Abstract
Immunization with live-attenuated Plasmodium sporozoites completely protects against malaria infection. Genetic engineering offers a versatile platform to create live-attenuated sporozoite vaccine candidates. We previously generated a genetically attenuated parasite (GAP) by deleting the P52 and P36 genes in the NF54 wild-type (WT) strain of Plasmodium falciparum (Pf p52−/p36− GAP). Preclinical assessment of p52−/p36− GAP in a humanized mouse model indicated an early and severe liver stage growth defect. However, human exposure to >200 Pf p52−/p36− GAP-infected mosquito bites in a safety trial resulted in peripheral parasitemia in one of six volunteers, revealing that this GAP was incompletely attenuated. We have now created a triple gene deleted GAP by additionally removing the SAP1 gene (Pf p52−/p36−/sap1− GAP) and employed flippase (FLP)/flippase recognition target (FRT) recombination for drug selectable marker cassette removal. This next-generation GAP was indistinguishable from WT parasites in blood stage and mosquito stage development. Using an improved humanized mouse model transplanted with human hepatocytes and human red blood cells, we show that despite a high-dose sporozoite challenge, Pf p52−/p36−/sap1− GAP did not transition to blood stage infection and appeared to be completely attenuated. Thus, clinical testing of Pf p52−/p36−/sap1− GAP assessing safety, immunogenicity, and efficacy against sporozoite challenge is warranted.
Introduction
Plasmodium parasites that cause malaria present a formidable threat to human health, primarily in resource-poor regions of the world.1 The tremendous morbidity and mortality inflicted by malaria infection could be dramatically diminished by a completely protective malaria vaccine.2 Fortunately, immunizations with live-attenuated sporozoites in animal models and humans have consistently demonstrated that a malaria vaccine conferring complete, protracted protection is possible.3,4,5,6,7,8,9 Recent work, involving immunizations with infectious Plasmodium falciparum sporozoites delivered by relatively small numbers of mosquito bites under concurrent chloroquine prophylaxis (chemoprophylaxis with sporozoites), showed robust, long-lasting protection against infectious sporozoite challenge.10 Furthermore, recent clinical studies with irradiation-attenuated P. falciparum sporozoites administered to humans intravenously showed complete protective efficacy against infectious sporozoite challenge at the highest-dose immunization regimen.11 This work built on previous studies showing that irradiation-attenuated P. falciparum sporozoites administered to humans by the bite of >1,000 infected mosquitoes conferred robust and near-complete protection against infectious sporozoite challenge.12 However, both chemoprophylaxis with sporozoites and irradiation-attenuated sporozoites do not allow for any intrinsic control over the design of the whole parasite immunogen. In contrast, engineered attenuation of parasites (genetically attenuated parasite (GAP)) offers a platform for controlled and consistent design of a whole parasite immunogen. Previous work with the rodent malaria parasites P. berghei and P. yoelii demonstrated the utility of genetic attenuation by deletion of the pre-erythrocytic stage-expressed genes called upregulated in infectious sporozoites (UIS)3 and UIS4, both of which resulted in early liver stage developmental arrest.13,14,15 Deletion of pre-erythrocytic stage-expressed genes P52 and P36 similarly resulted in an early liver stage developmental arrest.16,17 Immunization of mice with uis3−, uis4−, or p52− parasites induced complete long-lasting protection against infectious sporozoite challenge, demonstrating that rodent malaria GAPs are highly effective immunogens.13,14,15,18 Subsequently, a double gene deletion p52−/p36− GAP was generated in P. yoelii.19 Infection with Py p52−/p36− sporozoites did not cause blood stage infection in Balb/cJ mice, and immunization with Py p52−/p36− sporozoites conferred complete protection against intravenous infectious sporozoite challenge or infectious mosquito bite challenge.19 Deletion of P52 and P36 prevents the parasite from establishing or maintaining a parasitophorous vacuole membrane, which causes the parasite's attenuated phenotype. To assess orthologous genetic attenuation in P. falciparum, we previously generated a double gene deletion GAP lacking P52 and P36 (Pf p52−/p36−) in the NF54 strain.20 Analysis of the knockout (2 KO) parasites in vitro and in a humanized mouse model showed that the Pf p52−/p36− parasites suffered a liver stage growth defect early following hepatocyte infection. Subsequent clinical assessment showed that Pf p52−/p36− was not completely attenuated in human infection, and resulted in peripheral blood stage parasitemia in one of six volunteers exposed to >200 Pf p52−/p36−-infected mosquito bites.21 Therefore, the next step was to introduce a third gene deletion in these parasites that, when combined with the Pf p52−/p36− background, would potentially yield complete attenuation. In an earlier study, we identified the sporozoite asparagine-rich protein 1 (SAP1) to be essential for liver stage development of P. yoelii.22 The encoded protein localized to the cytoplasm of salivary gland sporozoites and deletion of SAP1 led to reduction in abundance of numerous UIS transcripts caused by increased RNA degradation.22,23 P. yoelii sap1− sporozoites exhibited a complete arrest in early liver stage development and the absence of blood stage parasitemia in mice injected with up to 2 × 106 sap1− sporozoites.22 Similar to P. yoelii, sap1− P. berghei also showed complete attenuation.24 Thus, we targeted P. falciparum SAP1 for deletion to generate a triple gene KO (3KO) GAP. Here, we report the successful generation of a Pf p52−/p36−/sap1− 3KO GAP and its characterization in preclinical assays.
Results
Complete attenuation of P. yoelii sap1− in the Balb/cByJ mouse strain
Our initial studies of the P. yoelii p52−/p36− GAP indicated complete attenuation because injection of 1 × 105 sporozoites in Balb/cJ mice did not lead to blood stage parasitemia (breakthrough).19 Similarly, no blood stage breakthrough infection was observed with the orthologous KO parasite in P. berghei (Pb p52−/p36−) in Balb/cJ mice.25 However, occasional breakthrough infection did occur in C57BL/6 mice injected with Pb p52−/p36− sporozoites, which are more susceptible to P. berghei infection.25 Thus, we tested if Py p52−/p36− could show breakthrough blood stage infection in a more susceptible mouse strain. We recently observed that Balb/cByJ mice, a congenic strain of Balb/cJ mice, carried significantly higher liver stage burden when compared to Balb/cJ mice (unpublished data). In light of the higher susceptibility of Balb/cByJ mice to P. yoelii, we infected Balb/cByJ mice with a high-dose (7.5 × 104) of P. yoelli p52−/p36− sporozoites and indeed observed breakthrough blood stage parasitemia in a fraction of these mice (Table 1). To evaluate the breakthrough capacity of Py sap1− parasites, we then tested the same dose of P. yoelii sap1− sporozoites in Balb/cByJ mice and observed no breakthrough blood stage parasitemia in any of the challenged mice (Table 1). This demonstrated that Py sap1− parasites are completely attenuated. Thus, we decided to pursue a SAP1 (PF3D7_1147000) deletion to achieve complete P. falciparum liver stage attenuation, reasoning that combining a deletion causing full arrest with two deletions causing severe but incomplete arrest in murine models would generate a parasite highly unlikely to cause breakthrough growth in humans.
Table 1. Infections of Plasmodium yoelii p52−/p36− and P. yoelii sap1− in the Balb/cByJ mouse model.
Generation of the P. falciparum p52−/p36−/sap1− triple gene KO parasite
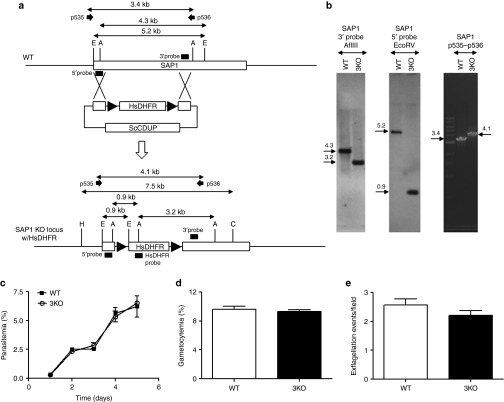
Our recent phase 1 clinical study with Pf p52−/p36− GAP showed that this 2KO parasite was severely but incompletely attenuated as one of six volunteers exposed to >200 Pf p52−/p36−-infected mosquito bites developed peripheral blood parasitemia.21 To further enhance the attenuated phenotype, we additionally targeted SAP1 in the Pf p52−/p36− genetic background. A clone of Pf p52−/p36− in which the positive selectable marker HsDHFR had been removed26 allowed for the introduction of a sequential gene deletion. SAP1 was deleted in this clone using the positive-negative selection strategy that was previously used to delete P52 and P36 (Figure 1).20 Following cloning of recombinant parasites by limiting dilution, Southern blotting of genomic DNA (Southern) and genotyping by polymerase chain reaction (PCR) were performed to confirm the successful deletion of SAP1 in this Pf p52−/p36−/sap1− 3KO as well as the absence of any residual Pf p52−/p36− parasites (Figure 1a,b).
Figure 1.
Strategy for targeted gene deletion of Pf SAP1, characterization, and phenotypic analysis of the Pf p52−/p36−/sap1−3KO. (a) Schematic of strategy for deleting the SAP1 gene in a Pf p52-/p36- KO clone lacking the HsDHFR marker.26 Enzymes and probes for Southern blotting and primers for polymerase chain reaction (PCR) are shown. Sizes of genomic DNA fragments and PCR products are indicated in kilobases. (b) Southern blotting (left and middle panels) and PCR (right panel) to show deletion of SAP1 in a Pf p52−/p36− KO clone (Pf p52−/p36−/sap1− 3KO). AflIII-digested genomic DNA was hybridized with a 3′ probe yielding a 4.3 and 3.2 kb band for wild type (WT) and 3KO DNA, respectively. Hybridization of EcoRV-digested genomic DNA with a 5′ probe detected a 5.2 and 0.9 kb band for WT and 3KO DNA, respectively. PCR on genomic DNA using primers p535 and p536 yielded a 3.4 and 4.1 kb band for WT and 3KO DNA, respectively. (c) Comparison of asexual blood stage growth rates, as measured by increase in parasitemia over time, between WT and 3KO. Cultures were initiated at 0.5% parasitemia and analyzed daily by Giemsa-stained thin blood smears until day 5. Growth assays were performed in triplicate for each line and parasitemia plotted as mean ± SEM. Mann–Whitney U-test was used for statistical analysis. (d) Comparison of % gametocytemia between WT and 3KO cultures at the time of feeding in vitro gametocyte cultures to mosquitoes (14–16 days postinitiation of in vitro gametocyte cultures). Gametocytemia was determined three independent times in duplicate cultures for each line and plotted as mean ± SEM. Mann–Whitney U-test was used for statistical analysis. (e) Comparison of number of male gamete exflagellation events between WT and 3KO gametocyte cultures at the time of feeding to mosquitoes. Exflagellation (plotted as mean ± SEM) was analyzed in several microscopic fields four independent times in duplicate and triplicate cultures for WT and 3KO, respectively. Mann–Whitney U-test was used for statistical analysis.
Pf p52−/p36−/sap1− parasites show normal blood stage growth and development in mosquitoes
Analysis of a the clonal population of the 3KO showed no observable defect during asexual blood stage replication (Figure 1c), in its ability to produce gametocytes (Figure 1d) or male gamete exflagellation (Figure 1e) when compared to wild type (WT). Evaluation of midgut infection in Anopheles stephensi mosquitoes fed with gametocyte cultures showed no significant differences between WT and 3KO parasites in oocyst prevalence (Figure 2a) and oocyst numbers per infected mosquito midgut (Figure 2b). Importantly, the invasion of mosquito salivary glands appeared unchanged in 3KO, as similar numbers of salivary gland sporozoites were isolated when compared to WT (Figure 2c). Additionally, 3KO sporozoites showed robust gliding motility on a solid substrate (Figure 2d).
Figure 2.
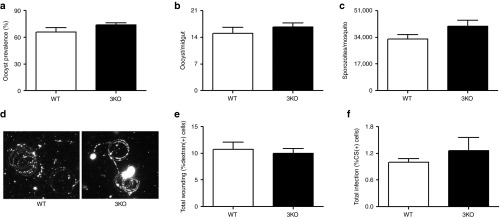
Mosquito stage development, and in vitro sporozoite host cell traversal and invasion assays. (a) Prevalence of oocysts in mosquito midguts infected with WT and 3KO parasites. Oocyst prevalence (plotted as mean ± SEM) was calculated by dividing the number of dissected midguts containing oocycts by the total number of midguts on day 7 postfeeding of mosquitoes with in vitro gametocyte cultures. Prevalence was determined in several mosquitoes four independent times in duplicate and quadruplicate for WT and 3KO, respectively. Mann–Whitney U-test was used for statistical analysis. (b) Comparison of average number of oocysts per mosquito midgut (plotted as mean ± SEM) between WT and 3KO on day 7 postfeeding of mosquitoes. Oocyts numbers were determined in several mosquitoes four independent times in duplicate and quadruplicate for WT and 3KO, respectively. Mann–Whitney U-test was used for statistical analysis. (c) Comparison of average number of sporozoites per mosquito (plotted as mean ± SEM) between WT and 3KO on 14–16 days postfeeding of mosquitoes. Sporozoite numbers were determined three independent times in at least duplicate for each line. Mann–Whitney U-test was used for statistical analysis. (d) Staining of CSP trails using Alexa 488-conjugated anti-PfCS 2A10 antibody in motility assays of salivary gland sporozoites from WT and 3KO. Sporozoites were collected 14–16 days postfeeding of mosquitoes. (e) Average total of HC-04 cells in traversal assays with WT and 3KO salivary gland sporozoites (plotted as mean ± SEM) as measured by the fraction of total HC-04 cells in the sample that had taken up FITC-dextran. Total dextran positive cells were determined four independent times in duplicate for each line. Mann–Whitney U-test was used for statistical analysis. (f) Average total infection of HC-04 cells by WT and 3KO salivary gland sporozoites (plotted as mean ± SEM) as measured by the fraction of total HC-04 cells in the sample that were positive for intracellular parasites as measured by CS staining. Total CS-positive cells were determined four independent times in duplicate for each line. Mann–Whitney U-test was used for statistical analysis CS, circumsporozoite; FITC, fluorescein isothiocyanate.
Pf p52−/p36−/sap1− sporozoites show normal cell traversal and host cell infection
We next investigated if the gene deletions in the 3KO clone would affect the ability of the parasite to traverse or invade hepatocytes, the two steps important for Plasmodium parasites to initiate liver infection. No significant differences were observed between WT and 3KO sporozoites in their ability to traverse hepatocytes in vitro (Figure 2e). Sporozoites of 3KO were also able to infect hepatocytes in vitro at levels comparable to WT (Figure 2f).
Pf p52−/p36−/sap1− parasites fail to complete liver stage development in FRG-HuHep mice
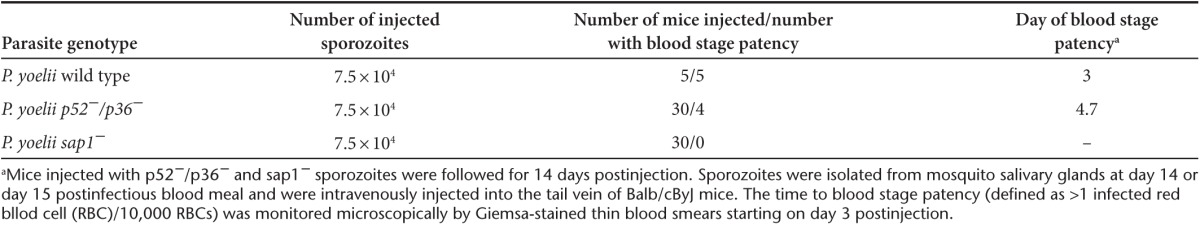
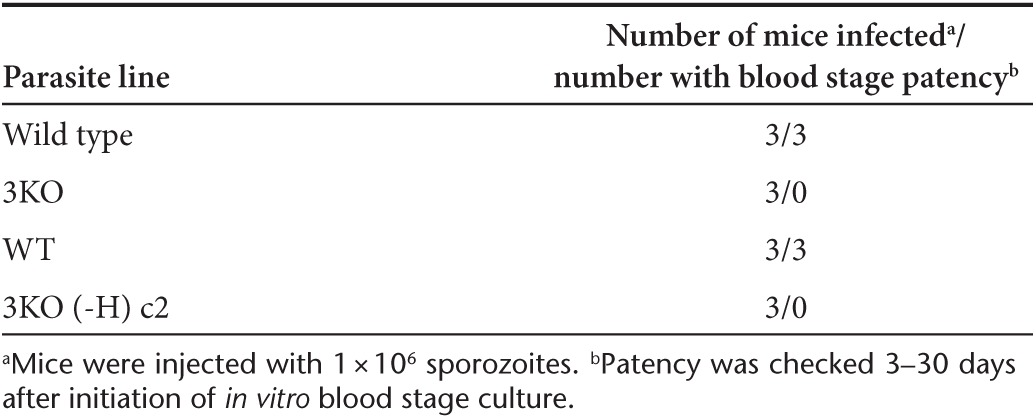
To evaluate whether 3KO parasites can complete liver stage development in vivo, FRG-HuHep (FRG) mice were injected intravenously with one million WT or 3KO sporozoites. Seven days after sporozoite infection, mice were injected with human red blood cells and subsequently sacrificed to collect the livers and peripheral blood. The blood was used for in vitro culture to detect the occurrence of blood stage infection.27 Blood collected from mice injected with WT sporozoites consistently produced patent parasitemia in culture. In contrast, none of the in vitro cultures of blood collected from the 3KO sporozoite-injected mice produced a positive blood stage parasite culture as evaluated by microscopic examination of blood smears for a period of 3 weeks postcollection (Table 2). Livers from these mice were also removed on day 7 and analyzed by immunofluorescence assay for presence of liver stage parasites. As expected, mature late stage parasites were detected in livers from WT sporozoite-injected mice (Supplementary Figure S2) at an average density of one parasite per 74 mm2 area of liver section (Supplementary Table S1). Conversely, no liver stages of any size were detected on day 7 in mice injected with 3KO sporozoites (Supplementary Table S1).
Table 2. Analysis of blood stage patency in FRG mice injected with wild type or Pf p52−/p36−/sap1− 3KO salivary gland sporozoites.
A next-generation GAP vaccine candidate for clinical studies
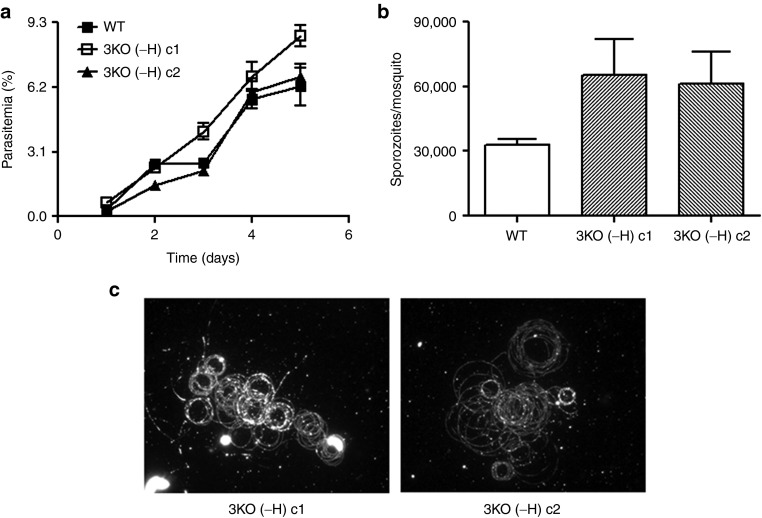
A GAP that could enter clinical development would need to be devoid of any extraneous DNA and not exhibit any drug resistance. Thus, we removed the HsDHFR selectable marker from the sap1− locus using the FLP/FRT system26 in 3KO parasites. Two clonal lines that were positive for Pf p52−/p36−/sap1− triple gene deletion were also devoid of any drug selectable marker cassettes (clones 3KO (-H) c1 and c2) by Southern and PCR analyses (Supplementary Figure S1 and Supplementary Table S2). These clones were analyzed for retention of vital characteristics needed to produce sporozoites for experimental vaccination. As with the parental 3KO parasite, no defects were observed in blood stage growth (Figure 3a), gametocyte production (data not shown), sporozoite production (Figure 3b), and gliding on solid substrates (Figure 3c). Additionally, 3KO (-H) c1 and c2 were susceptible to the drugs WR99210 and blasticidin, further demonstrating that the cassettes containing selectable markers conferring resistance to these drugs were no longer retained (Supplementary Table S3). Three FRG mice were injected with sporozoites of one of the 3KO (-H) clones to determine if the parasites showed the same degree of liver stage attenuation as observed for the marker-containing 3KO. As expected, no liver stage parasites were detected by immunofluorescence assays at day 7 post-3KO(-H) sporozoite injection, and in vitro culturing of blood collected from the 3KO(-H)-infected FRG mice did not show occurrence of blood stage infection during 3 weeks of culture (Table 2). Control mice injected with WT sporozoites had detectable liver stage infection and in vitro blood cultures were consistently parasitemic shortly after blood transfer from infected FRG mice (Table 2). Finally, we used reverse transcriptase polymerase chain reaction (RT-PCR) analysis to test for the presence of P. falciparum 18S A-type transcripts in day 7 liver samples from FRG mice infected with either WT or 3KO (-H) salivary gland sporozoites. While we detected a strong signal for 18S in FRG mice infected with WT sporozoites at day 7, no signal for 18S was detected in liver samples from three independent mice that had been injected with 3KO (-H) sporozoites (Supplementary Figure S3).
Figure 3.
Phenotypic analysis of Pf p52−/p36−/sap1−3KO (-H) clones. (a) Comparison of asexual blood stage growth rates between wild type (WT) and 3KO (-H) clones. Cultures were initiated at 0.5% parasitemia and analyzed daily until day 5. Growth assays were performed in triplicate for each line and parasitemia plotted as mean ± SEM. Mann–Whitney U-test was used for statistical analysis. (b) Comparison of average number of sporozoites (plotted as mean ± SEM) per mosquito between WT and 3KO (-H) clones 14–16 days postfeeding of mosquitoes. Sporozoite numbers were determined three independent times in at least duplicate for each line. Mann–Whitney U-test was used for statistical analysis. (c) Staining of CS trails using Alexa Fluor 488-conjugated anti-PfCSP 2A10 antibody in motility assays of salivary gland sporozoites of WT and 3KO (-H) clones.
Discussion
Despite the enormous importance of malaria, the goal of developing a P. falciparum vaccine that demonstrates high efficacy and confers long-lasting protection remains unrealized.28,29 Recent data however renewed optimism that a highly protective vaccine might be attainable when based on live-attenuated sporozoites. Seder et al.11 showed that intravenous immunization with repeated high doses of irradiated, cryopreserved P. falciparum sporozoites protected six out of six volunteers against infectious P. falciparum challenge. This is in agreement with historical data showing that intravenous immunizations with irradiation-attenuated sporozoites in animal models and by mosquito bite delivery in human volunteers induce sterile protection against subsequent sporozoite challenges. The efficacy of these whole live-attenuated sporozoite immunizations have so far been unmatched by any subunit malaria vaccines in development, including the most advanced CSP-based RTS,S vaccine candidate currently being tested in phase 3 clinical studies, which showed 30–50% protective efficacy and a relatively short duration of protection.30
Targeted gene deletion that allows for producing intrinsically and uniformly attenuated Plasmodium sporozoites with a potential of increased vaccination potency, is a design-based alternative strategy to irradiation-based parasite attenuation.31 In our previous study, deletion of two pre-erythrocytic stage-expressed genes (P52 and P36) in P. falciparum negatively affected the ability of sporozoites to create functional hepatocyte infection and initiate liver stage development, resulting in an attenuated phenotype.20 Phenotypic analysis of the P52 and P36 gene deletions in rodent malaria parasites implicated the lack of a parasitophorous vacuole membrane surrounding the parasites early in hepatocyte infection as the cause for the profound developmental defect in the p52−/p36− parasites.19,20 Despite this defect, however, the double gene deletion did not completely attenuate Pf p52−/p36− infections. This came to light in a first-in-human proof-of-concept safety study in which one of six volunteers developed peripheral blood parasitemia after exposure to >200 Pf p52−/p36− GAP-infected mosquito bites.21 Interestingly, it was recently shown that P. berghei rodent malaria p52−/p36− parasites could, in rare instances, develop within the hepatocyte without parasitophorous vacuole membrane formation,32 providing a potential explanation for the occurrence of breakthrough blood stage infections. We have here substantiated this potential for breakthrough in the P. yoelii model by demonstrating the infrequent occurrence of blood stage parasitemia in highly susceptible Balb/cByj mice challenged with Py p52−/p36− sporozoites. Thus, this model will be an important experimental addition to the toolbox that evaluates GAP phenotypes.
To optimize attenuation and prevent blood stage breakthrough of those rare parasites that could develop without P52 and P36 expression, we introduced an additional gene deletion into Pf p52−/p36− parasites. The deletion was selected to complete attenuation based on data from rodent malaria parasite models, which showed that Py and Pb sap1− parasites suffered complete attenuation of early liver stage growth and did not show breakthrough blood stage parasitemia.22,24 We corroborated this here with the highly sensitive P. yoelii-Balb/cByJ model. SAP1 was also selected for deletion as the putative function of the encoded protein significantly differs from the functions of P52 and P36. While SAP1 is a cytoplasmic protein involved in regulating RNA stability and as such impacts sporozoite gene expression,22 P52 and P36 are secreted proteins both involved in the formation of the parasitophorous vacuole membrane.19 Deletions of genes that are involved in independent biological processes should improve the robustness of complete attenuation and further reduce the possibility of compensatory changes in the parasite that might lead to loss of attenuation. We demonstrated here that the Pf p52−/p36−/sap1− triple gene deletion had no significant effect on gametocytogenesis, mosquito infectivity, or sporozoite production. Also, despite undergoing extensive sequential genetic manipulation and drug selection in vitro, Pf p52−/p36−/sap1− 3KO sporozoites showed neither reduced viability nor altered characteristics of initial infection, as measured by the ability of the sporozoites to glide on a solid substrate, traverse and infect hepatocytes.
To directly assess attenuation of the Pf p52−/p36−/sap1− 3KO parasite, we employed the robust humanized FRG mouse model harboring human hepatocytes and human red blood cells that allow for complete development of P. falciparum liver stages and supports liver stage-to-blood stage transition.27 Unlike WT parasites, 3KO parasites were undetectable in the livers of infected FRG mice at day 7 after sporozoite infection. Strikingly, no liver-to-blood transition of infection was observed for the 3KO, while WT infections reliably showed this transition. Long-term in vitro culture of the isolated blood also did not result in detectable parasitemia for 3KO. This further suggests that the 3KO parasites are fully attenuated and cannot undergo liver stage development. The removal of the drug resistance marker was achieved using the flippase (FLP)/flippase recognition target (FRT) system, which allowed for complete excision of all exogenous DNA from the 3KO genome. As a result, the 3KO (-H) clones are devoid of any drug resistance markers, which is currently a requirement for the use of genetically engineered agents in advanced clinical testing. It is also important to note that several rounds of genetic manipulation and prolonged in vitro culturing did not affect the fitness and viability of the 3KO (-H) clones. This further emphasizes that the development of P. falciparum genetically attenuated sporozoites for vaccination is feasible.
With the pursuit of a triple gene deletion Pf GAP, we have focused on achieving robust sporozoite production, viability, and complete attenuation, which is a prerequisite for the use of attenuated sporozoites in human vaccination. A recent clinical study with Pf p52−/p36− GAP showed that they induce substantial immune responses including functional antibody responses that can effectively block sporozoite infection in vitro.21,33 These data, together with extensive evidence that sap1− and p52−/p36− rodent GAPs engender sterile protection against sporozoite challenge in mice, give reasons to predict that the Pf p52−/p36−/sap1− triple gene deletion GAP will induce protective immune responses in humans.19,22,24 However, early liver stage arresting GAPs might not yet constitute the optimal live-attenuated immunogen. It was previously shown that late liver stage–arresting P. yoelii GAPs, created by gene deletions in the fatty acid biosynthesis pathway (FASII), produce superior immune responses and protection in mice.34,35 However, recent analysis of FASII gene deletions in P. falciparum showed an unexpected detrimental effect on sporozoite formation in oocysts, thus currently precluding the production of FASII KO GAPs for human immunization.36
In conclusion, we have developed a next-generation triple gene deletion GAP strain of P. falciparum. We used state-of-the-art preclinical tools to evaluate its degree of attenuation and found that 3KO produced viable, infectious sporozoites that arrested early, could not complete liver stage development and could not transition to blood stage infection. A proof-of concept clinical study with the 3KO GAP assessing safety, induction of cellular and humoral immune responses as well as preliminary efficacy against infectious sporozoite challenge is thus warranted.
Materials and Methods
Ethics statement. All animal studies were approved by the Institutional Review Board at Seattle BioMed.
In vitro culturing of parasite lines. The WT P. falciparum NF54 strain and KO lines were propagated in vitro in O+ human blood (Interstate Blood Bank, Memphis, TN) in custom-made Roswell Park Memorial Institute medium containing hypoxanthine, sodium bicarbonate, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Invitrogen, Life Technologies, Grand Island, NY) supplemented with 0.4% Albumax (Invitrogen) and gentamycin (Invitrogen) according to standard procedures for in vitro P. falciparum culturing.
Blood stage growth assay. In vitro cultures of parasites were set up in triplicate in 12-well plates at 0.5% starting parasitemia and 4% hematocrit (HCT). Media was replaced and parasitemia was determined daily for 5 days by Giemsa (Sigma-Aldrich, St Louis, MO)-stained thin smears to determine rate of growth.
Design of gene-targeting constructs and generation of KO parasite lines. Oligonucleotide primers used to generate 5′ and 3′ homologous recombination regions (5′ and 3′ flanks, respectively) for simultaneous deletion of P52 and P36 genes were as follows: P52 5′flank: 5′ CCGCGGGGATCTCTATAAATGCATGAGG (sense) and 5′; ACTAGTGAAGTTCCTATACTTTCTAGAGAATAGGAACTTCCTGGGTGAGTTTTTGCCGTAGTACTAAAAGCATCATTC (antisense); P36 3′ flank: 5′ CCTAGGGAA-GTTCCTATTCTCTAGAAAGTATAGGAACTTCGGGAATTTACATGCCATTCTATG (sense) and 5′ GGCGCCCCTATACCCTTCCCTTGTG (antisense). For P52/P36 targeting plasmid, the 5′ and 3′ flanks were cloned into SacII-SpeI and AvrII-SfoI, respectively in the pCC1 plasmid.37 Oligonucleotide primers used to generate 5′ and 3′ flanks for deleting SAP1 gene were as follows: 5′ flank: 5′ CCGCGGTGAAGAAAAGGGAAACCAAGACATGTG (SAP1 start codon mutated; italicized; sense) and 5′ ACTAGTATAACTTCGTATAGCATACATTATACGAAGTTATGGTGTATTATAACTTTGTGGTGTATTATAAC (antisense); 3′ flank: 5′ GAATTCATAACTTCGTATAATGTATGCTATACGAAGTTATCAGAATCAAAATTATATAACCAACC (sense) and 5′ CCTAGGCGTTGTTAAGATGTGGGTCTATATACG (antisense). 5′ and 3′ flanks for SAP1 targeting plasmid were cloned into SacII-SpeI and EcoRI-AvrII, respectively in pCC1. WT parasites were transfected (see below) to generate the p52−/p36− double KO parasites.20,26 Knockout parasites were cloned by limiting dilution. HsDHFR marker was removed by transfecting a clone of p52−/p36− with p-Tet-BSD-FLP plasmid.26 SAP1 was deleted in a clone of p52−/p36− lacking the HsDHFR marker yielding the p52−/p36−/sap1− triple KO. This line was further cloned by limiting dilution. One clone (3KO) was selected and transfected with the pTet-BSD-FLP plasmid generating a 3KO population lacking the HsDHFR marker in the SAP1 locus. This population was cloned again by limiting dilution and two clones (3KO (-H) c1 and c2) were picked.
Transfection of P. falciparum with KO plasmid constructs. Plasmid DNA was extracted by maxi prep (Qiagen, Valencia, CA). P. falciparum NF54 (WT) parasites were sorbitol synchronized38 and transfected with 30–40 µg plasmid by electroporation at 0.31 kV and 950 µF using a BioRad Gene Pulser (BioRad, Hercules, CA).39 To select for transfectants, cultures were placed under either WR99210 (WR; Jacobus Pharmaceuticals, Princeton, NJ) or Blasticidin (Invivogen, San Diego, CA) 48 hours after transfection depending on whether HsDHFR or AtBSD used as selectable marker, respectively.26 WR and Blasticidin were used at 2.5 nmol/l and 2.5 µg/ml, respectively.
Negative selection for KO parasites. Knockout parasites were generated by positive-negative drug selection.37 Briefly, transfectants positively selected using WR were propagated without WR to enrich for parasites that had lost the episomal KO plasmid. Thereafter, WR pressure was reapplied to select for parasites with the KO plasmid integrated in the genome. These parasites were placed under weekly cycles with or without 5-fluorocytosine (770 nmol/l) (Sigma-Aldrich) to select for KO parasites that had undergone target gene deletion by double crossover homologous recombination. 5-fluorocytosine-resistant parasites were genotyped by Southern and PCR.
Parasite cloning by limiting dilution. Knockout parasites were cloned by limiting dilution in 96-well flat bottom plates. Parasitemia (using Giemsa-stained thin smears) and HCT of cultures were accurately determined. Cultures were diluted and plated at a density of 0.5 parasite per well in a 200-µl volume at 2% HCT. Cultures were fed once a week with media containing fresh blood at 0.5% HCT. Parasitemia in wells was checked starting 14 days postinitiation of cloning.
Southern blotting and PCR. Southern blotting for the P52/P36 locus was performed by hybridizing HindIII-ClaI-digested genomic DNA from WT and KO lines with a 3′ probe. AflIII or EcoRV-digested DNA was hybridized with 3′ or 5′ probe, respectively to characterize WT and SAP1 KO locus. Digested DNA was run on a 0.7% Tris-acetate-EDTA agarose gel at 55 V and transferred to Hybond-N membrane (Amersham, GE Healthcare Life Sciences, Pittsburgh, PA) in 20× SSC overnight at room temperature (RT). DNA was UV crosslinked to the membrane and hybridized with digoxygenin-labeled probes prepared using the DIG kit (Roche Diagnostics, Indianapolis, IN). A P52/P36 locus 3′ probe was generated using oligonucleotide primers 5′ TATGTACATGTGAAAGTAGCAAAGAC (sense) and 5′ TTCCCTTGTGGGAAATTACAATGAC (antisense). 3′ probe for SAP1 locus was generated with oligonucleotide primers 5′ ATTATGAACATGACAATACAACTAACG (sense) and 5′ CATATTTATGCTACTGTCAGGGATAG (antisense). 5′ probe for SAP1 locus was generated using oligonucleotide primers 5′ CTAAAATACATAATATACGAAAAAAGTATG (sense) and 5′ TCATATGGCATATAAGATTGTATATCC (antisense). HsDHFR probe was generated with primers 5′ CCTGGCCACCGCTCAGGAACG (sense) and 5′ TCCTTGTCACAAATAGTTTAAGATGG (antisense). Primers 5′ CTCAAGAAGAATCCACCCTCATTG (sense) and 5′ CCACACATAACCAGAGGGCAGC (antisense) were used to make a BSD probe. Primers 5′ CAACCTGCAAAATCTAAATTGGT (sm002; sense) and 5′ GTAAATATATAAAACACTACAAATAGTAC (mo041; antisense) were used for PCR genotyping the P52/P36 locus, and 5′ TCCAAAAATTGACATTCAGAGTTATAG (p353; sense) and 5′ ACACTTATATGTATAGAAATAGTGTTAC (p536; antisense) for the SAP1 locus.
Mosquito infections. Gametocyte cultures of WT and KO lines were propagated in O+ human blood in custom-made Roswell Park Memorial Institute medium containing supplemented pooled human A+ serum (Interstate Blood Bank). Culture media was changed daily and culture volume was maintained around 35 ml. Gametocytogenesis was checked by Giemsa-stained thick smears. Exflagellation was checked by phase contrast microscopy at ×40 magnification beginning 12 days postinitiation of gametocyte cultures. The cultures were fed to mosquitoes when majority of the gametocytes were morphologically mature and vigorously exflagellating. Female A. stephensi mosquitoes aged 4–7 days were starved for 1–2 hours and fed for at least 30 minutes at 37 °C on a Baudruche membrane feeder apparatus (Joseph Long, Belleville, NJ). Each cage with 250–300 mosquitoes was fed with concentrated erythrocytes from the 35 ml gametocyte culture mixed with an equal volume of fresh red blood cells and 2 volumes of A+ serum. Oocysts prevalence was determined by microdissecting whole midguts and examining them at ×10 magnification using a phase contrast microscope. Midgut and salivary gland sporozoite numbers were determined by microdissecting and grinding whole midguts and salivary glands from mosquitoes on day 7 and days 14–16 postfeeding with in vitro gametocyte cultures, respectively, and counting using a hemocytometer.
Traversal and invasion. Traversal and invasion assays were performed as previously described.40 Briefly, HC-04 cells were plated at 300K cells/well in a 24-well plate the day before in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% heat-inactivated fetal bovine serum (Sigma-Aldrich), penicillin (200 IU/ml)/streptomycin (200 µg/ml) (Corning, Corning, NY), and 5 ml of amphotericin B (Fungizone) (Corning). Salivary gland sporozoites of WT and KO lines were activated in Roswell Park Memorial Institute medium and 20% fetal bovine serum at RT for 15 minutes. Sporozoites were transferred to HC-04-coated chamber slides at 100K/well (3:1 ratio of cells:sporozoites). Fluorescein isothiocyanate-dextran (Invitrogen) was added to the appropriate wells to assess total wounding by sporozoites. The slides were centrifuged at 1,500 rpm for 3 minutes at RT and incubated at 37 °C for 1.5–2 hours. Media was removed and cells were fixed and permeabilized in Cytofix/Cytoperm (Becton Dickinson, Franklin Lakes, NJ). Cells were stained with anti-PfCS mouse monoclonal antibody (2A10) and analyzed by flowcytometry for total wounding (dextran+ cells) and total infection (CS+ cells).
Motility. Glass coverslips (VWR International, Radnor, PA) were precoated with 10 ng/ml 2A10 antibody in phosphate-buffered saline overnight at RT. Salivary gland sporozoites of WT and KO parasites were activated in Roswell Park Memorial Institute medium containing 20% fetal bovine serum and allowed to glide on antibody-coated coverslips at 37 °C for 2 hours. Coverslips were fixed in 10% neutral buffered formalin (Sigma-Aldrich), blocked in 2% bovine serum albumin–phosphate-buffered saline, stained with Alexa 488-conjugated 2A10 antibody, and mounted in ProLong Gold Antifade Reagent (Life Technologies). Motility was assessed by detecting CS protein shed in gliding trails on the coverslips.
Patency. FRG-HuHep mice (Yecuris Corporation, Tualatin, OR) were injected intravenously with 1 × 106 each of WT and KO salivary gland sporozoites. On days 6 and 7, these mice were injected with 400 µl of washed O+ human blood at 50% HCT. On day 7, 3–4 hours following injection of human blood, the mice were sacrificed, and peripheral blood was collected by cardiac puncture, washed three times, and in vitro cultures were set up to determine progression of infection from liver stage to blood stage. Blood stage patency was assessed starting day 2 postblood collection by Giemsa-stained thin smears.
Indirect immunofluorescence assay. After collecting peripheral blood for in vitro culturing, the livers of infected mice were perfused with phosphate-buffered saline, dissected out, washed with phosphate-buffered saline, and fixed in 10% neutral buffered formalin. Fifty micrometer sections were cut using a Vibratome apparatus (Ted Pella, Redding, CA). Immunofluorescence assays were performed as previously described.41 Primary antibodies used were anti-BiP (monoclonal) and anti-ACP (polyclonal).
RT-PCR assay. Liver samples were collected in TRIzol (Life Technologies) from FRG mice day 7 postinfection with 1 × 106 intravenously injected either WT or 3KO (-H) salivary gland sporozoites. Total RNA was extracted using the Direct-zol MiniPrep Kit (Zymo Research, Irvine, CA). cDNA synthesis was performed using the QuantiTect Reverse Transcription Kit (Qiagen). PCR cycling conditions used for amplification of cDNA were 92 °C for 30 seconds for DNA denaturation, 54 °C for 30 seconds for primer annealing, and 62 °C for 1 minute for extension (35 cycles). P. falciparum 18S A-type rRNA was amplified using primers 5′ CCAGTAGTCATATGCTTGTCTC and 5′ GAAGCGTATTAAAGCGAAAAGC (~700 bp product). Human ApoA1 was amplified using primers 5′ AGCGTGACCTCCACCTTCAG and 5′ CCTTCACCTCCTCCAGATCCTT (~150 bp product).
SUPPLEMENTARY MATERIAL Figure S1. Strategy for removal of HsDHFR marker from the SAP1 KO locus in 3KO. Figure S2. Immunofluorescence of in vivo liver stage development. Figure S3. RT-PCR of RNA collected on day 7 of liver stage development. Table S1. Density of liver stage infection in mice injected with WT or 3KO salivary gland sporozoites. Table S2. Sizes of fragments detected by Southern and PCR products for WT, 3KO and 3KO (-H) clones. Table S3. Assay for retention of drug resistance markers in 3KO and 3KO(-H) clones.
Acknowledgments
The research was partially funded by a Grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (Grant ID: 1481). This research and development program was also made possible by a cooperative agreement that was awarded and administered by the US Army Medical Research & Materiel Command and the Telemedicine & Advanced Technology Research Center, at Fort Detrick, MD, under contract number: W81XWH-11-2-0184. The authors declare no conflict of interest. S.H.I.K. is an inventor listed on US Patent No. 7,22,179, US Patent No. 7,261,884, and international patent application PCT/US2004/043023, each titled “Live Genetically Attenuated Malaria Vaccine.”
Supplementary Material
Strategy for removal of HsDHFR marker from the SAP1 KO locus in 3KO.
Immunofluorescence of in vivo liver stage development.
RT-PCR of RNA collected on day 7 of liver stage development.
Density of liver stage infection in mice injected with WT or 3KO salivary gland sporozoites.
Sizes of fragments detected by Southern and PCR products for WT, 3KO and 3KO (-H) clones.
Assay for retention of drug resistance markers in 3KO and 3KO(-H) clones.
References
- World Health Organization Malaria Control Department. (2013). Malaria report 2013 . http://www.who.int/malaria/publications/world_malaria_report_2013/en
- Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- Clyde DF, McCarthy VC, Miller RM, Hornick RB. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266:398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24:397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1974;68:258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH, Beaudoin RL, Cassells JS, Sell KW. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull World Health Organ. 1979;57 suppl. 1:261–265. [PMC free article] [PubMed] [Google Scholar]
- Rieckmann KH. Human immunization with attenuated sporozoites. Bull World Health Organ. 1990;68:13–16. [PMC free article] [PubMed] [Google Scholar]
- Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. VRC 312 Study Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, Franetich JF, et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS One. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- Douradinha B, van Dijk M, van Gemert GJ, Khan SM, Janse CJ, Waters AP, et al. Immunization with genetically attenuated P52-deficient Plasmodium berghei sporozoites induces a long-lasting effector memory CD8+ T cell response in the liver. J Immune Based Ther Vaccines. 2011;9:6. doi: 10.1186/1476-8518-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuskirk KM, O'Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci USA. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, Labaied M, et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol. 2008;69:152–163. doi: 10.1111/j.1365-2958.2008.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH. SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol Microbiol. 2011;79:929–939. doi: 10.1111/j.1365-2958.2010.07497.x. [DOI] [PubMed] [Google Scholar]
- Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 2008;4:e1000086. doi: 10.1371/journal.ppat.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoura T, Ploemen IH, van Schaijk BC, Sajid M, Vos MW, van Gemert GJ, et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine. 2012;30:2662–2670. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]
- O'Neill MT, Phuong T, Healer J, Richard D, Cowman AF. Gene deletion from Plasmodium falciparum using FLP and Cre recombinases: implications for applied site-specific recombination. Int J Parasitol. 2011;41:117–123. doi: 10.1016/j.ijpara.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PE, Sahu T, Akue A, Milman N, Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Expert Rev Vaccines. 2012;11:1261–1280. doi: 10.1586/erv.12.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Douglas AD, Draper SJ. Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum Vaccin Immunother. 2013;9:1831–1840. doi: 10.4161/hv.25383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, et al. RTS,S Clinical Trials Partnership A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. Genetic engineering of attenuated malaria parasites for vaccination. Curr Opin Biotechnol. 2012;23:908–916. doi: 10.1016/j.copbio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Ploemen IH, Croes HJ, van Gemert GJ, Wijers-Rouw M, Hermsen CC, Sauerwein RW. Plasmodium berghei Δp52&p36 parasites develop independent of a parasitophorous vacuole membrane in Huh-7 liver cells. PLoS One. 2012;7:e50772. doi: 10.1371/journal.pone.0050772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney OC, Keitany GJ, Smithers H, Kaushansky A, Kappe S, Wang R. Immunization with genetically attenuated P. falciparum parasites induces long-lived antibodies that efficiently block hepatocyte invasion by sporozoites. Vaccine. 2014;32:2135–2138. doi: 10.1016/j.vaccine.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9:451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaijk BC, Kumar TR, Vos MW, Richman A, van Gemert GJ, Li T, et al. Type II Fatty Acid Biosynthesis Is Essential for Plasmodium falciparum Sporozoite Development in the Midgut of Anopheles Mosquitoes. Eukaryot Cell. 2014;13:550–559. doi: 10.1128/EC.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier AG, Braks JA, Waters AP, Cowman AF. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol Biochem Parasitol. 2006;150:118–121. doi: 10.1016/j.molbiopara.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Wu Y, Sifri CD, Lei HH, Su XZ, Wellems TE. Transfection of Plasmodium falciparum within human red blood cells. Proc Natl Acad Sci USA. 1995;92:973–977. doi: 10.1073/pnas.92.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky A, Rezakhani N, Mann H, Kappe SH. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol Biochem Parasitol. 2012;183:100–103. doi: 10.1016/j.molbiopara.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Mikolajczak SA, Camargo N, Lakshmanan V, Kennedy M, Lindner SE, et al. A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol Biochem Parasitol. 2012;186:143–147. doi: 10.1016/j.molbiopara.2012.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strategy for removal of HsDHFR marker from the SAP1 KO locus in 3KO.
Immunofluorescence of in vivo liver stage development.
RT-PCR of RNA collected on day 7 of liver stage development.
Density of liver stage infection in mice injected with WT or 3KO salivary gland sporozoites.
Sizes of fragments detected by Southern and PCR products for WT, 3KO and 3KO (-H) clones.
Assay for retention of drug resistance markers in 3KO and 3KO(-H) clones.