Abstract
Premise of the study:
Here we present a series of protocols for RNA extraction across a diverse array of plants; we focus on woody, aromatic, aquatic, and other chemically complex taxa.
Methods and Results:
Ninety-one taxa were subjected to RNA extraction with three methods presented here: (1) TRIzol/TURBO DNA-free kits using the manufacturer’s protocol with the addition of sarkosyl; (2) a combination method using cetyltrimethylammonium bromide (CTAB) and TRIzol/sarkosyl/TURBO DNA-free; and (3) a combination of CTAB and QIAGEN RNeasy Plant Mini Kit. Bench-ready protocols are given.
Conclusions:
After an iterative process of working with chemically complex taxa, we conclude that the use of TRIzol supplemented with sarkosyl and the TURBO DNA-free kit is an effective, efficient, and robust method for obtaining RNA from 100 mg of leaf tissue of land plant species (Embryophyta) examined. Our protocols can be used to provide RNA of suitable stability, quantity, and quality for transcriptome sequencing.
Keywords: aquatic plants, aromatic plants, extraction methods, RNA, transcriptome, woody plants
Next-generation sequencing (NGS) offers numerous research opportunities to the plant systematics and evolutionary biology communities (e.g., Godden et al., 2012; Strickler et al., 2012; Soltis et al., 2013). To apply NGS to whole transcriptome sequencing, high-quality RNA must be reliably obtained, often from diverse taxa. However, high levels of compounds such as flavonoids, tannins, waxes, and other secondary metabolites found in the tissues of aromatic, woody, and aquatic plants can make it difficult to extract RNA in sufficient quantity and quality for NGS. To circumvent these problems, many different methods have been developed for RNA extraction from plant tissues (e.g., Johnson et al., 2012; Yockteng et al., 2013; Zhu et al., 2013).
During our involvement in the 1KP project (One Thousand Plants Initiative, see www.onekp.com), we developed two RNA isolation protocols that were used to obtain high-quality and -quantity RNA from a diverse set of plant species. Here we present two protocols (Options 1 and 2) in detail and compare their success rate with a third protocol (Option 3) that is standardly used for many species (e.g., Buggs et al., 2009; Johnson et al., 2012). An earlier version of our Option 1 can be found in the appendix of Johnson et al. (2012) in Protocol 14, but is presented here with further refinements that improve RNA quality and quantity. Option 2 is presented for the first time.
In this manuscript, we quantify RNA extraction success among the methods with Bioanalyzer metrics, not transcriptome sequencing success as was done in Johnson et al. (2012), by quantifying number of scaffolds and resulting assemblies. An extraction was considered successful if it met the quantity and quality set by the 1KP consortium agreement: ≥30 μg of total RNA, RNA integrity number (RIN) higher than 5, and rRNA ratio (26S/18S) greater than 1. Although Johnson et al. (2012) conclude that transcriptome assemblies were mostly dependent on the NGS sequencing platform, RNA quality (RIN and optical density [OD] ratios) and quantity also had significant effects. The two preferred protocols presented here result in RNA isolations of the quality and quantity required for transcriptome sequencing in a wide range of plant taxa.
METHODS AND RESULTS
Selection of material
The plants selected for the 1KP project were meant to circumscribe the entire green plant clade (Viridiplantae), including chlorophyte algae (see Johnson et al., 2012; Matasci et al., 2014). RNA from the majority of taxa in the 1KP project were extracted with protocols outlined in Johnson et al. (2012). The protocols presented here were developed to handle samples for which those methods had failed to provide good-quality RNA extractions. Most are angiosperms and many have characteristics (aromatic, woody, aquatic) known to present challenges to RNA extraction (Appendix 1).
Overview of protocols
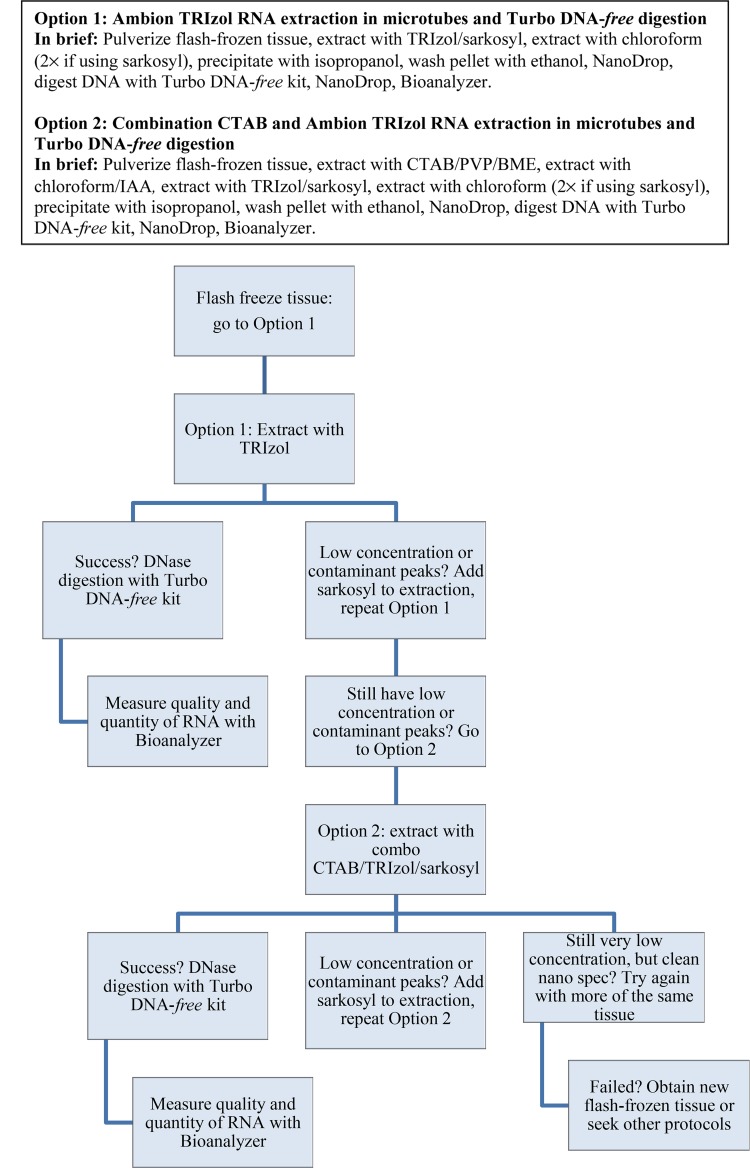
Option 1 is the manufacturer’s protocol for RNA extraction with TRIzol (Ambion, Life Technologies, Carlsbad, California, USA), with the addition of sarkosyl to the extraction solution. Option 2 combines traditional cetyltrimethylammonium bromide (CTAB) extraction (Doyle and Doyle, 1987) followed by the use of TRIzol with sarkosyl (i.e., CTAB followed by Option 1). Both Option 1 and 2 used the TURBO DNA-free kit (Ambion) for DNA digestion, which proved to be superior for RNA stability compared to the on-column digestion (Appendix 2 for taxa that degraded [gray cells]). Option 3 combines the CTAB extraction method followed by the use of half of the QIAGEN RNeasy Plant Mini Kit for on-column DNA digestion (QIAGEN, Valencia, California, USA). Option 3 was the protocol used during the initial phase of the 1KP project, and in other projects in our laboratory (e.g., Buggs et al., 2009), but after multiple failures, Options 1 and 2 were developed to deal with difficult plant materials. These methods are briefly outlined below, and detailed bench-ready protocols can be found in Appendices 3–6). A flow chart can be found in Fig. 1. Option 1 is recommended as a starting point when working with unstudied taxa because it has fewer steps. If this fails, researchers should then move to Option 2. The protocols here can be adapted to processing few samples or relatively high-throughput extractions of 12 to 24 samples at a time. The use of 2-mL microcentrifuge tubes and an automatic shaker for tissue pulverization is ideal (see Appendix 3 for details on tissue collection and processing). These methods can still be used with tissue pulverization in a mortar and pestle if an automatic shaker is not available or the tissue will not pulverize by beads alone, then transferred to the 2-mL tubes (i.e., some Poaceae and succulent species).
Fig. 1.
Flow chart describing the iterative process to successfully obtain high-quality and -quantity RNA using Option 1 and Option 2. (Option 3 is excluded, as it is not recommended.)
The addition of sarkosyl in the TRIzol extraction step in either Option 1 or 2 was integral for improving quality and yield in some taxa, but was not required for all taxa (data not shown). The addition of sarkosyl to lysis solutions is not new; it has been used in both plant and animal nucleic acid and protein isolations since the 1970s (Kingston, 2010) and is still used today for various applications (Huang et al., 2012). It is commonly recommended as an addition to isolation buffers containing guanidine (Kingston, 2010), an ingredient in the TRIzol solution. The addition of sarkosyl to the Ambion TRIzol extraction kit was used to deal with plants with high amounts of organic compounds or other complexities (typically indicated by a brown color in the aqueous layer). After centrifugation, the lightweight sarkosyl layer rested at the interface between the upper aqueous solution and the lower organic layer. Following TRIzol and sarkosyl extraction, a 100% chloroform extraction was always done once, sometimes twice, depending on whether a whitish interface was present between the organic and the aqueous layers.
RNA precipitation and pelleting
In all of the options, the method of RNA precipitation was similar (in Option 3, 5 M NaCl was also added). The tube containing the aqueous phase was filled with 100% isopropanol at room temperature, gently inverted, then incubated at −20°C for 10 min for Options 1 and 2, and 20 min for Option 3. Overnight incubation did not increase the yield, and the best RNA was obtained when no cloudiness or white precipitate was formed after addition of isopropanol. After incubation, in Options 1 and 2 the samples were centrifuged at 4°C for 20 min to pellet the RNA, typically producing pellets of 0.25–1 cm long (the expected size as indicated by Ambion). The RNA was not pelleted in Option 3. The pellet was washed with 75% ethanol and air-dried for no longer than 10 min (otherwise resuspension was difficult), and resuspended in RNase-free water by incubation at 37°C on an orbital shaker for 10 min. If the pellet was not dissolved by the end of this incubation period, in most cases, it was not worth proceeding, and the process was started from the beginning with a new sample (Fig. 1). For Option 3, if the NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA) gave poor results (data not shown), the sample was also discarded. Therefore, these failed attempts do not have Bioanalyzer metrics as others listed in Appendix 2.
Extraction success: Quality, quantity, and stability
Extraction success was measured from Bioanalyzer metrics for the quality and quantity of RNA isolated (Agilent Technologies, Santa Clara, California, USA) (Fig. 2, Appendix 2, and Appendix S1 (107.1KB, pdf) for those that were analyzed with the Bioanalyzer). Over the course of this study, 382 separate RNA extractions were attempted from leaf tissues, of which 138 were successful (Table 1). Many of the failures were due to the iterative process of developing the protocols presented here and multiple unsuccessful attempts with Option 3 before alternatives were sought. Table 1 summarizes the levels of extraction success of each method during this process, and the number of taxa for which RNA was successfully extracted in the end. Using our methods, we finally successfully extracted RNA from 77 unique taxa of 91 attempted (85%). Option 1 was tested on 74 of the 91 taxa in this data set, Option 2 was tested on 41 of the 91 taxa, Option 3 was used on 68 out of 91 taxa. Not all methods were tried on all taxa, as the goal of our work was to obtain pure RNA for the 1KP project as quickly as possible, not to test all methods across all taxa. Options 1 and 2 often succeeded with material that failed to yield RNA with Option 3 (Appendix 2). Nine taxa were unsuccessful in extraction with any of the three methods, perhaps due to the chemical composition of the plant, or perhaps tissue quality (Appendix 2). Two taxa yielded good-quality and -quantity RNA, but the library construction failed at BGI (Shenzen, China) (for details see Johnson et al. [2012] and Appendix 2). Most of the extractions that were partially successful with Option 3 were of low RNA quality, and often degraded (gray cells in Appendix 2), but the nondegraded isolations from Option 3 were sometimes ultimately suitable for transcriptome sequencing (Appendix 2).
Fig. 2.
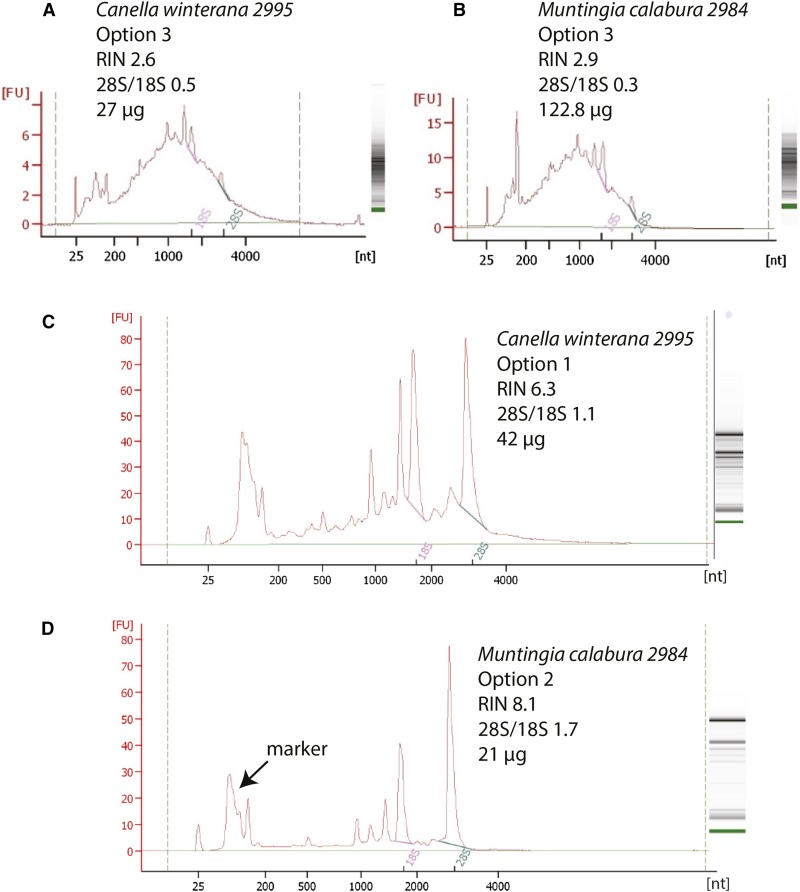
Examples of Agilent 2100 Bioanalyzer spectra of total RNA showing improvement with Options 1 and 2 compared to Option 3. Each graph shows the intensity of the peaks of the ribosomal RNA subunits: nuclear large-28S, small-18S, cytoplasmic, mitochondrial, and chloroplastic (smaller subunits). The electrophoretic gel for each sample is shown to the right indicating the subunit bands or degradation (i.e., smear). nt = number of estimated nucleotides based on ladder; FU = fluorescence unit (i.e., intensity of peak). (A) Degraded ribosomal RNA subunits of Canella winterana (L.) Gaertn. extracted with Option 3 that resulted in an estimation of 27 μg of RNA, but the subunits are degraded. (B) A second example using Option 3, Muntingia calabura L., also shows an inflated quantity reading with degraded subunits. (C) Canella winterana, extracted with Option 1, indicating peaks for intact ribosomal RNA subunits. (D) Muntingia calabura extracted with Option 2.
Table 1.
Success of RNA extraction for each method. Success is defined by the Bioanalyzer results and final quantity of pure RNA that was sequenced. Final concentration was estimated from the Bioanalyzer and the known volume of the final extraction. Some samples were extracted multiple times and pooled (i.e., “repeats”). Note that most samples were initially tried with Option 3; those that failed were then attempted with Options 1 and 2. Thus, not all samples were extracted with all options.
| Method | No. of successful RNA extractions/total RNA extractions tried | % of successful RNA extractions | No. of taxa with successful RNA extractions/tried taxa | % of taxa with successful RNA extractions | Avg. μg of total RNA |
| Option 1: TRIzol/TURBO DNA-free | 56/119 | 47 | 34/74 | 46 | 51.1 |
| Option 2: CTAB/ TRIzol/TURBO DNA-free | 43/63 | 68 | 31/41 | 76 | 77.6 |
| Option 3: CTAB/QIAGEN Kit/on-column digestion | 39/200 | 20 | 18/68 | 26 | 33.8 |
| Overall totals | 138/382 | 36 | 83/183 (without repeats 77/91) | 45 (without repeats 85) | Total average 54.2 |
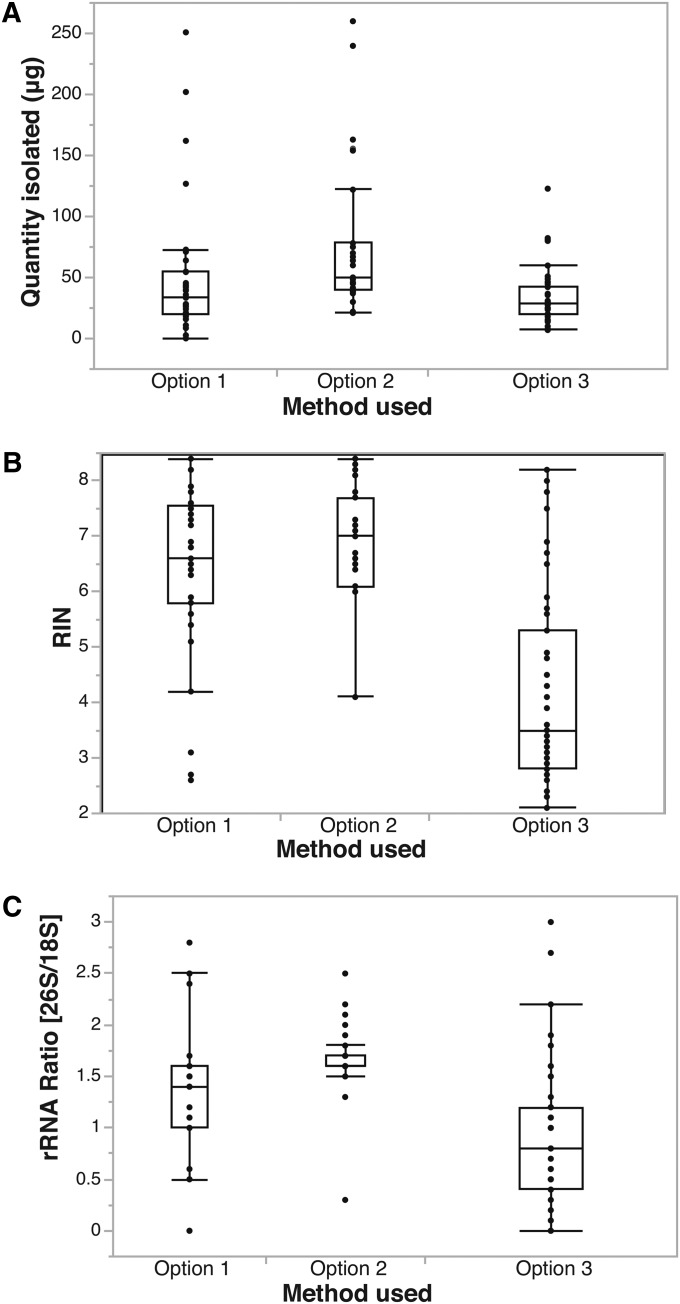
Bioanalyzer metrics for the total quantity of RNA isolated, RIN, and OD ratio were not the same across the different extraction options (Fig. 3). Given that the data set was compiled as trial and error, but not formally designed for statistical measures, only the distribution of the data is shown (Appendix S1 (107.1KB, pdf) ). For the purposes of the 1KP project, 30 μg of total RNA was requested for each taxon from BGI for a full transcriptome sequence (Johnson et al., 2012). However, today, 30 μg of RNA is not necessarily needed to generate transcriptomes. For the 18 taxa successfully extracted with Option 3 in this study, multiple extractions (2–4) had to be done and pooled to reach the desired amount (using ∼100 mg of frozen tissue per extraction). Out of the successful extractions made with Options 1 and 2, only 33% of the taxa gave less than 30 μg in the first extraction attempt, and at most two extractions were needed to obtain the quantity desired. The majority of the time for Option 2 (67% of the extractions), the quantity averaged 77 μg of RNA from only 100 mg of tissue in the first extraction attempt, so no pooling was needed. The maximum amount was 250 μg of RNA in one extraction.
Fig. 3.
Distribution of RNA quantity and quality. Box plots illustrate (A) total micrograms of RNA isolated by each method, (B) RNA integrity number resulting from each method, and (C) rRNA (OD) ratio resulting from each method. The error bars and the boxes indicate the quantiles of the data (JMP Pro 11.0, SAS Institute).
To NanoDrop or not
NanoDrop or similar equipment for RNA (or DNA) concentration measures can be used to obtain an estimate for total amount of nucleic acids. The NanoDrop cannot detect the presence of intact ribosomal-RNA subunits, and therefore is not able to detect if the RNA is degraded. Additionally, the NanoDrop cannot reliably measure the concentration in impure samples from chemically complex plant extractions, giving an inflated reading. However, as most laboratories do not have easy access or funds to use a Bioanalyzer, the use of the NanoDrop (or Qubit, Life Technologies) is still a laboratory necessity. We advise that using spectrophotomic methods such as NanoDrop or fluorescent-dye methods (e.g., Qubit) are rough and easy ways to assess if the samples are “on track,” but should not be used as an absolute measure for concentration and cannot give a reading of the quality of the ribosomal RNA subunits to prepare for transcriptome sequencing. Low-quality RNA can still be sequenced, but the resulting sequences will be poor, and only determined so after the transcriptome is assembled (R. Cronn, personal communication).
RNA stability: Storing and shipping pure RNA
As with most molecular biological materials, a freeze/thaw process can damage a sample. We found that RNA samples that were not digested immediately with DNase could be stored at 4°C for 2 to 3 d before digestion with no apparent change in quality (data not shown). Once the DNA was digested, a 3-μL aliquot was run on the Agilent 2100 Bioanalyzer, and the remaining samples were placed at −80°C for storage until it was mailed. The pure RNA was sent in the mail for library construction and sequencing after drying down onto specially coated tubes (i.e., GenVault, now renamed as GenTegra; IntegenX, Pleasanton, California, USA) that inhibit RNase activity and stabilize the RNA at room temperature. In general, samples extracted with Options 1 and 2 were more likely to make it through storage, GenVault shipping, and resuspension at BGI than were samples extracted with Option 3. The Option 3 samples were often degraded beyond use upon resuspension at the BGI sequencing facility (Fig. 2A, B and Appendix 2 [gray cells]).
CONCLUSIONS
Use of the TRIzol supplemented with sarkosyl followed by removal of DNA with the TURBO DNA-free kit (Option 1) is an efficient and effective means of extracting RNA from a diverse array of plants, especially those that are woody, aromatic, or aquatic. With the addition of the traditional CTAB method prior to the TRIzol (Option 2), even the most stubborn taxa were mostly successful and gave consistent RNA quality measures. Option 3, which has been used successfully in many laboratories (including our own, e.g., Buggs et al., 2009; Johnson et al., 2012) for RNA isolation, is not the most efficient or robust method for obtaining high-quantity and -quality RNA in transcriptomics across the Embryophyta. Despite the success of the protocols described here, our methods were not successful for some plants that contain high amounts of mucilage, such as Opuntia sp.
Supplementary Material
Appendix 1.
List of taxa from which RNA was extracted. Voucher information for the accessions includes the collector, collection number, herbarium acronym, collection location, and georeferenced coordinates of the collection site.a
| Taxon | Family | Voucher ID | Herbarium | Collection information | Geographic coordinates |
| Agrimonia eupatoria L. | Rosaceae | Chase 38775 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Alluaudiopsis marnieriana Rauh | Didiereaceae | Soltis and Miles 2981 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Alnus serrulata (Aiton) Willd. | Betulaceae | Soltis and Miles 2964 | FLAS | U of Florida campus | 29.64, −82.34 |
| Amelanchier canadensis (L.) Medik. | Rosaceae | Chase 38778 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Anisacanthus quadrifidus (Vahl) Nees | Acanthaceae | Soltis and Miles 2970 | FLAS | U of Florida campus | 29.64, −82.34 |
| Antirrhinum majus L. | Plantaginaceae | Soltis and Miles 2963 | FLAS | U of Florida campus | 29.65, −82.34 |
| Aristida stricta Michx. | Poaceae | Jackie Rice JDR10 | FLAS | Morningside Nature Center, Gainesville, FL | 29.65, −82.27 |
| Aruncus dioicus (Walter) Fernald | Rosaceae | Chase 38772 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Astilbe chinensis (Maxim.) Franch. & Sav. | Saxifragaceae | Chase 38784 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Bacopa caroliniana (Walter) B. L. Rob. | Plantaginaceae | Soltis and Miles 2974 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Bischofia javanica Blume | Phyllanthaceae | Soltis and Miles 2978 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Bixa orellana L. | Bixaceae | Soltis and Miles 2985 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Boykinia jamesii var. heucheriformis (Rydb.) Engl. | Saxifragaceae | Chase 38782 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Canella winterana (L.) Gaertn. | Canellaceae | Soltis and Miles 2995 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Castanea pumila (L.) Mill. | Fagaceae | Soltis and Miles 2977 | FLAS | U of Florida campus | 29.64, −82.34 |
| Ceratopteris sp. Brongn. | Pteridaceae | Soltis and Miles 3005 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Cercocarpus ledifolius Nutt. | Rosaceae | Chase 38780 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Cicerbita plumieri Kirschl. | Asteraceae | Edward Schilling 11-48 | TENN | U of Tennessee campus | 35.95, −83.92 |
| Corynocarpus laevigatus J. R. Forst. & G. Forst. | Corynocarpaceae | Soltis and Miles 2986 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Cotoneaster transcaucasicus Pojark. | Rosaceae | Chase 38779 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Cryptocarya alba (Molina) Looser | Lauraceae | Soltis and Miles 2998 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Deutzia scabra Thunb. | Hydrangeaceae | Soltis and Miles 2965 | FLAS | U of Florida campus | 29.64, −82.34 |
| Disporopsis pernyi (Hua) Diels | Asparagaceae | Mike Heaney JMH2546 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Dryas octopetala L. | Rosaceae | Chase 38781 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Epifagus virginiana (L.) W. P. C. Barton | Orobanchaceae | Soltis and Miles 2996 (Paul Manos 1800) | DUKE | Duke University campus | 36.00, −78.94 |
| Forestiera segregata (Jacq.) Krug & Urb. | Oleaceae | Soltis and Miles 2969 | FLAS | U of Florida campus | 29.64, −82.34 |
| Frullania sp. Raddi | Jubulaceae | Von Konrat 10021 | F | Chile, NW shore of Isla Londonderry, SW arm of Bahia Isabel | −54.98, −70.87 |
| Geum quellyon Sweet | Rosaceae | Chase 38770 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Gunnera chilensis Lam. | Gunneraceae | Soltis and Miles 2966 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Gunnera manicata Linden ex André | Gunneraceae | Soltis and Miles 2938 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Hilleria latifolia (Lam.) H. Walter | Phytolaccaceae | Soltis and Miles 2976 | FLAS | U of Florida campus | 29.64, −82.34 |
| Hydrocotyle umbellata L. | Araliaceae | Soltis and Miles 2959 | FLAS | U of Florida campus | 29.64, −82.34 |
| Illicium floridanum J. Ellis | Schisandraceae | Soltis and Miles 2960 | FLAS | Gainesville, FL, home garden; Soltis | 29.67, −82.36 |
| Kerria japonica (L.) DC. | Rosaceae | Chase 38777 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Kigelia africana (Lam.) Benth. | Bignoniaceae | Soltis and Miles 2992 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Kirkia wilmsii Engl. | Kirkiaceae | Soltis and Miles 2982 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Krameria lanceolata Torr. | Krameriaceae | Soltis and Miles 2991 | FLAS | Rte. 24, 0.5 mi. W of Alachua Co. line; N side of rd.; sandhill | 29.46, −82.61 |
| Lachnanthes caroliniana (Lam.) Dandy | Haemodoraceae | Soltis and Miles 2988 | FLAS | Rt 24, N of roadside, 10 mi W of Alachua Co. line | 29.41, −82.67 |
| Lactuca graminifolia Michx. | Asteraceae | Edward Schilling LG-1 | TENN | U of Tennessee campus | 35.95, −83.92 |
| Lagerstroemia indica L. | Lythraceae | Soltis and Miles 2971 | FLAS | U of Florida campus | 29.64, −82.34 |
| Licania michauxii Prance | Chrysobalanaceae | Soltis and Miles 2990 | FLAS | Gainesville, FL, home garden; Judd | 29.57, −82.42 |
| Lindera benzoin (L.) Blume | Lauraceae | Soltis and Miles 2968 | FLAS | U of Florida campus | 29.64, −82.34 |
| Malus baccata (L). Borkh. var. jackii Borkh. | Rosaceae | Chase 38773 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Mammea americana L. | Calophyllaceae | Soltis and Miles 3003 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Melia azedarach L. | Meliaceae | Soltis and Miles 2961 | FLAS | U of Florida campus | 29.64, −82.35 |
| Michelia maudiae Dunn | Magnoliaceae | Soltis and Miles 2954 | FLAS | U of Florida campus | 29.64, −82.34 |
| Micranthes geum (L.) Small | Saxifragaceae | Chase 38790 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Microtea debilis Sw. | Phytolaccaceae | Soltis and Miles 2997 | FLAS | University of Cambridge campus | 52.20, −0.116 |
| Muntingia calabura L. | Muntingiaceae | Soltis and Miles 2984 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Myrica pumila (Michx.) Small | Myricaceae | Jackie Rice JDR9 | FLAS | Morningside Nature Center, Gainesville, FL | 29.65, −82.27 |
| Nandina domestica Thunb. | Berberidaceae | Soltis and Miles 2972 | FLAS | U of Florida campus | 29.64, −82.34 |
| Nolina bigelovii (Torr.) S. Watson | Asparagaceae | Mike Heaney JMH2984 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Opuntia austrina Small | Cactaceae | Lucas Majure LCM_3450 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Opuntia pusilla (Haw.) Haw. | Cactaceae | Lucas Majure LCM_753 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Peliosanthes minor Yamam. | Asparagaceae | Mike Heaney JMH2549 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Peltoboykinia watanabei (Yatabe) H. Hara | Saxifragaceae | Chase 38787 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Persea borbonia (L.) Spreng. | Lauraceae | Soltis and Miles 2980 | FLAS | U of Florida campus | 29.64, −82.34 |
| Philadelphus inodorus L. | Hydrangeaceae | Soltis and Miles 2953 | FLAS | U of Florida campus | 29.64, −82.34 |
| Phoradendron leucarpum (Raf.) Reveal & M. C. Johnst. | Santalaceae | Soltis and Miles 2957 | FLAS | U of Florida campus | 29.64, −82.34 |
| Physocarpus opulifolius (L.) Maxim. | Rosaceae | Chase 38776 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Podostemum sp. Michx. | Podostemaceae | Soltis and Miles 2994 | FLAS | Massachusetts | NA |
| Pogostemon sp. Desf. | Lamiaceae | Grant Godden GGT4 | FLAS | Gainesville, FL, home garden; Godden | 29.65, −82.31 |
| Poliomintha bustamanta B. L. Turner | Lamiaceae | Grant Godden GGT1 | FLAS | Gainesville, FL, home garden; Godden | 29.65, −82.31 |
| Polypremum procumbens L. | Tetrachondraceae | Soltis and Miles 2989 | FLAS | Gainesville, FL, home garden; Judd | 29.57, −82.42 |
| Prunus prostrata Labill. | Rosaceae | Chase 38785 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Pteris ensiformis Burm. f. | Pteridaceae | Soltis and Miles 3001 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Punica granatum L. | Lythraceae | Soltis and Miles 2973 | FLAS | U of Florida campus | 29.64, −82.34 |
| Pyrus calleryana Decne. | Rosaceae | Chase 38791 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Rhamnus caroliniana Walter | Rhamnaceae | Soltis and Miles 2952 | FLAS | U of Florida campus | 29.64, −82.34 |
| Rivina humilis L. | Phytolaccaceae | Soltis and Miles 2975 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Rodgersia podophylla A. Gray | Saxifragaceae | Chase 38783 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Ruellia brittoniana Leonard | Acanthaceae | Soltis and Miles 2962 | FLAS | Gainesville, FL, home garden; Soltis | 29.67, −82.36 |
| Salvinia sp. Ség. | Salviniaceae | Soltis and IEJ-T 3013 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Sambucus canadensis L. | Adoxaceae | Soltis and Miles 2955 | FLAS | U of Florida campus | 29.64, −82.34 |
| Sanguisorba minor Scop. | Rosaceae | Chase 38771 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Saxifraga geum L. var. gracilis | Saxifragaceae | Chase 38790 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Schlegelia parasitica (Sw.) Miers ex Griseb. | Schlegeliaceae | Soltis and Miles 2983 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Sorbus koehneana C. K. Schneid. | Rosaceae | Chase 38774 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Sprekelia formosissima (L.) Herb. | Amaryllidaceae | Garcia 4381 | K | U of Florida greenhouse | 29.64, −82.34 |
| Strobilanthes dyerianus | Acanthaceae | Soltis and Miles 2958 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Talinum sp. Adans. | Talinaceae | Soltis and Miles 2979 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Tellima breviflora Rydb. | Saxifragaceae | Chase 38789 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Teucrium chamaedrys L. | Lamiaceae | Grant Godden GGT3 | FLAS | Gainesville, FL, home garden; Godden | 29.65, −82.31 |
| Thymus vulgaris L. | Lamiaceae | Grant Godden GGT2 | FLAS | Gainesville, FL, home garden; Godden | 29.65, −82.31 |
| Tiarella polyphylla D. Don | Saxifragaceae | Chase 38786 | K | RBG Kew, Living Collection | 51.47, 0.295 |
| Tragopogon castellanus Levier | Asteraceae | Soltis and Miles 2993 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Traubia modesta Ravenna | Amaryllidaceae | Garcia 3014 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Uniola paniculata L. | Poaceae | Richard Hodel RGJH1001 | FLAS | St. Augustine Beach, FL | 29.86, −81.26 |
| Utricularia sp. L. | Lentibulariaceae | Soltis and Miles 2987 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Zephyranthes citrina Baker | Amaryllidaceae | Garcia 4376 | FLAS | U of Florida greenhouse | 29.64, −82.34 |
| Ziziphus jujuba Mill. | Rhamnaceae | Soltis and Miles 2956 | FLAS | U of Florida campus | 29.64, −82.34 |
Note: NA = not available.
All garden-grown individuals in this study are from unknown seed or stock source and cannot be traced to the possible wild collection site as they are either established landscape plants or part of a permanent greenhouse collection with the main purpose of teaching botany.
Appendix 2.
List of taxa from which RNA was extracted, the method used, and the Agilent 2100 Bioanalyzer quality indicators of the final extraction that was sequenced.a
| Option 1 | Option 2 | Option 3 | ||||||||||
| Family | Taxon | Material isolated (μg) | RINb | rRNA Ratio 26S/18S | Material isolated (μg) | RINb | rRNA Ratio 26S/18S | Material isolated (μg) | RINb | rRNA Ratio 26S/18S | Sequenced? | Voucher ID |
| Acanthaceae | Anisacanthus quadrifidus (Vahl) Nees | 40 | 6.4 | Missing | — | — | — | 46.1 | 3.9 | 1.2 | Option 1 | Soltis and Miles 2970 |
| Acanthaceae | Ruellia brittoniana Leonard | 43 | 7.6 | Missing | — | — | — | 29.2 | 2.4 | 0 | Option 1 | Soltis and Miles 2962 |
| Acanthaceae | Strobilanthes dyerianus | — | — | — | — | — | — | 25* | 4.9 | 2.7 | Option 3 | Soltis and Miles 2958 |
| Adoxaceae | Sambucus canadensis L. | 45.5 | 4.2 | 1.4 | — | — | — | — | — | — | Option 1 | Soltis and Miles 2955 |
| Amaryllidaceae | Sprekelia formosissima (L.) Herb. | — | — | — | 22.1 | 3.2 | 0.2 | — | — | — | Library construction failed | Garcia 4381 |
| Amaryllidaceae | Traubia modesta Ravenna | — | — | — | 40 | 6 | 1.6 | — | — | — | Option 2 | Garcia 3014 |
| Amaryllidaceae | Zephyranthes citrina Baker | — | — | — | 22 | 4.5 | 0.3 | — | — | — | Library construction failed | Garcia 4376 |
| Araliaceae | Hydrocotyle umbellata L. | — | — | — | — | — | — | 30* | 5.9 | 1.1 | Option 3 | Soltis and Miles 2959 |
| Asparagaceae | Disporopsis pernyi (Hua) Diels | 33.7 | 7.2 | 1.4 | — | — | — | — | — | — | Option 1 | Mike Heaney JMH2546 |
| Asparagaceae | Nolina bigelovii (Torr.) S. Watson | 33.45 | 7.9 | 1.5 | — | — | — | — | — | — | Option 1 | Mike Heaney JMH2984 |
| Asparagaceae | Peliosanthes minor Yamam. | 21.1 | 6.9 | 1.2 | — | — | — | — | — | — | Option 1 | Mike Heaney JMH2549 |
| Asteraceae | Cicerbita plumieri Kirschl. | 21 | 7.6 | 2.5 | — | — | — | — | — | — | Option 1 | Edward Schilling 11-48 |
| Asteraceae | Lactuca graminifolia Michx. | 54.85 | 7.6 | 2.4 | — | — | — | — | — | — | Option 1 | Edward Schilling LG-1 |
| Asteraceae | Tragopogon castellanus Levier | 19.3 | 6.3 | 1 | — | — | — | 36.7 | 2.1 | 0 | Option 1 | Soltis and Miles 2993 |
| Berberidaceae | Nandina domestica Thunb. | — | — | — | — | — | — | 30* | 5.7 | 1 | Option 3 | Soltis and Miles 2972 |
| Betulaceae | Alnus serrulata (Aiton) Willd. | — | — | — | — | — | — | 20* | 8.2 | 2.2 | Option 3 | Soltis and Miles 2964 |
| Bignoniaceae | Kigelia africana (Lam.) Benth. | 250.9 | 5.1 | 2.8 | — | — | — | — | — | — | Option 1 | Soltis and Miles 2992 |
| Bixaceae | Bixa orellana L. | — | — | — | — | — | — | 30* | 4.8 | 1.6 | Option 3 | Soltis and Miles 2985 |
| Cactaceae | Opuntia austrina Small | — | — | — | 7.7 | 8.2 | 1.4 | — | — | — | Low conc.; library not tried | Lucas Majure LCM_3450 |
| Cactaceae | Opuntia pusilla (Haw.) Haw. | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Lucas Majure LCM_753 |
| Calophyllaceae | Mammea americana L. | — | — | — | 50 | 6 | 1.6 | — | — | — | Option 2 | Soltis and Miles 3003 |
| Canellaceae | Canella winterana (L.) Gaertn. | 42.0 | 6.3 | 1.1 | — | — | — | 28.5 | 2.6 | 0.5 | Option 1 | Soltis and Miles 2995 |
| Chrysobalanaceae | Licania michauxii Prance | — | — | — | 22 | 7 | 2 | — | — | — | Option 2 | Soltis and Miles 2990 |
| Corynocarpaceae | Corynocarpus laevigatus J. R. Forst. & G. Forst. | 126.8 | 7.3 | Missing | — | — | — | 42.2 | 3.4 | 0.8 | Option 1 | Soltis and Miles 2986 |
| Didiereaceae | Alluaudiopsis marnieriana Rauh | 15.9 | 5.9 | Missing | — | — | — | 27.7 | 3.3 | 0.8 | Option 1 | Soltis and Miles 2981 |
| Fagaceae | Castanea pumila (L.) Mill. | — | — | — | — | — | — | 25* | 6.5 | 1.5 | Option 3 | Soltis and Miles 2977 |
| Gunneraceae | Gunnera chilensis Lam. | — | — | — | — | — | — | 25* | 6.7 | 1.6 | Option 3 | Soltis and Miles 2966 |
| Gunneraceae | Gunnera manicata Linden ex André | — | — | — | — | — | — | 81 | 5.6 | 1 | Option 3 | Soltis and Miles 2938 |
| Haemodoraceae | Lachnanthes caroliniana (Lam.) Dandy | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Soltis and Miles 2988 |
| Hydrangeaceae | Deutzia scabra Thunb. | — | — | — | 1.75 | 2.6 | 0 | 82.3 | 2.9 | 0.5 | Option 3 | Soltis and Miles 2965 |
| Hydrangeaceae | Deutzia scabra Thunb. | Low conc.; library not tried | Soltis and Miles 2965 | |||||||||
| Hydrangeaceae | Philadelphus inodorus L. | — | — | — | — | — | — | 35* | 4.9 | 1.1 | Option 3 | Soltis and Miles 2953 |
| Jubulaceae | Frullania sp. Raddi | — | — | — | 40 | 6.1 | 1.6 | — | — | — | Option 2 | Von Konrat 10021 |
| Kirkiaceae | Kirkia wilmsii Engl. | — | — | — | — | — | — | 13.8* | 6.9 | 0.7 | Option 3 | Soltis and Miles 2982 |
| Krameriaceae | Krameria lanceolata Torr. | — | — | — | — | — | — | 23.8 | 4.5 | 1.1 | Option 3 | Soltis and Miles 2991 |
| Lamiaceae | Pogostemon sp. Desf. | 39.35 | 7.5 | 1.6 | — | — | — | — | — | — | Option 1 | Grant Godden GGT4 |
| Lamiaceae | Poliomintha bustamanta B. L. Turner | — | — | — | 46 | 6 | 1.6 | — | — | — | Option 2 | Grant Godden GGT1 |
| Lamiaceae | Teucrium chamaedrys L. | 72.85 | 7.4 | 1.7 | — | — | — | — | — | — | Option 1 | Grant Godden GGT3 |
| Lamiaceae | Thymus vulgaris L. | 71.1 | 7.5 | 1.4 | — | — | — | — | — | — | Option 1 | Grant Godden GGT2 |
| Lauraceae | Cryptocarya alba (Molina) Looser | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Soltis and Miles 2998 |
| Lauraceae | Lindera benzoin (L.) Blume | 2.7 | 2.6 | 0 | 48.5 | 6 | 1.6 | 27.8 | 2.7 | 0.2 | Option 2 | Soltis and Miles 2968 |
| Lauraceae | Persea borbonia (L.) Spreng. | — | — | — | — | — | — | 18* | 5.3 | 1.2 | Option 3 | Soltis and Miles 2980 |
| Lentibulariaceae | Utricularia sp. L. | — | — | — | — | — | — | 30.9 | 4.3 | 1.2 | Option 3 | Soltis and Miles 2987 |
| Lythraceae | Lagerstroemia indica L. | — | — | — | — | — | — | 7 | 8 | 1.8 | Option 3 | Soltis and Miles 2971 |
| Lythraceae | Punica granatum L. | — | — | — | — | — | — | 20* | 7.5 | 1.9 | Option 3 | Soltis and Miles 2973 |
| Magnoliaceae | Michelia maudiae Dunn | — | — | — | — | — | — | 28.5* | 4.1 | 0.6 | Option 3 | Soltis and Miles 2954 |
| Malvaceae | Grewia occidentalis | 14.04 | 3.2 | 1 | Degraded | Missing | ||||||
| Meliaceae | Melia azedarach L. | 202 | 6.5 | 1.5 | — | — | — | — | — | — | Option 1 | Soltis and Miles 2961 |
| Muntingiaceae | Muntingia calabura L. | — | — | — | 21 | 8.1 | 1.7 | 122.8 | 2.9 | 0.3 | Option 2 | Soltis and Miles 2984 |
| Myricaceae | Myrica pumila (Michx.) Small | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | 14.5 | 3.1 | 1.3 | All extractions failed | Jackie Rice JDR9 |
| Oleaceae | Forestiera segregata (Jacq.) Krug & Urb. | 27.0 | 6.5 | Missing | — | — | — | 24.5 | 3.0 | 0.5 | Option 1 | Soltis and Miles 2969 |
| Orobanchaceae | Epifagus virginiana (L.) W. P. C. Barton | 10.9 | 3.1 | 0.6 | 38.3 | 7.2 | 1.6 | 46.5 | 2.1 | 0 | Option 2 | Soltis and Miles 2996 (Paul Manos 1800) |
| Phyllanthaceae | Bischofia javanica Blume | — | — | — | — | — | — | 10* | 7.8 | 3 | Option 3 | Soltis and Miles 2978 |
| Phytolaccaceae | Hilleria latifolia (Lam.) H. Walter | 24.1 | 5.8 | 0.5 | — | — | — | 35.6 | 2.7 | 0.1 | Option 1 | Soltis and Miles 2976 |
| Phytolaccaceae | Microtea debilis Sw. | 43.9 | 6.3 | 1 | — | — | — | 29.4 | 3.0 | 0.4 | Option 1 | Soltis and Miles 2997 |
| Phytolaccaceae | Rivina humilis L. | 19.45 | 6.6 | 1 | — | — | — | 28.42 | 2.8 | 0.4 | Option 1 | Soltis and Miles 2975 |
| Plantaginaceae | Antirrhinum majus L. | 64.0 | 5.4 | 0.5 | — | — | — | 50.9 | 4.1 | 0.8 | Option 1 | Soltis and Miles 2963 |
| Plantaginaceae | Bacopa caroliniana (Walter) B. L. Rob. | 26.0 | 7.8 | 1.4 | — | — | — | 28.4 | 3.5 | 1.5 | Option 1 | Soltis and Miles 2974 |
| Poaceae | Aristida stricta Michx. | 54.5 | 5.6 | Missing | — | — | — | — | — | — | Option 1 | Jackie Rice JDR10 |
| Poaceae | Uniola paniculata L. | 35.85 | 7.6 | 2.5 | — | — | — | — | — | — | Option 1 | Richard Hodel RGJH1001 |
| Podostemaceae | Podostemum sp. Michx. | — | — | — | 23.9 | 4.7 | 0.8 | 44.7 | 2.1 | 0 | Library construction failed | Soltis and Miles 2994 |
| Pteridaceae | Ceratopteris sp. Brongn. | — | — | — | Missing | Missing | Missing | — | — | — | Option 2 | Soltis and Miles 3005 |
| Pteridaceae | Pteris ensiformis Burm. f. | 28 | 8.4 | 1.6 | — | — | — | — | — | — | Option 1 | Soltis and Miles 3001 |
| Rhamnaceae | Rhamnus caroliniana Walter | 162 | 5.8 | 1.1 | — | — | — | 80 | 3.1 | 0.5 | Option 1 | Soltis and Miles 2952 |
| Rhamnaceae | Ziziphus jujuba Mill. | 0.15 | 2.7 | 0 | 45 | 6.5 | 1.6 | 25* | 3.6 | 0.6 | Option 2 | Soltis and Miles 2956 |
| Rosaceae | Agrimonia eupatoria L. | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Chase 38775 |
| Rosaceae | Amelanchier canadensis (L.) Medik. | — | — | — | 50 | 7 | 1.7 | — | — | — | Option 2 | Chase 38778 |
| Rosaceae | Aruncus dioicus (Walter) Fernald | — | — | — | 122 | 6.4 | 1.6 | — | — | — | Option 2 | Chase 38772 |
| Rosaceae | Cercocarpus ledifolius Nutt. | — | — | — | 40 | 7.1 | 2.5 | — | — | — | Option 2 | Chase 38780 |
| Rosaceae | Cotoneaster transcaucasicus Pojark. | — | — | — | 154 | 8.2 | 1.5 | — | — | — | Option 2 | Chase 38779 |
| Rosaceae | Dryas octopetala L. | — | — | — | 67 | 7.3 | 1.6 | — | — | — | Option 2 | Chase 38781 |
| Rosaceae | Geum quellyon Sweet | — | — | — | 78.2 | 7.7 | 1.6 | — | — | — | Option 2 | Chase 38770 |
| Rosaceae | Kerria japonica (L.) DC. | — | — | — | 260 | 8.4 | 1.8 | — | — | — | Option 2 | Chase 38777 |
| Rosaceae | Malus baccata (L). Borkh. var. jackii Borkh. | — | — | — | 75 | 6.7 | 1.9 | — | — | — | Option 2 | Chase 38773 |
| Rosaceae | Physocarpus opulifolius (L.) Maxim. | — | — | — | 163 | 8.3 | 2.2 | — | — | — | Option 2 | Chase 38776 |
| Rosaceae | Prunus prostrata Labill. | — | — | — | 155.6 | 7.1 | 1.7 | — | — | — | Option 2 | Chase 38785 |
| Rosaceae | Pyrus calleryana Decne. | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Chase 38791 |
| Rosaceae | Sanguisorba minor Scop. | — | — | — | 239.8 | 8.2 | 1.6 | — | — | — | Option 2 | Chase 38771 |
| Rosaceae | Sorbus koehneana C. K. Schneid. | — | — | — | 38.7 | 6.6 | 1.3 | — | — | — | Option 2 | Chase 38774 |
| Salviniaceae | Salvinia sp. Ség. | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Soltis and IEJ-T 3013 |
| Santalaceae | Phoradendron leucarpum (Raf.) Reveal & M. C. Johnst. | — | — | — | 37 | 4.1 | 0.3 | 59.8 | 2.3 | 0 | Option 2 | Soltis and Miles 2957 |
| Saxifragaceae | Astilbe chinensis (Maxim.) Franch. & Sav. | — | — | — | 60 | 7 | 1.6 | — | — | — | Option 2 | Chase 38784 |
| Saxifragaceae | Boykinia jamesii var. heucheriformis (Rydb.) Engl. | — | — | — | 63.9 | 6 | 2.1 | — | — | — | Option 2 | Chase 38782 |
| Saxifragaceae | Micranthes geum (L.) Small | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Chase 38790 |
| Saxifragaceae | Peltoboykinia watanabei (Yatabe) H. Hara | — | — | — | 70 | 7 | 1.7 | — | — | — | Option 2 | Chase 38787 |
| Saxifragaceae | Rodgersia podophylla A. Gray | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Chase 38783 |
| Saxifragaceae | Saxifraga geum L. var. gracilis | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | Tried but failed | All extractions failed | Chase 38790 |
| Saxifragaceae | Tellima breviflora Rydb. | — | — | — | 41.5 | 7.1 | 1.5 | — | — | — | Option 2 | Chase 38789 |
| Saxifragaceae | Tiarella polyphylla D. Don | — | — | — | 30 | 7.8 | 1.6 | — | — | — | Option 2 | Chase 38786 |
| Schisandraceae | Illicium floridanum J. Ellis | — | — | — | — | — | — | 25* | 4.8 | 0.8 | Option 3 | Soltis and Miles 2960 |
| Schlegeliaceae | Schlegelia parasitica (Sw.) Miers ex Griseb. | 18.2 | 8.2 | 1.5 | — | — | — | 7.8 | 2.1 | 0 | Option 1 | Soltis and Miles 2983 |
| Talinaceae | Talinum sp. Adans. | 8.6 | 7.3 | Missing | — | — | — | 15.4 | 2.3 | 0 | Option 1 | Soltis and Miles 2979 |
| Tetrachondraceae | Polypremum procumbens L. | 28.3 | 6.8 | 1.5 | — | — | — | 48.8 | 2.8 | 0.5 | Yes | Soltis and Miles 2989 |
Note: — = method was not tried on the species.
Gray cells are those with degraded RNA and either the library was not attempted or it failed. All samples were extracted from 60 to 100 mg of plant leaf tissue (for aquatics and succulents, 200 mg). The taxa that failed with all attempts were: Agrimonia eupatoria L. (Rosaceae), Cryptocarya alba (Molina) Looser (Lauraceae), Lachnanthes caroliniana (Lam.) Dandy (Haemodoraceae), Micranthes geum (L.) Small (Saxifragaceae), Opuntia pusilla (Haw.) Haw. (Cactaceae), Pyrus calleryana Decne. (Rosaceae), Rodgersia podophylla A. Gray (Saxifragaceae), Salvinia sp. Ség. (Salviniaceae), and Saxifraga geum L. var. gracilis (Saxifragaceae). Failed library construction despite having satisfactory metrics: Sprekelia formosissima (L.) Herb. (Amaryllidaceae) and Zephyranthes citrina Baker (Zephranthaceae).
RIN (RNA integrity number) is a measure from the Agilent 2100 Bioanalyzer that combines concentration and expected presence of subunits.
Marks the samples where an isolate was extracted from two to four extractions and pooled to be sequenced. (Not all extractions in Table 1 are in this table as not all were ran on the Bioanalyzer.)
Appendix 3.
Protocols in brief and notes about tissue collection (except for mucilaginous ones) and RNA handling. Prepared by Ingrid Jordon-Thaden (Soltis Laboratory, Department of Biology, University of Florida, Gainesville, Florida, USA).
Option 1: Ambion TRIzol RNA extraction in microfuge tubes with TURBO DNA-free digestion
In brief: Pulverize flash-frozen tissue, extract with TRIzol (optional sarkosyl), extract with chloroform, precipitate with isopropanol, wash pellet with ethanol, NanoDrop, digest DNA with TURBO DNA-free kit by Ambion, NanoDrop, Agilent 2100 Bioanalyzer.
Option 2 (for more difficult species): Combination CTAB and Ambion TRIzol RNA extraction in microfuge tubes with TURBO DNA-free digestion
In brief: Pulverize flash-frozen tissue, extract with CTAB/PVP/BME, extract with chloroform/IAA, extract with TRIzol/sarkosyl, extract with chloroform, precipitate with isopropanol, wash pellet with ethanol, NanoDrop, digest DNA with TURBO DNA-free kit by Ambion, NanoDrop, Agilent 2100 Bioanalyzer.
Option 3: Combination CTAB, QIAGEN RNeasy Plant Mini Kit, on-column digest
In brief: Grind flash-frozen tissue, extract with CTAB/PVP/BME, extract with chloroform/IAA, extract with phenol/chloroform/IAA repeating until aqueous layer is clear, extract with chloroform/IAA, precipitate with isopropanol and salt, purify with QIAGEN spin columns, digest with QIAGEN on-column kit, NanoDrop, combine aliquots of RNA, Agilent 2100 Bioanalyzer.
Note: High salts (recommended by Ambion: 250 μL of isopropanol, 250 μL of 0.8 M sodium citrate, and 1.2 M NaCl) in precipitation were tried, and the resulting yield or quality was not improved. The addition of sarkosyl significantly improved both yield and quality.
Note: Ideally the best is to do 12 or fewer samples per batch. Times between steps are small and running consecutive batches results in cleaner RNA.
Regarding voucher specimens
Depending on your project, it is imperative to document the collection of an individual or a population with a voucher specimen. A voucher specimen is held in a herbarium where the information is databased and stored permanently. When collecting an individual for RNA, if the specimen is large enough, part of the plant can be frozen, while another part can be pressed in a plant press to produce a herbarium voucher. If the plant is too small for this, then personal discretion must be used to select a representative individual that will stand as the voucher for your frozen sample. If you are collecting populations of individuals, it is general practice to create one voucher per population. The vouchers for this set of data are mainly held at the University of Florida Museum of Natural History, Gainesville, Florida, and Royal Botanic Gardens, Kew, Richmond, Surrey, United Kingdom (Appendix 1).
Choosing an option
Options 1 and 2 eliminate the mortar and pestle by grinding the tissue in 2-mL Eppendorf tubes (Eppendorf, Hauppauge, New York, USA) (if tissue does not pulverize a mortar and pestle may still be needed). The methods illustrated here are almost directly from the Ambion manufacturer’s protocol (Ambion, Life Technologies, Carlsbad, California, USA) and could be used on a 96-well system (after tissue pulverization). Options 1 and 2 worked well for species that proved to be difficult to extract with other methods (i.e., woody, aquatic, aromatic) and were tested on 93 different species. Two different methods for tissue collection are listed below: one directly in Eppendorf tubes, and one in 50-mL Falcon tubes. It is not recommended to use Option 3 for difficult species.
General procedural notes
Workplace preparation: Proper preparation of the laboratory workspace reduced the chances of RNA degradation by RNase contamination in the environment (i.e., RNA-clean, relying heavily on Ambion RNaseZap [Life Technologies]). All homemade buffer solutions were prepared in a clean environment and filtered to ensure they were RNase-free (e.g., with Millipore Stericup [Millipore, Billerica, Massachusetts, USA]). It was noted that 100% chloroform melts most plastics (except for polypropylene, such as Eppendorf Safe-Lock tubes [Eppendorf, Hamburg, Germany], which can handle this), which meant it could not be filtered. Therefore, we maintained a separate chloroform bottle that was kept only for RNA work. We found that we could use plastic pipette tips for 100% chloroform, but only if we moved very quickly to avoid liquid loss.
Tissue homogenization: Tissues were pulverized in 2-mL Eppendorf tubes containing baked zirconia beads, using an automatic shaker (Mini-Beadbeater-96; BioSpec Products, Bartlesville, Oklahoma, USA). We found it was best to use approximately 100 mg of frozen tissue (but as little as 50 mg is possible). For aquatic or succulent plants, we used 200 mg of tissue because the plant cells are mostly filled with water. Because the tubes were held within a block in the shaker, it was necessary to freeze the block at −80°C prior to use to keep the tubes frozen longer. We found that the tubes stayed frozen for 2 min of shaking. If the samples needed further grinding, the block with the tubes was placed back into liquid nitrogen, then shaken again. The pulverized tissue was not allowed to thaw before the buffer was added, as this could have triggered RNA degradation. When the extraction buffer (TRIzol or CTAB) was added to the freshly pulverized tissue, the sample was immediately vortexed to hydrate the tissue completely with buffer. For some samples, it was necessary to use an RNase-clean spatula to assist in the process of mixing the buffer with tissue. In-tube pulverization was usually successful, but use of a mortar and pestle was necessary in some cases (i.e., some succulent or aquatic plants that formed a block of ice and one grass species tested).
For the duration of the protocols, the samples were kept on ice (4°C) unless otherwise incubated at another temperature. During incubation, for the TRIzol, it was important to have a second tube rack available at room temperature in which to place the samples for their 5-min incubation, as it took almost 10 min for a plastic tube rack to warm from 4°C to room temperature (∼25°C). The times between steps were best kept as short as possible (i.e., minutes), and we found that running consecutive, smaller batches of 24 or fewer samples resulted in cleaner RNA. For all three options, the entire process took between 3 and 5 h, depending on the number of extraction steps needed for each taxon (see Appendices 4–6). We also found that ample time was needed to prepare the RNA-clean space and pre-label the tubes (2–4 h); additionally, the time needed for checking the samples on the NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA) was approximately 1 h, depending on the number of samples.
Storing pure RNA: As with most molecular biological materials, a freeze/thaw process can damage a sample. We found that RNA samples that were not digested with DNase could be stored at 4°C for 2 to 3 days before digestion with no apparent change in quality. Once the DNA was digested, a 3-μL aliquot was run on the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA), and the remaining samples were placed at −80°C for storage. The pure RNA was sent in the mail for library construction and sequencing after drying down onto specially coated tubes (i.e., GenVault, now renamed as GenTegra; IntegenX, Pleasanton, California, USA) that inhibited RNase activity and stabilized the RNA at room temperature.
Leaf collection in microfuge tubes
In some experiments, it may be desired to collect RNA from many plants in a short period of time. For example, in a greenhouse one may have numerous individuals from different genetic lineages all growing simultaneously, and the goal of the experiment is to observe the expression levels between individuals. In these sort of studies, it is best that the leaves all be frozen within a small bracket of time, all in the same day, with the same light and temperature. This is the sort of large-scale project for which using microfuge tubes is ideal. For these collections, one leaf or part of one leaf that weighs between 50 and 200 mg can be placed in one microfuge tube to produce enough RNA for most genotyping studies and other transcriptomic-type analyses.
For large-scale projects, using a mortar and pestle is not practical because each mortar and pestle must be baked at 200°C for 4 h prior to use. If you are collecting hundreds of individuals, dealing with many mortar and pestles is a misuse of time considering the actual volume of RNA generated is more than necessary. Therefore, for larger-scale projects, it is recommended that microfuge tubes be used instead. We recommend Eppendorf Safe-Lock tubes as they are less likely to fail during frozen pulverization and better withstand chemical exposure.
Pre-label RNase-free 2-mL tubes, place five zirconia baked beads (which have been baked at 200°C for 4 h) in each tube, and place the tubes in 81-place plastic boxes stored inside clean plastic bags. The tubes must be pre-labeled before going to the collection site as you will be under time constraint with the liquid nitrogen. Bring the boxes to the collection site with the following supplies: liquid nitrogen in a Dewar flask, large ice chest with dry ice, small scissors (enough for each worker who will be collecting), winter stretch gloves, nitrile gloves to wear over winter gloves, RNaseZap bottles, 20% bleach in squirt bottle, 95% ethanol in spray bottle, Kimwipes (Kimberly Clark Professional, Roswell, Georgia, USA), and numerous small coolers to hold the liquid nitrogen while collecting. Cut leaf tissue and put it into the tube, close the tube very tightly, and then place the tube directly into small cooler with liquid nitrogen in a matter of seconds. When you have reached a convenient end point for a batch (e.g., one rack of plants), transfer the tubes with the frozen leaves into the plastic boxes that are sitting cold on the dry ice inside the large ice chest, then reuse the small cooler with liquid nitrogen for the next batch. They will stay frozen on the dry ice until the dry ice evaporates (one large cooler can take ∼48 h for the dry ice to fully evaporate). Collect a specimen of a representative individual plant for the plant press to create a voucher for the population or the greenhouse collection. At the end of the on-site collection, store the boxes with the leaves in the −80°C freezer.
Leaf collection in 50-mL Falcon tubes
In the case of field collections of individuals for obtaining enough material to repeat the RNA extraction many times, you can harvest a large amount of tissue from one individual. This was the standard method used for the 1KP project, allowing for numerous attempts at extracting RNA from the same leaf tissue. However, this method of collecting in 50-mL Falcon tubes is enough to repeat the RNA extraction as many as 100 to 200 times.
To do this type of collection, bring the following into the field or greenhouse: Dewar flask of liquid nitrogen, small cooler with plastic 50-mL (or 15-mL) Falcon tube holder, scissors, 50-mL (or 15-mL) Falcon tubes, Sharpie pen (any color but blue, which rubs off too easily when frozen; Sharpie, Downers Grove, Illinois, USA), 95% ethanol, RNaseZap, stretch gloves, nitrile gloves, Kimwipes, and plant press. Identify the plant to collect, assign it a collection number, and write the number on the Falcon tube. Use a scissor or pin to cut a hole in the top of the tube so the liquid nitrogen has the ability to volatize out of the tube without exploding. Fill the tube with liquid nitrogen and place in the cooler with liquid nitrogen and rack. Clean the scissors with ethanol and RNaseZap. Cut the youngest leaf tissue and immediately put into the Falcon tube for freezing. Close the lid when you feel like you have enough tissue. If possible, collect a specimen of the same individual plant for the plant press to create a voucher. Store on dry ice until you can place the tubes in a −80°C freezer until you are ready to start extracting.
Please consult the following Ambion protocols: TRIzol (http://tools.invitrogen.com/content/sfs/manuals/TRIzol_reagent.pdf); TURBO DNA-free (http://tools.invitrogen.com/content/sfs/manuals/cms_055740.pdf).
Appendix 4.
Bench-ready protocol for Option 1: Ambion TRIzol RNA extraction in microfuge tubes with TURBO DNA-free digestion. Prepared by Ingrid Jordon-Thaden (Soltis Laboratory, Department of Biology, University of Florida, Gainesville, Florida, USA).
Reagents:
20% bleach in squirt bottle
95% ethanol in spray or squirt bottle
RNaseZap bottle (Ambion, Life Technologies, Carlsbad, California, USA)
Liquid nitrogen in a Dewar flask
TRIzol (also called Tri-Reagent, Ambion; store at 4°C)
20% sarkosyl (optional for particularly difficult species, high 2° metabolites)
100% chloroform
100% isopropanol
75% ethanol
RNA/RNase-free water
- TURBO DNA-free kit (Ambion; store at −20°C)
- 10× TURBO DNase buffer
- TURBO DNase
- DNase inactivation reagent
Materials:
Zirconia beads, prebaked at 200°C for 4 h in glass beaker covered with foil
Small metal spatula, scissors, and forceps, prebaked at 200°C for 4 h wrapped with foil
P100 and P1000 RNase-free barrier tips; RNase-free pipettes: P1000, P100
Stretch or thin winter gloves that can be worn under the nitrile gloves
Nitrile gloves (to wear over winter gloves, latex will crack in liquid nitrogen)
Two large plastic 2-mL microfuge tube racks (96-well)
Sample tube block (for automatic shaker/pulverizer machine)
One ice bucket to hold the 2-mL microfuge tube rack on ice
One small ice bucket with liquid nitrogen to hold the sample tube block
One small ice bucket with liquid nitrogen to hold the tubes while weighing
24, 2-mL RNase-free Eppendorf tubes (Eppendorf, Hauppauge, New York, USA) (to freeze the tissue in, don’t use cheap tubes, they will crack) (need one tube for each sample)
72, 1.7-mL RNase-free Eppendorf tubes (three batches of 24, prelabeled; 96 tubes if using sarkosyl, this adds one more chloroform extraction)
Waste bin for tips
Kimwipes (Kimberly Clark Professional, Roswell, Georgia, USA), for regular cleaning of items/surfaces with RNaseZap
Tray to hold waste tubes after supernatant removal
Freshly prepared aliquots in three 50-mL Falcon tubes: 100% chloroform (only immediately prior to use, will melt), 100% isopropanol (also called 2-propanol), and 75% ethanol
Equipment:
Automatic shaker machine (e.g., Beadbeater-96; BioSpec Products, Bartlesville, Oklahoma, USA)
24-place centrifuge cooled to 4°C
Vortexer
- Incubator at 37°C with orbital shaker
- Note: The default temperature for RNA samples is 4°C (i.e., on ice). Always keep the samples cool unless the step requires incubation at a different temperature.
Procedure:
- I. RNA extraction
- A. Preparation for extraction
- 1. Precool the centrifuge to 4°C.
- 2. Gather required samples, beads, labeled tubes, chemicals, and equipment.
- 3. Fill a Dewar flask with liquid nitrogen.
- B. Set up the hoods
- 4. Remove all unneeded items from the hood.
- 5. Clean all surfaces and equipment with 20% bleach, 95% ethanol, and then RNaseZap.
- 6. Place listed supplies in the hood.
- 7. Put liquid nitrogen in small cooler with black shaker block.
- 8. Get samples from −80°C and place them in the sample tube block (if already in tubes).
- 9. Be sure to check the labels on the tubes to avoid any mislabeling issues.
- C. Preparing the frozen tissue in tubes (if tissue is not in them already)
- 10. Fill two small coolers with liquid nitrogen.
- 11. Place a 100-place plastic box in one of the coolers floating in the nitrogen.
- 12. Place the labeled tubes with baked beads in the 100-place plastic box, tube lids on tight.
- 13. Working in batches of about three or four plants at a time to prevent thawing, use forceps or a spatula to place 60–100 mg of tissue into the Beadbeater sample tubes from the 50-mL Falcon tubes (for aquatic plants use 200 μg due to the weight of the internal water). It is best to work near the balance with a small cooler of liquid nitrogen holding your samples and tubes.
- 14. Weigh a cold, blank tube with five beads first.
- 15. Get a weight for each of the samples.
- 16. If you need to add more, do so right away.
- 17. Write the sample weight in the laboratory book.
- 18. Repeat steps 13–17 until you have finished all of the samples.
- D. Pulverize the frozen tissue
- 19. Check each tube for beads, making sure they easily move within the tube.
- 20. Taking the sample tube block with the 24 samples, place tightly into the automatic shaker, doing this quickly as to not allow thawing.
- 21. Shake for 2 min; they will stay frozen.
- 22. Place block back into the liquid nitrogen cooler if needed to shake another 2 min, then shake a second time after the block appears to be frozen.
- 23. Keep on liquid nitrogen, until TRIzol is added.
- E. Lyse the cells and separate the polysaccharides from the nucleic acids
- 24. Add 1 mL of TRIzol solution to each sample tube.
- a. Optional adjustment is to add 50–100 μL of 20% sarkosyl to each sample with the TRIzol. Results in three layers; take only the top water layer. (I usually used 50 μL for 1 mL of TRIzol.)
- b. Before opening the tubes, tap the bottom of the tube on the bench to remove most of the leaf tissue out of the cap.
- c. If the tissue does not dislodge from the cap, you can use the vortexer with the solution added to force it out of the cap, or carefully pull it with a clean spatula.
- 25. After each tube has had the TRIzol solution added, vortex immediately, both the top and bottom of the tube, until all tissue is hydrated. Vortexing for more than 2 min can be common.
- 26. Place tube with hydrated sample on ice.
- 27. Do this sequentially until you have all 24 tubes finished.
- 28. Once the batch is ready, incubate in a new rack at room temperature (RT) for 5 min (a new RT rack is needed here as the cold rack takes too long to come to RT).
- F. Extract the nucleic acids and purify the extraction
- 29. Centrifuge at 12,000 × g for 10 min at 4°C.
- 30. Pipette aqueous solution (top layer) to a new 1.7-mL tube (will be ∼900 μL).
- a. If sarkosyl was used, be aware it will be a thick, viscous layer at the interface. Try not to pull any into the aqueous layer.
- 31. Add 200 μL of 100% chloroform to each tube. (Do not change this volume or more protein will be forced into the aqueous layer.)
- 32. Vortex for 10–15 s (solution should be milky colored).
- 33. Incubate at RT for 10 min.
- 34. Centrifuge at 12,000 × g for 15 min at 4°C.
- 35. Remove upper aqueous layer (should be clear), 500–700 μL, and put in a new 1.7-mL tube; place on ice.
- 36. If you suspect the sample does not look “clean,” repeat steps 31–35.
- Note: Wait to discard the contents of the tubes, to avoid cross contamination between samples.
- G. Precipitate and pellet the nucleic acids
- 37. Add 500 μL (or more, you want to fill up the tube) of isopropanol.
- 38. Mix by inverting the rack.
- 39. Incubate at −20°C for 10 min.
- a. Extractions work best when the solution stays clear.
- b. If a white precipitate forms, a pellet will result, but it will not be as clean. You can make a note in your book that a precipitate was seen.
- c. If the whole solution instantly turns cloudy, this extraction will probably not work at all. Do not expect the pellet to dissolve in the final steps. Discard this solution and repeat the extraction with the addition of sarkosyl if you did not do it the first time. If you get the same result, use less tissue in the initial extraction and try again.
- 40. Pellet the RNA by centrifuging at 12,000 × g for 20 min at 4°C.
- 41. Pour off the supernatant into a waste beaker.
- 42. Wash pellet by adding 1 mL of 75% ethanol and vortex until the pellet is loose.
- 43. Centrifuge at 8900 × g for 5 min at 4°C.
- 44. Pour off the ethanol into a waste beaker and tap the tube on a tissue to pull as much ethanol off as you can. If the pellet is very clean, it is better to pull it off with a pipette so not to lose the pellet when pouring, as it will not adhere to the side of the tube as well.
- 45. Centrifuge the tube with the pellet for 2 min at 4°C.
- 46. Using a pipette, pull off excess ethanol collected at the bottom of the tube (∼25 μL).
- a. The final pellet should be clear, gel like, and 3–5 mm long.
- b. If it is white, then it may still have salts or other naturally occurring contaminants and you can repeat the ethanol wash a second time; however, the DNA removal step below seems to also remove salt contamination.
- 47. Let the pellet dry for 2 min at RT, but no more than 10 min. Leave lids open, cover tray with foil loosely.
- 48. Redissolve the pellet in 50 μL of RNase-free water. If the RNA is pure, it should dissolve instantly.
- Note: To aid in dissolution, incubate at 37°C for 10 min on an orbital shaker. If the pellet does not dissolve after this heating, it should be discarded.
- 49. Check RNA with the NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA), or similar device, for concentration. It is recommended by the manufacturer’s instructions to dilute the samples to be less than 200 ng/μL before proceeding to the DNase digestion step. However, I have done the following steps with samples that are 700–1500 ng/μL and had success. I suggest only diluting when you have 2000–3000 ng/μL of RNA.
- 50. Store the RNA at 4°C if you plan on doing the DNA removal the next day. Do not freeze and thaw the RNA.
- II. Removal of DNA using TURBO DNA-free kit
- Note: Do not continue with the DNA digestion step if you have difficulty dissolving the pellet or the NanoDrop gives high levels of contamination (indicated by unusual peak structure) or very low quantity. It is best to repeat with a new extraction and save the DNA removal kit for ideal samples. However, the TURBO DNA-free kit also cleans the sample partially.
- 51. Add 0.1 volume of 10× TURBO DNase buffer, vortexing before use (usually 5 μL if no dilution of RNA was made).
- 52. Add 1 μL of TURBO DNase (vortex before use) to the RNA and mix gently.
- 53. Incubate at 37°C while on the orbiter shaker inside the incubation oven for 30 min.
- a. For highly concentrated samples, incubation time could be longer, but I never needed to do this.
- 54. Add resuspended DNase Inactivation Reagent, vortexing before use (typically 0.1 volume; 5 μL if no dilution of RNA was made) and mix well (vortex briefly). At this step, I would recommend transferring the sample to a new tube if you are still in 2-mL tubes. (A Dolphin tube would be ideal if available.)
- 55. Incubate at RT for 2 min, vortexing every 30 s.
- 56. Centrifuge at 10,000 × g for 2 min and transfer to a new tube, being very careful not to disturb the gel-like inactivation buffer at the tip of the tube.
- 57. Check the concentration of the RNA with NanoDrop again.
- a. Ideally you want at least 100 ng/μL. Expect some loss from the TURBO kit. Also, if the first time the spectra appeared contaminated, this step may have partially cleaned it. The A260/A280 ratio should be 1.8–2.2.
- 58. Can add sterile water to dilute RNA stock if you like. Store at –80°C.
- III. Checking for integrity of RNA with Agilent 2100 Bioanalyzer
- 59. If transcriptome sequencing is desired for the RNA, it will be required to determine the quality of the extracted RNA, especially its 18S and 28S subunits.
- 60. Preferably, the RIN number that is calculated should be between 5 and 10, and the rRNA ratio (28S/18S) should be higher than 2, but as low as 1.3 is still acceptable.
- 61. The Agilent 2100 Bioanalyzer uses 2–5-μL aliquots of the samples; consult with your core facility on the desired volume. It necessary to know the approximate concentration of the samples prior to using the Bioanalyzer, thus the NanoDrop is still needed prior to this step.
- IV. Preparing samples in the GenVault tubes for mailing
- 62. Prepare tubes with labels.
- 63. Thaw the RNA samples, but keep on ice when thawed.
- 64. Set up the evaporator with either a cold trap by putting the vapor collection flask in a cooler of ice or ice packs, or by using a refrigeration unit.
- 65. Place sample in the GenVault tube and place the lid carefully in a clean place.
- 66. Put GenVault tubes in the evaporator.
- 67. It should take no more than 1 h to evaporate 50 μL of water. If you have combined two successful extractions to the same tube, then expect 2 h. If this is still not evaporating, you are risking damage to your sample.
- 68. Do not over dry the samples.
- 69. These tubes are stable at room temperature for many days. See GenVault manual for full description on how the tubes work.
Appendix 5.
Bench-ready protocol for Option 2: Combination CTAB and Ambion TRIzol RNA extraction in microfuge tubes with TURBO DNA-free digestion. Prepared by Ingrid Jordon-Thaden (Soltis Laboratory, Department of Biology, University of Florida, Gainesville, Florida, USA).
Reagents:
20% bleach in squirt bottle
95% ethanol in spray or squirt bottle
RNaseZap bottle (Ambion, Life Technologies, Carlsbad, California, USA)
Liquid nitrogen in a Dewar flask
CTAB (hexadecyltrimethylammonium bromide)
10% PVP (polyvinylpyrrolidone)
BME (β-mercaptoethanol) (added right before use) (1 μL per 0.5 mL of CTAB buffer)
chloroform:IAA (24:1) (isoamyl alcohol)
TRIzol (also called Tri-Reagent, Ambion; store at 4°C)
20% sarkosyl (optional for particularly difficult species, high 2° metabolites)
100% chloroform
100% isopropanol
75% ethanol
RNA/RNase-free water
- TURBO DNA-free kit (Ambion; store at −20°C)
- 10× TURBO DNase buffer
- TURBO DNase
- DNase inactivation reagent
Materials:
Zirconia beads, prebaked at 200°C for 4 h in glass beaker covered with foil
Small metal spatula, scissors, and forceps, prebaked at 200°C for 4 h wrapped with foil
P100 and P1000 RNase-free barrier tips; RNase-free pipettes: P1000, P100
Stretch or thin winter gloves that can be worn under the nitrile gloves
Nitrile gloves (to wear over winter gloves, latex will crack in liquid nitrogen)
Two large plastic 2-mL microfuge tube racks (96-well)
Sample tube block (for automatic shaker/pulverizer machine)
One ice bucket to hold the 2-mL microfuge tube rack on ice
One small ice bucket with liquid nitrogen to hold the sample tube block
One small ice bucket with liquid nitrogen to hold the tubes while weighing
96, 2-mL RNase-free Eppendorf tubes (Eppendorf, Hauppauge, New York, USA) (to freeze the tissue in, don’t use cheap tubes, they will crack) (use four tubes for each sample)
72, 1.7-mL RNase-free Eppendorf tubes (three batches of 24, prelabeled; 96 tubes if using sarkosyl, this adds one more chloroform extraction)
Waste bin for tips
Kimwipes (Kimberly Clark Professional, Roswell, Georgia, USA), for regular cleaning of items/surfaces with RNaseZap
Tray to hold waste tubes after supernatant removal
Freshly prepared aliquots in three 50-mL Falcon tubes: 100% chloroform (only immediately prior to use, will melt), 100% isopropanol (also called 2-propanol), and 75% ethanol
Equipment:
Automatic shaker machine (e.g., Beadbeater-96; BioSpec Products, Bartlesville, Oklahoma, USA)
24-place centrifuge at room temperature
24-place centrifuge cooled to 4°C
Vortexer
Water bath at 55°C
- Incubator at 37°C with orbital shaker
- Note: The default temperature for RNA samples is 4°C (i.e., on ice). Always keep the samples cool unless the step requires incubation at a different temperature.
Procedure:
- I. RNA extraction
- A. Preparation for extraction
- 1. Precool the centrifuge to 4°C.
- 2. Gather required samples, beads, labeled tubes, chemicals, and equipment.
- 3. Dissolve PVP in CTAB (0.3 g in 30 mL final). Add PVP to a clean 50-mL Falcon tube, add 20 mL of CTAB and place tube in water bath at 60°C until PVP is fully dissolved. When PVP is dissolved, add the remaining CTAB to ∼30 mL. Add the BME later, after the hood is completely set. Leave water bath at 60°C for sample incubation later.
- 4. Fill a Dewar flask with liquid nitrogen.
- B. Set up the hoods
- 5. Remove all unneeded items from the hood.
- 6. Clean all surfaces and equipment with 20% bleach, 95% ethanol, and then RNaseZap.
- 7. Place listed supplies in the hood.
- 8. Put liquid nitrogen in small cooler with sample tube block.
- 9. Get samples from −80°C and place them in the black shaker block (if already in tubes).
- 10. Be sure to check the labels on the tubes to avoid any mislabeling issues.
- C. Preparing the frozen tissue in tubes (if tissue is not in them already)
- 11. Fill two small coolers with liquid nitrogen.
- 12. Place a 100-place plastic box in one of the coolers floating in the nitrogen.
- 13. Place your labeled tubes with baked beads in the 100-place plastic box, tube lids on tight.
- 14. Working in batches of about three or four plants at a time to prevent thawing, use forceps or a spatula to place 60–100 mg of tissue into the Beadbeater sample tubes from the 50-mL Falcon tubes (for aquatic plants use 200 μg due to the weight of the internal water). It is best to work near the balance with a small cooler of liquid nitrogen holding your samples and tubes.
- 15. Weigh a cold, blank tube with five beads first.
- 16. Get a weight for each of the samples.
- 17. If you need to add more, do so right away.
- 18. Write the sample weight in the laboratory book.
- 19. Repeat steps 14–18 until you have finished all of the samples.
- D. Pulverize the frozen tissue
- 20. Check each tube for beads, making sure they easily move within the tube.
- 21. Taking the sample tube block with the 24 samples, place tightly into the automatic shaker, doing this quickly as to not allow thawing.
- 22. Shake for 2 min; they will stay frozen.
- 23. Place block back into the liquid nitrogen cooler if needed to shake another 2 min, then shake a second time after the block appears to be frozen.
- 24. Keep on liquid nitrogen, until CTAB is added.
- E. Lyse the cells and separate the polysaccharides from the nucleic acids
- 25. Add 900 μL of the premixed solution of CTAB, PVP, and BME to each sample tube.
- a. Before opening the tubes, tap the tube on the bottom on the bench to empty most of the leaf tissue that is in the lid from the shaking process.
- b. If the tissue does not dislodge from the cap, you can use the vortexer with the solution added to force it out of the cap, or carefully pull it with a clean spatula.
- 26. After each tube has had the CTAB/PVP/BME solution added, vortex immediately, both the top and bottom of the tube, until all tissue is hydrated. Vortexing for more than 2 min can be common.
- 27. Then place tube with hydrated sample on ice.
- 28. Do this sequentially until you have all 24 tubes finished.
- 29. Once the batch is ready, incubate in a water bath at 55°C for 8 min.
- F. Extract the nucleic acids and purify the extraction
- 30. Centrifuge at 13,000 × g for 5 min at room temperature (RT).
- 31. Pipette aqueous solution to a new 2-mL tube (will be 900–1000 μL).
- 32. Add 900 μL of chloroform :IAA (24:1).
- 33. Mix by inverting the tubes two or three times.
- 34. Spin at 13,200 × g for 5 min at 4°C.
- 35. Pipette aqueous solution to a new 2-mL tube (will be 900–1000 μL).
- 36. Add 900 μL of TRIzol solution and 50 μL of 20% sarkosyl to each tube. Results in three layers.
- 37. Centrifuge at 12,000 × g for 10 min at 4°C.
- 38. Pipette aqueous solution (top layer) to a new 2-mL tube (will be 900–1000 μL).
- 39. Add 200 μL of 100% chloroform to each tube (do not change this volume or more protein will be forced into the aqueous layer).
- 40. Vortex for 10–15 s (solution should be milky colored).
- 41. Incubate at RT for 10 min.
- 42. Centrifuge at 12,000 × g for 15 min at 4°C.
- 43. Remove upper aqueous layer (should be clear), 500–700 μL, and put in a new 2-mL tube.
- 44. If you suspect the sample does not look “clean,” repeat steps 39–43.
- Note: Wait to discard the contents of the tubes, to avoid cross contamination between samples.
- G. Precipitate and pellet the nucleic acids
- 45. Add 500 μL (or more, you want to fill up the tube) of isopropanol.
- 46. Mix by inverting the rack.
- 47. Incubate at −20°C for 10 min.
- a. Extractions work best when the solution stays clear.
- b. If a white precipitate forms, a pellet will result, but it will not be as clean. You can make a note in your book that a precipitate was seen.
- c. If the whole solution instantly turns cloudy, this extraction will probably not work at all. Do not expect the pellet to dissolve in the final steps. Discard this solution and repeat the extraction with the addition of sarkosyl if you did not do it the first time. If you get the same result, use less tissue in the initial extraction and try again.
- 48. Pellet the RNA by centrifuging at 12,000 × g for 20 min at 4°C.
- 49. Pour off the supernatant into a waste beaker.
- 50. Wash pellet by adding 1 mL of 75% ethanol and vortex until the pellet is loose.
- 51. Centrifuge at 8900 × g for 5 min at 4°C.
- 52. Pour off the ethanol into a waste beaker and tap the tube on a tissue to pull as much ethanol off as you can. If the pellet is very clean, it is better to pull it off with a pipette so not to lose the pellet when pouring, as it will not adhere to the side of the tube as well.
- 53. Centrifuge the tube with the pellet for 2 min at 4°C
- 54. Using a pipette, pull off excess ethanol collected at the bottom of the tube (∼25 μL).
- a. The final pellet should be clear, gel like, and 3–5 mm long.
- b. If it is white, then it may still have salts or other naturally occurring contaminants and you can repeat the ethanol wash a second time; however, the DNA removal step below seems to also remove salt contamination.
- 55. Let the pellet dry for 2 min at RT, but no more than 10 min. Leave lids open, cover tray with foil loosely.
- 56. Redissolve the pellet in 50 μL of RNase-free water. If the RNA is pure, it should dissolve instantly.
- Note: To aid in dissolution, incubate at 37°C for 10 min on an orbital shaker. If the pellet does not dissolve after this heating, it should be discarded.
- 57. Check RNA with the NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA), or similar device, for concentration. It is recommended by the manufacturer’s instructions to dilute the samples to be less than 200 ng/μL before proceeding to the DNase digestion step. However, I have done the following steps with samples that are 700–1500 ng/μL and had success. I suggest only diluting when you have 2000–3000 ng/μL of RNA.
- 58. Store the RNA at 4°C if you plan on doing the DNA removal the next day. Do not freeze and thaw the RNA.
- II. Removal of DNA using TURBO DNA-free kit
- Note: Do not continue with the DNA digestion step if you have difficulty dissolving the pellet or the NanoDrop gives high levels of contamination (indicated by unusual peak structure) or very low quantity. It is best to repeat with a new extraction and save the DNA removal kit for ideal samples. However, the TURBO DNA-free kit also cleans the sample partially.
- 59. Add 0.1 volume of 10× TURBO DNase buffer, vortexing before use (usually 5 μL if no dilution of RNA was made).
- 60. Add 1 μL of TURBO DNase (vortex before use) to the RNA and mix gently.
- 61. Incubate at 37°C while on the orbiter shaker inside the incubation oven for 30 min.
- a. For highly concentrated samples, incubation time could be longer, but I never needed to do this.
- 62. Add resuspended DNase Inactivation Reagent, vortexing before use (typically 0.1 volume; 5 μL if no dilution of RNA was made) and mix well (vortex briefly). At this step, I would recommend transferring the sample to a new tube if you are still in 2-mL tubes. (A Dolphin tube would be ideal if available.)
- 63. Incubate at RT for 2 min, vortexing every 30 s.
- 64. Centrifuge at 10,000 × g for 2 min and transfer to a new tube, being very careful not to disturb the gel-like inactivation buffer at the tip of the tube.
- 65. Check the concentration of the RNA with NanoDrop again.
- a. Ideally you want at least 100 ng/μL. Expect some loss from the TURBO kit. Also, if the first time the spectra appeared contaminated, this step may have partially cleaned it. The A260/A280 ratio should be 1.8–2.2.
- 66. Can add sterile water to dilute RNA stock if you like. Store at −80°C.
- III. Checking for integrity of RNA with Agilent 2100 Bioanalyzer
- 67. If transcriptome sequencing is desired for the RNA, it will be required to determine the quality of the extracted RNA, especially its 18S and 28S subunits.
- 68. Preferably, the RIN number that is calculated should be between 5 and 10, and the rRNA ratio (28S/18S) should be higher than 2, but as low as 1.3 is still acceptable.
- 69. The Agilent 2100 Bioanalyzer uses 2–5-μL aliquots of the samples; consult with your core facility on the desired volume. It necessary to know the approximate concentration of the samples prior to using the Bioanalyzer, thus the NanoDrop is still needed prior to this step.
- IV. Preparing samples in the GenVault tubes for mailing
- 70. Prepare tubes with labels.
- 71. Thaw the RNA samples, but keep on ice when thawed.
- 72. Set up the evaporator with either a cold trap by putting the vapor collection flask in a cooler of ice or ice packs, or by using a refrigeration unit.
- 73. Place sample in the GenVault tube and place the lid carefully in a clean place.
- 74. Put GenVault tubes in the evaporator.
- 75. It should take no more than 1 h to evaporate 50 μL of water. If you have combined two successful extractions to the same tube, then expect 2 h. If this is still not evaporating, you are risking damage to your sample.
- 76. Do not over dry the samples.
- 77. These tubes are stable at room temperature for many days. See GenVault manual for full description on how the tubes work.
Appendix 6.
Bench-ready protocol for Option 3: CTAB extraction, QIAGEN RNeasy Plant Mini Kit, and on-column digestion kits (not recommended for difficult species). By Richard Buggs, modified by Ingrid Jordon-Thaden (Soltis Laboratory, Department of Biology, University of Florida, Gainesville, Florida, USA).
Reagents:
20% bleach in squirt bottle
95% ethanol in spray or squirt bottle
RNaseZap bottle (Ambion, Life Technologies, Carlsbad, California, USA)
Liquid nitrogen in a Dewar flask
CTAB (hexadecyltrimethylammonium bromide) with 1% PVP (polyvinylpyrrolidone; 1 mL per sample, but make 30 mL with 0.3 g PVP and 60 μL of BME [β-mercaptoethanol]); (CTAB, PVP, 0.1 g per 10 mL of CTAB buffer), BME (added right before use) (1 μL of BME per 0.5 mL of CTAB buffer)
Cold (4°C) phenol:chloroform:IAA (25:24:1) (isoamyl alcohol)
Chloroform:IAA (24:1)
5 M NaCl
Cold (−20°C) 100% ethanol
Materials:
P1000 RNase-free filter tips
P100 RNase-free filter tips
RNase-free pipettes: P1000, P100, P20, P10
Stretch or thin winter gloves to wear under the nitrile gloves
Nitrile gloves
Two large plastic Eppendorf tube racks (to hold 2-mL tubes; Eppendorf, Hauppauge, New York, USA)
Sample tube block (for automatic shaker machine)
One ice bucket to hold the Eppendorf tube rack on ice
One small ice bucket with liquid nitrogen to hold the black shaker block
72, 2-mL tubes (prelabeled)
1 plastic serological pipette (25 mL)
1 glass serological pipette (25 mL)
Pipette bulb
Waste bin for tips
Kimwipes (Kimberly Clark Professional, Roswell, Georgia, USA), for regular cleaning of items/surfaces with RNaseZap
Tray to hold waste tubes after supernatant removal
Three 50-mL Falcon tubes: CTAB/PVP/BME (30 mL), to catch waste of phenol:chloroform :IAA from glass pipette (do not prepour), and chloroform:IAA, for each extraction 30 mL
QIAGEN RNeasy Plant Mini Kit (QIAGEN, Valencia, California, USA; only using the DNA digestion columns and reagents)
Equipment:
Automatic shaker machine (e.g., Beadbeater-96; BioSpec Products, Bartlesville, Oklahoma, USA)
24-place centrifuge at room temperature (RT) and later at 4°C
Vortexer
- Water bath at 60°C in hood
- Note: The default temperature for RNA samples is 4°C (i.e., on ice). Always keep the samples cool unless the step requires incubation at a different temperature.
Procedure:
- I. RNA extraction
- A. Preparation for extraction
- 1. Put three sets of 24, 2-mL prelabeled tubes in an 81-place rack.
- 2. Dissolve PVP in CTAB (0.3 g in 30 mL final). Add PVP to a clean 50-mL Falcon tube, add 20 mL of CTAB and place tube in water bath at 60°C until PVP is fully dissolved. When PVP is dissolved, add the remaining CTAB to ∼30 mL. Add the BME later, after the hood is completely set. Leave water bath at 60°C for sample incubation later.
- 3. Fill a Dewar flask with liquid nitrogen.
- B. Set up the hoods
- 4. Remove all unneeded items from the hood.
- 5. Clean all surfaces and equipment with 20% bleach, 95% ethanol, and then RNaseZap.
- 6. Place listed supplies in the hood.
- 7. Put liquid nitrogen in small cooler with sample tube block.
- 8. Get samples from −80°C and place them in the sample tube block (if already in tubes).
- 9. Be sure to check the labels on the tubes before going any further to avoid any mislabeling issues.
- C. Preparing the frozen tissue in tubes (if tissue is not in them already)
- 10. Fill two small coolers with liquid nitrogen.
- 11. Place a 100-place plastic box in one of the coolers floating in the nitrogen.
- 12. Place your labeled tubes with baked beads in the 100-place plastic box, tube lids on tight.
- 13. Working in batches of about three or four plants at a time to prevent thawing, use forceps or a spatula to place 60–100 mg of tissue into the Beadbeater sample tubes from the 50-mL Falcon tubes (for aquatic plants use 200 μg due to the weight of the internal water). It is best to work near the balance with a small cooler of liquid nitrogen holding your samples and tubes.
- 14. Weigh a cold, blank tube with five beads first.
- 15. Get a weight for each of the samples.
- 16. If you need to add more, do so right away.
- 17. Write the sample weight in the laboratory book.
- 18. Repeat steps 13–17 until you have finished all of the samples.
- D. Pulverize the frozen tissue
- 19. Check each tube for beads, making sure they easily move within the tube.
- 20. Taking the sample tube block with the 24 samples, place tightly into the automatic shaker, doing this quickly as to not allow thawing.
- 21. Shake for 2 min; they will stay frozen.
- 22. Place sample tube block back into the liquid nitrogen cooler if needed to shake another 2 min, then shake a second time after the block appears to be frozen.
- E. Lyse the cells and separate the polysaccharides from the nucleic acids
- 23. Add 1 mL of the CTAB/1% PVP/BME mixture to each sample tube.
- a. Before opening the tubes, tap the tube on the bottom on the bench to empty most of the leaf tissue that is in the lid.
- b. If the tissue does not dislodge from the cap, you can use the vortexer with the solution added to force it out of the cap, or carefully pull it with a clean spatula.
- 24. After each tube has had the CTAB solution added, vortex immediately, both the top and bottom of the tube, until all tissue is hydrated. Then place tube on ice and continue with the remaining tubes.
- 25. Incubate the batch at 60°C in a water bath (or thermoblock for 2-mL tubes) for 7 min and 30 s.
- F. Extract nucleic acids from the proteins and other metabolites
- 26. Pour 25 mL of chloroform:IAA into a 50-mL Falcon tube.
- 27. Add 1 mL of the solution to the tubes.
- 28. Invert the tubes gently by holding an empty rack over the full rack of tubes. Invert the tubes four or five times until you see there is a definite layer between the organic and the aqueous solutions, and there is a color difference between the two layers. You will see three layers at this point, the middle layer being other plant debris that will be centrifuged into a thick layer between the aqueous and organic layer.
- 29. To purify the aqueous solution from the cell tissue and other debris, centrifuge the samples for 5 min at 13,200 × g at RT.
- 30. Carefully pull the aqueous (water) supernatant (top) off and place into a new 2-mL tube (about 950 μL). Leave the water layer that lays directly on the debris at the separation layer; this is less than 100 μL of the sample and is most likely not very clean.
- G. Extract again to clean the aqueous layer that is holding the nucleic acids
- 31. Use the same technique except use a glass 25-mL pipette, add 1 mL of phenol:chloroform:IAA solution to each tube.
- 32. Invert the tubes gently as above, with an empty rack over the full rack, four or five times.
- 33. Centrifuge the samples at 13,200 × g for 5 min at RT.
- 34. Carefully pull the aqueous supernatant off and place into a new 2-mL tube. Be careful not to pull the milky white protein that sits between the layers.
- Note: Wait to discard the contents of the tubes, to avoid cross contamination between samples.
- H. Extract one last time
- 35. Repeat the chloroform:IAA extraction from steps 26–30.
- 36. Place the supernatant into a new 2-mL tube.
- I. Precipitate the nucleic acids
- 37. Add 1/20th volume of 5 M NaCl to each tube; this allows the negatively charged phosphates to be attracted to the NaCl, leaving the nucleic acids phosphate free, which assists in the precipitation (30.5 μL of NaCl for 700 μL of sample; or 25 μL for 500 μL of sample).
- 38. Add ∼2 volumes of cold 100% ethanol to each tube (fill the tube).
- 39. Gently invert the tubes to mix.
- 40. You can continue directly to the next step, or choose to incubate the solution for 20 min at −20°C. Some have reported incubating overnight, but this is not recommended as it can pull down more contamination.
- 41. Do not pellet the RNA.
- II. Clean the RNA from the DNA with on-column DNA digestion kit from QIAGEN
- 42. Using the QIAGEN Plant Mini Kit, refer to the manual for the DNA digestion steps.
- 43. Label both the columns and the collection tubes with the sample names.
- 44. Apply sample (sequentially add 650 μL at a time until entire sample is passed through the column) to an RNeasy mini column (pink color) placed in a 2-mL collection tube.
- a. If the sample volume exceeds 700 μL, which it always does, centrifuge two or three extractions in the same RNeasy spin column.
- b. Discard the flow-through after each centrifugation until the entire sample is loaded onto the column membrane.
- c. Centrifuge for 15–20 s at >10,000 rpm, discard the flow-through.
- d. Reuse the collection tube.
- 45. Once the entire sample is loaded onto the membrane, pipette 350 μL Buffer RW1 onto the RNeasy column.
- 46. Centrifuge for 15 s at ≥8000 × g (≥10,000 rpm), discard the flow-through.
- 47. Add 10 μL DNase I stock solution to 70 μL Buffer RDD (per sample).
- a. For 24 samples, use 240 μL of DNase I stock solution and 1680 μL of Buffer RDD (DNase aliquots can be stored in the −20°C freezer; Buffer RDD is kept at 4°C).
- b. Homogenize the DNAse mix without vortexing.
- 48. Pipette the DNase I incubation mix (80 μL) directly onto the RNeasy silica-gel membrane, and incubate on bench (20–30°C) for 15 min.
- 49. Set the centrifuge to 4°C.
- 50. Pipette 350 μL of Buffer RW1 onto the RNeasy column.
- 51. Centrifuge for 15 s at ≥8000 × g (≥10,000 rpm), discard the flow-through.
- 52. Pipette 500 μL Buffer RPE onto the RNeasy column.
- 53. Centrifuge for 15 s at ≥8000 × g (≥10 000 rpm), discard the flow-through.
- Note: Buffer RPE is supplied as a concentrate. Ensure that ethanol is added to Buffer RPE before use (see “Things to do before starting” in the QIAGEN manual).
- 54. Add another 500 μL Buffer RPE.
- 55. Centrifuge for 2 min at ≥8000 × g to dry the membrane.
- 56. Place the RNeasy column into a new 2-mL collection tube and discard the old collection tube with the flow-through.
- 57. Centrifuge for 1 min at full speed.
- 58. To elute the RNA, transfer the RNeasy column to a new 1.5-mL RNase-free collection tube.
- 59. Add 70 μL of RNase-free water directly onto the RNeasy silica-gel membrane and centrifuge for 20 s at 20,000 rpm.
- 60. This elution should be checked by the NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA).
- 61. Move the columns into a new tube for the second elution.
- 62. Add 70 μL more of RNase-free water directly onto the RNeasy silica-gel membrane and centrifuge for 1 min at ≥8000 × g (≥10,000 rpm) to elute.
- 63. Store this second elution for emergency use; it is not as pure as the first one.
- III. Checking for integrity of RNA with Agilent 2100 Bioanalyzer
- 64. If transcriptome sequencing is desired for the RNA, it will be required to determine the quality of the extracted RNA, especially its 18S and 28S subunits.
- 65. Preferably, the RIN number that is calculated should be between 5 and 10, and the rRNA ratio (28S/18S) should be higher than 2, but as low as 1.3 is still acceptable.
- 66. The Agilent 2100 Bioanalyzer uses 2–5-μL aliquots of the samples; consult with your core facility on the desired volume. It necessary to know the approximate concentration of the samples prior to using the Bioanalyzer, thus the NanoDrop is still needed prior to this step.
- IV. Preparing samples in the GenVault tubes for mailing
- 67. Prepare tubes with labels.
- 68. Refer to the user manual of the evaporator to better understand the machine.
- 69. Thaw the RNA samples, but keep on ice when thawed.
- 70. Set up the evaporator with either a cold trap by putting the vapor collection flask in a cooler of ice or ice packs, or by using a refrigeration unit.
- 71. Place sample in the GenVault tube and place the lid carefully in a clean place.
- 72. Put GenVault tubes in the evaporator.
- 73. It should take no more than 1 h to evaporate 50 μL of water. If you have combined two successful extractions to the same tube, then expect 2 h. If this is still not evaporating, you are risking damage to your sample.
- 74. Do not over dry the samples.
- 75. These tubes are stable at room temperature for many days. See the GenVault manual for a full description on how the tubes work.
LITERATURE CITED
- Buggs R. J. A., Doust A. N., Tate J. A., Koh J., Soltis K., Feltus F. A., Paterson A. H., et al. 2009. Gene loss and silencing in Tragopogon miscellus (Asteraceae): Comparison of natural and synthetic allotetraploids. Heredity 103: 73–81. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Doyle J. L. 1987. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Godden G. T., Jordon-Thaden I. E., Chamala S., Crowl A. A., Garcia N., Germain-Aubrey C. C., Heaney J. M., et al. 2012. Making next-generation sequencing work for you: Approaches and practical considerations for marker development and phylogenetics. Plant Ecology & Diversity 5: 427–450. [Google Scholar]
- Huang C., Picimbon J. F., Li H. Q., Li Z., Liu Q., Liu W. 2012. An efficient method for total RNA extraction from peanut seeds. Russian Journal of Plant Physiology 59: 129–133. [Google Scholar]
- Johnson M., Tian Z., Carpenter E., Bruskiewich B. J., Carrigan C., Chase M., Clarke N., et al. 2012. Evaluating methods for isolating total RNA and predicting the success of sequencing phylogenetically diverse plant transcriptomes. PLoS ONE 7: e50226. 10.1371/journal.pone.0050226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E. 2010. Preparation and analysis of RNA. In Current protocols in molecular biology 4.0.1-4.0.2. John Wiley & Sons, Hoboken, New Jersey, USA. [Google Scholar]
- Matasci N., Hung L.-H., Yan Z., Carpenter E. J., Wickett N. J., Mirarab S., Nguyen N., et al. 2014. Data access for the 1,000 Plants (1KP) project. GigaScience 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis D. E., Gitzendanner M., Stull G., Chester M., Chanderbali A., Chamala S., Jordon-Thaden I., et al. 2013. The potential of genomics in plant systematics. Taxon 62: 886–898. [Google Scholar]
- Strickler S. R., Bombarely A., Mueller L. A. 2012. Designing a transcriptome next-generation sequencing project for a nonmodel plant species. American Journal of Botany 99: 257–266. [DOI] [PubMed] [Google Scholar]
- Yockteng R., Almeida A. M. R., Yee S., Andre T., Hill C., Specht C. D. 2013. A method for extracting high-quality RNA from diverse plants for next-generation sequencing and gene expression analyses. Applications in Plant Sciences 1: 1300070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Q., Wu W. J., Xiao H. W., Chen H. B., Zheng Y., Zhang Y. J., Wang H. X., Huang L. Q. 2013. A generic plant RNA isolation method suitable for RNA-Seq and suppression subtractive hybridization. Genetics and Molecular Research 12: 5537–5546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.