic assessment using ovary culture in cycling rats: effects of bis(2-diethylhexyl)phthalate on ovarian steroid production. Reprod Toxicol. 1993;7:25–33. doi: 10.1016/0890-6238(93)90006-s. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Hirosawa N, Yano K, Suzuki Y, Sakamoto Y. Endocrine disrupting effect of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics. 2006;6:958–971. doi: 10.1002/pmic.200401344. [DOI] [PubMed] [Google Scholar]
- Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194:603–609. doi: 10.1677/JOE-07-0238. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhang Y, Pei X, Duan Z. Effects of di-(2-ethylhexyl) phthalate exposure on reproductive development and PPARs in prepubertal female rats Wei Sheng Yan Jiu 2011. 40 688 692, 697 [PubMed] [Google Scholar]
- Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242:224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect. 1998;106:551–557. doi: 10.1289/ehp.98106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ. Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect. 1997;105:734–742. doi: 10.1289/ehp.97105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, Matthews JB. Examination of the in vitro and in vitro estrogenic activities of eight commercial phthalate esters. Toxicol Sci. 1998;46:282–293. doi: 10.1006/toxs.1998.2505. [DOI] [PubMed] [Google Scholar]
- Kruger T, Long M, Bonefeld-Jorgensen EC. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 2008;246:112–123. doi: 10.1016/j.tox.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Barbieri RLSJI. Yen and Jaffe's Reproductive Endocrinology Philadelphia, Pennsylvania: Elsevier; 2009. [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Kim JY. Control of ovarian primordial follicle activation. Clin Exp Reprod Med. 2012;39:10–14. doi: 10.5653/cerm.2012.39.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356:24–30. doi: 10.1016/j.mce.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Takai R, Hayashi S, Kiyokawa J, Iwata Y, Matsuo S, Suzuki M, Mizoguchi K, Chiba S, Deki T. Collaborative work on evaluation of ovarian toxicity. 10) Two- or four-week repeated dose studies and fertility study of di-(2-ethylhexyl) phthalate (DEHP) in female rats J Toxicol Sci 2009. 34( suppl 1): Sp111 Sp119 [DOI] [PubMed] [Google Scholar]
- Long Jae HM. The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922:1–148. [Google Scholar]
- Hartman CG. Some new observations on the vaginal smear of the rat. Yale J Biol Med. 1944;17:99–112. [PMC free article] [PubMed] [Google Scholar]
- Everett JW. Neurobiology of reproduction in the female rat. A fifty-year perspective. Monogr Endocrinol. 1989;32:1–133. [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Stokoe D. The phosphoinositide 3-kinase pathway and cancer. Expert Rev Mol Med. 2005;7:1–22. doi: 10.1017/S1462399405009361. [DOI] [PubMed] [Google Scholar]
- Masters RA, Crean BD, Yan W, Moss AG, Ryan PL, Wiley AA, Bagnell CA, Bartol FF. Neonatal porcine endometrial development and epithelial proliferation affected by age and exposure to estrogen and relaxin. Domest Anim Endocrinol. 2007;33:335–346. doi: 10.1016/j.domaniend.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- Paulose T, Hernandez-Ochoa I, Basavarajappa MS, Peretz J, Flaws JA. Increased sensitivity of estrogen receptor alpha overexpressing antral follicles to methoxychlor and its metabolites. Toxicol Sci. 2011;120:447–459. doi: 10.1093/toxsci/kfr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18:58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li L, Qin XS, Zhou Y, Zhang XF, Wang LQ, De Felici M, Chen H, Qin GQ, Shen W. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ Mol Mutagen. 2014;55:343–353. doi: 10.1002/em.21847. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen. 2013;54:354–361. doi: 10.1002/em.21776. [DOI] [PubMed] [Google Scholar]
- Moyer B, Hixon ML. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP) Reprod Toxicol. 2012;34:43–50. doi: 10.1016/j.reprotox.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–2954. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezele PR, Nilsson EE, Skinner MK. Insulin but not insulin-like growth factor-1 promotes the primordial to primary follicle transition. Mol Cell Endocrinol. 2002;192:37–43. doi: 10.1016/s0303-7207(02)00114-4. [DOI] [PubMed] [Google Scholar]
- Rogers R, Ouellet G, Brown C, Moyer B, Rasoulpour T, Hixon M. Cross-talk between the Akt and NF-kappaB signaling pathways inhibits MEHP-induced germ cell apoptosis. Toxicol Sci. 2008;106:497–508. doi: 10.1093/toxsci/kfn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Qin Q, Zhang W, Zhang Y, Zheng H, Liu C, Yang Y, Xiong W, Yuan J. Activation of the PI3K-AKT-mTOR signaling pathway promotes DEHP-induced Hep3B cell proliferation. Food Chem Toxicol. 2013;59:325–333. doi: 10.1016/j.fct.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zheng J, Xiao X, Zheng S, Dong K, Liu J, Wang Y. Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol Rep. 2010;23:129–139. [PubMed] [Google Scholar]