Abstract
Objective
Pathological gaits have been shown to limit transfer between potential (PE) and kinetic (KE) energy during walking, which can increase locomotor costs. The purpose of this study was to examine whether energy exchange would be limited in people with knee osteoarthritis (OA).
Methods
Ground reaction forces during walking were collected from 93 subjects with symptomatic knee OA (self-selected and fast speeds) and 13 healthy controls (self-selected speed) and used to calculate their center of mass (COM) movements, PE and KE relationships, and energy recovery during a stride. Correlations and linear regressions examined the impact of energy fluctuation phase and amplitude, walking velocity, body mass, self-reported pain, and radiographic severity on recovery. Paired t-tests were run to compare energy recovery between cohorts.
Results
Symptomatic knee OA subjects displayed lower energetic recovery during self-selected walking speeds than healthy controls (p=0.0018). PE and KE phase relationships explained the majority (66%) of variance in recovery. Recovery had a complex relationship with velocity and its change across speeds was significantly influenced by the self-selected walking speed of each subject. Neither radiographic OA scores nor subject self-reported measures demonstrated any relationship with energy recovery.
Conclusions
Knee OA reduces effective exchange of PE and KE, potentially increasing the muscular work required to control movements of the COM. Gait retraining may return subjects to more normal patterns of energy exchange and allow them to reduce fatigue.
Keywords: Knee osteoarthritis, Energy recovery, Mechanical work, Locomotor costs
Introduction
It is well understood that the movements of the center of mass (COM) of a person during normal walking on a relatively stiff leg (with limited knee flexion) follow the cycle of an inverted pendulum and that this pattern influences the exchange of energy and muscular work required to accelerate and decelerate (strictly defined as positive or negative accelerate) the COM1-5. In walking, the stored gravitational potential energy (PE) of the COM is at its highest during midstance, when the kinetic energy (KE) of the COM is at its lowest. As it leaves this midstance position and the COM descends, PE is converted to KE, and the horizontally directed component of KE moves the body forward to land on the contralateral limb. After this footfall, the COM again moves upward (as long as the limb remains relatively straight) driven partly by KE and stores PE that can again be returned as KE at the next step-to-step transition. The efficiency of this energy exchange between PE and KE can be as high as 70% during normal human walking at preferred speeds. When the exchange is efficient, it can reduce the amount of muscular effort needed to accelerate and decelerate the COM1, 6. Several studies have separately indicated that the metabolic cost of walking is primarily allocated towards raising the COM throughout the gait cycle7-9. Thus, this mechanism of exchanging KE and PE may serve to reduce the metabolic cost of locomotion by reducing the muscular effort required to accelerate and decelerate the COM2.
Some studies have considered the mechanical energy required to raise the COM in various populations affected by pathologies, such as cerebral palsy10 and hemiplegia11-13 that lead to gait dysfunction and have been shown to increase metabolic costs. In all cases, subjects have been shown to have abnormal patterns of energy exchange and recovery. To date, however, few studies have examined the biomechanics of the COM and gait efficiency of people affected with osteoarthritis, and those studies have to date been limited12-15.
Knee osteoarthritis affects over 9.2 million people over the age of 26 in the United States alone16, making it the most widespread form of OA17. People affected by knee OA are more likely to report general fatigue and pain after daily activities18, 19, walk at reduced speeds20, 21, and exhibit lower maximal isometric strength in knee extension and flexion with quicker muscular fatigue22 when compared to those without OA. Previous studies have tested the physiological costs of gait in people with knee OA, but have focused on cardiac and ventilatory costs rather than on mechanical costs23, 24. The only study to date to specifically examine mechanical work in patients with knee OA was conducted by Detrembleur, et al15 reported on a group of 8 patients with mild to moderate OA who were still able to walk without the use of an assistive device. This study reported that these OA patients had an energy recovery of 44% without intervention and had an improvement of approximately 10% with a pharmacologic intervention15. The study of Detrembleur and colleagues sets the stage for the current work15. Although their sample size was small, the results suggest that energy recovery may be a considerable physiological problem for patients with significant levels of knee OA. Yet the underlying mechanisms—what mechanical factors are driving low energy recovery in subjects with OA—associated with low energy recovery remain unknown. Nor is it known how walking speed will influence energy recovery in this population. The goal of the present study is to examine patterns of energy recovery in OA subjects and to examine the aspects of COM movements that are driving energy recovery values in this population.
In that context, it is worth reviewing the factors that contribute to effective exchange of potential and kinetic energy. Percentage recovery can be affected by fluctuations in the relative magnitude of PE and KE and the phase relationships between KE and PE (the amount of time during the stride in which PE and KE curves changed in the same direction), also described as congruity (Fig. 1) 25, 26. Both the magnitude of PE and KE as well as the phase relationship can be influenced by mechanical factors. For example, if the knee is flexed more deeply during stance the rise of the COM could be reduced and therefore follow a flatter path. This in turn could lower the amount of gravitational potential energy stored in the system and reduce the percentage recovery. Similarly, if toe-off were delayed until the COM was rising (long double support phase) then congruity may be increased which would result in a decrease in recovery. The pattern of knee and ankle motion described above have been reported for subjects with OA22, 27. But these gait parameters have not been examined in the context of COM movements and costs of locomotion. As such, this study has the potential to fill a significant gap in the literature on OA gait mechanics by looking at the consequences of gait changes on ambulatory function and mobility.
Fig 1.
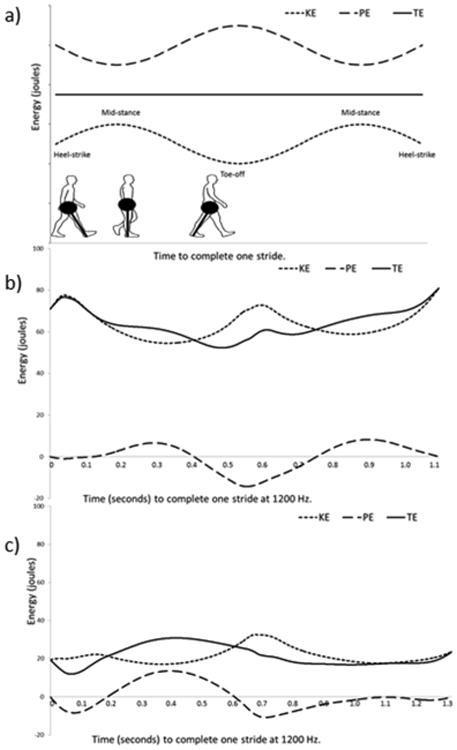
The association of Potential Energy (PE), Kinetic Energy (KE), and Total Energy (TE) of the center of mass in a theoretical model of 100% recovery (a) and two examples from osteoarthritis (OA) subjects from the current study (b and c). In (a) PE and KE are of the same magnitude and 100% out of phase (0% congruity) leading to a pattern of TE that does not fluctuate. Recovery (as calculated following the methods described in the text) would be 100%. The points in the gait cycle are indicated and in the lower left is found a schematic of human walking with a pendular models of the human lower limb (as a massless rod) with the mass of the body concentrated at one end. In (b) PE and KE fluctations are highly out of phase with low congruity (10%) and high recovery (67%). In (c) PE and KE flucations are relatively in phase (congruity of 36%) compared to the subject in (b) and also differ in magnitude, resulting in a low recovery value (38%)
In addition to kinematic parameters, changes in walking speed can directly alter recovery6, 9. Previous work in knee OA has reported a significant decrease in walking speed when compared to a healthy control group20. Previous studies have suggested that the relationship between recovery, the efficiency of PE and KE energy exchange, and speed in a healthy individual is parabolic with relatively low values at speeds that are exceptionally slower or faster than preferred walking speed (i.e. recovery drops off by 10% or more when walking speed is less than 1 m/s or greater than 2 m/s when preferred speed is 1.5 m/s) 1, 6. Since KE primarily incorporates energy from forward velocity and PE primarily represents energy from COM vertical displacements, which can be influenced by stride length, one explanation for this pattern may be a disproportionate magnitude of PE fluctuations relative to KE fluctuations at slower than preferred speeds and the reverse at speeds higher than preferred.
As a result of the predictive (albeit parabolic) relationship between speed and recovery, it may be possible to understand the influence of speed versus other OA-associated disability factors (i.e. pain and range of motion) on energy recovery. The recognition of this complex relationship between recovery and walking speed leads to a model in which subjects should be tested at their preferred speed and the fastest speed at which they are comfortable. An analysis of recovery and speed should explicitly compare those values relative to expectations for speed and recovery in an asymptomatic population in order to better understand whether the energy recovery results are due to walking speed or could be associated with other gait limitations in pathologic populations.
With this framework in mind, the following hypotheses were developed and tested. First, it was hypothesized that individuals with knee OA would exhibit a decreased energy recovery during level walking at all speeds when compared to normal, healthy individuals. Second, it was hypothesized that change in energy recovery from self-selected to fast speeds would be dependent on self-selected velocity, as described above, and allow us to account for the effect of subject walking speed. Finally, we hypothesized that the energy recovery of subjects with knee OA would be negatively correlated with the radiographic severity of the most affected limb28. Since KL grade is also associated with self-report of pain, we expect a similar negative relationship between energy recovery and self-reported measures of pain28. Therefore, subjects with more severe OA based on radiographic assessment and/or self-report would have increased gait disability and lower energy recovery. It is important to note that both radiographic assessment and self-report are instruments that cannot capture all aspects of OA-related pain and disability. Both approaches are blunt measurements. The first involves a rough 1 – 4 scale of OA development. This is necessary and appropriate for organizing broad patterns of OA but this system misses nuance in OA severity. Similarly, self-report relies on people's subjective sense of both pain and disability and, in this case, data for each knee were not reported. Nonetheless, these tools are standard measures in both clinical and research settings and some measures of gait disability have been shown to be reflected in self-report29.
Methods
Subjects
Ninety-three subjects (18 men, 75 women) with symptomatic knee OA were examined in this study. Subjects were participating in a larger clinical trial29-32 in which they had to exhibit chronic knee pain, be overweight or obese (BMI between 25 and 42kg/m2), meet the American College of Rheumatology criteria for symptomatic knee OA, and have no other weight bearing joint symptomatically affected by OA. ACR criteria for referral to this study by a diagnosing physician include knee pain, joint stiffness, crepitus, joint enlargement, and joint tenderness. Radiographic estimations of joint OA severity were completed after the subject was referred to the research study. As a result, in some cases, baseline gait data was collected before radiographic scores were reported. Subjects were excluded from the study if they had a significant medical condition that would increase the risk of an adverse experience, were involved in regular exercise, had an abnormal cardiac response to exercise, had a non-OA inflammatory arthropathy, were morbidly obese, or if they regularly used corticosteroids.
Radiographic estimations of OA severity and self-reported measures of pain and disability
Weight bearing, fixed-flexion (30°) posterior-anterior radiographs of both knees were examined by a reader experienced in diagnosing OA severity and graded on the Kellgren/Lawrence scale33. For bilaterally affected subjects, the most affected limb was used in all analyses. The goal of this study was to determine changes in energy recovery in patients with osteoarthritis. The subject pool include persons with bilateral or unilateral disease because the analysis was performed on the entire stride and the presence of any OA will affect the entire stride patterns and therefore will influence recovery. In this sample, we did not have a self-reported measure of pain or disability associated with a specific limb, therefore, we chose to use the knee with the greatest radiographic OA severity as the KL score for the subject, which we expected to have the greatest influence on gait. At the time of testing, each OA subject completed self-reported assessments of pain, including the Arthritis Impact Measurement Scales (AIMS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and a Visual Analog Pain Scale (VAS).34 The specific protocol used in this study for filling out the questionnaires is discussed in detail in a previous study by Nebel et al29.
Subject pool demographics and metrics
Prior to participation, each subject read and signed a consent form approved by the Institutional Review Board. At the time of testing, the OA subjects had a mean age of 58.9±10.7 years, a mean BMI of 32.9±4.7 kg/m2, and a median radiographically determined K/L score of 3 on the most affected knee (Table 1). Thirteen healthy control subjects (8 men, 5 women), that were recruited for a separate study in our lab35, were included in the study analysis. These controls were between the age of 40 and 70 and had to be pain free at the time of testing, have no previous history of lower extremity surgeries, and no clinical diagnosis of OA in any other lower extremity joint aside from the knee. At the time of testing, the control subjects had a mean age of 49.8±7.5 years and a mean BMI of 26.3±8.7 kg/m2.
Table 1. Number of subjects in each of the K/L score groups for both the fast and normal walking speeds.
| K/L Score | Self Selected Speed (N) | Fast Speed (N) |
|---|---|---|
| 0 | 4 | 4 |
| 1 | 6 | 9 |
| 2 | 11 | 8 |
| 3 | 43 | 32 |
| 4 | 17 | 17 |
| Unscored | 1 | 0 |
The population under study here, like many populations of subjects with knee OA, are mildly to moderately overweight. In that sense the results of this study are potentially influenced by two factors—weight and OA—but we control for some of that by having records of both radiographic severity and self-report of pain as well as by testing for the effect of BMI on recovery. Soft tissue movement is a concern in kinematic studies of overweight persons. But in this case only the sacral marker was used for any analysis. The sacral marker was used to test for acceleration and deceleration within a stride and provide a conservative exclusion criterion.
Gait Data
An eight-camera motion analysis system sampling at 120Hz (Motion Analysis Corporation, Santa Rosa, CA) was used in conjunction with four force plates embedded in the walkway sampling at 1200Hz (AMTI, Watertown, MA) to collect ground reaction forces during level walking. A single reflective marker was placed at the sacrum in order to track the speed of the COM (walking speed) within each stride during each of the walking trials. This marker was used in order to evaluate whether strides met steady-state criteria of no notable acceleration or deceleration. Only steps in which no obvious acceleration or deceleration over the time of the stride were selected for analysis. This steady state was determined by calculating whether or not a single marker placed at the sacrum exhibited anterior-posterior acceleration or deceleration across the stride with an average value not significantly different from zero.
Five walking trials were collected along a 10m walkway at both a self-selected preferred and a self-selected fast walking speed for the symptomatic subjects and at only a self-selected preferred walking speed for the healthy control group. For each of the self-selected preferred walking trials, subjects were asked to walk at the speed at which they typically perform their daily activities. For the fast speeds, subjects were asked to walk as fast as they felt comfortable. Gait speed was measured during data collection using two wireless infrared photocell timing devices placed 5m apart in the middle of the 10m walkway. This walking speed data was used to ensure that subjects completed all walking trials within ±5% of their average walking speed for 3 practice walking trials before the self-selected preferred and self-selected fast walking speeds.
Calculations
Stored gravitational potential energy (PE) and kinetic energy (KE) were calculated from all three components of force output using MATLAB software version R2010a (The Mathworks Inc., Natick, Massachusetts, United States) following the computations set forth originally by Cavagna et al.1 and used in subsequent studies6, 10, 12, 13, 15, 25, 26. All calculations were carried out for walking trials in which a complete stride was obtained with one clean foot strike of each leg on different force plates in order to acquire complete ground reaction forces for both legs and the ability to reconstruct the forces over the entire stride. This requirement, along with the steady state requirement for integration as described below, limited the number of subjects who could be included at preferred and fast speeds. As a result, of the 93 total participants, 82 subjects could be included in the analysis of the self-selected preferred speed, while 70 subjects could be included in the analysis of the self-selected fast speed. Of these two groups, 59 subjects overlapped and were therefore included in both analyses.
The force plate outputs were combined to generate the force values for the entire stride. The output was divided by mass, and acceleration due to gravity was removed from the vertical component resulting in curves representing the acceleration of the COM in all three orthogonal planes of motion (vertical, anterior-posterior, medial-lateral). Integration of the acceleration data provided velocity, while double integration provided the displacement of the COM, which were both used to calculate the KE of the COM in all three planes and the PE of the COM in the vertical plane.
Following previous methods of integrating the acceleration and velocity curves1, 26, it was assumed that average vertical velocity and displacement during a complete stride were both zero, which served as the integration constant for the vertical plane analyses. The same assumption was made for the medial-lateral motion. However, for the anterior-posterior calculations of KE, the average horizontal velocity of the subject served as the integration constant. The horizontal velocity was determined based on the velocity of the sacral marker that was collected using three dimensional motion capture. As much as was possible with a pathological population, only steps that could be considered steady state were used based on inclusion criteria described above. The percent of COM energy recovery was calculated according to the formula 26 (KE=kinetic energy, PE=potential energy, TE=total energy):
| (1) |
Recovery is influenced by the shape of the PE and KE curves, the degree to which PE and KE peaks are out of phase (PE is high when KE is low), and the difference in amplitude between the oscillation of KE and PE1, 25, 26. Percentage congruity, the percent of time throughout the stride in which PE and KE changed in the same direction, was calculated following Ahn et al.'s25 equation for congruity and the determination of the proportion of positive values across time.
| (2) |
Percentage congruity was used as a measure of the phase relationship between the two energy curves. High congruity suggests curves that are highly in-phase and should reflect reduced recovery. Amplitude differences between KE and PE oscillations were calculated by determining the amplitude differences (KE - PE) between the oscillation peaks throughout the stride and then averaging the differences.
Statistical Analysis
Each variable was averaged for each subject across trials. Parametric Pearson Correlation analyses were performed to examine the associations between COM percent recovery and congruity, energy oscillation amplitude differences, BMI, K/L score, and velocity. Regression lines and R2 values were calculated for relevant variables. Furthermore, COM percent recovery was averaged across 0.25 m/s increments of velocity from 0.5-2.0 m/s and compared to values previously reported by Mian et al.6. Independent t-tests were run to compare the mean recoveries for the symptomatic subjects and the healthy controls at the self-selected walking speed. An alpha level of 0.05 was used to indicate statistically significant differences between groups. All statistical analyses were performed using JMP, Version Pro 10.0.0 (SAS Institute Inc., Cary, NC).
Results
Preferred Walking Velocity
For this analysis we needed full, steady state strides with each limb in contact with separate plates (see methods for further detail). Not all subjects provided datasets for both preferred and fast walking speeds. Therefore, of the original 93 subjects, 82 provided data that could be analyzed at self-selected preferred walking speeds. These subjects had an average walking velocity of 1.04±0.18 m/s. The means for the mechanical energy variables calculated during self-selected preferred walking indicated a 55.6±10.5% COM percent recovery, 16.4±8.8% congruity, and PE had on average a higher amplitude than KE (-3.24±3.46 J) (Table 2).
Table 2. Means and standard deviations for each cohort tested and comparative values of previously collected data.
| N | Age (yrs) | BMI (kg/m2) | Velocity (m/s) | Recovery (%) | Congruity (%) | Energy Difference (J) | |
|---|---|---|---|---|---|---|---|
| Knee OA | 82 | 59.57±10.96 | 32.69±4.78 | 1.04±0.18 | 55.64±10.52 | 16.35±8.82 | -3.24±3.48 |
| Preferred Speed | (57.17,61.98) | (31.64,33.74) | (0.99,1.08) | (53.33,57.96) | (14.41,18.29) | (-4.01,-2.48) | |
| Knee OA | 70 | 58.61±11.05 | 32.98±4.71 | 1.38±0.28 | 54.06±10.90 | 15.99±9.75 | -0.46±3.54 |
| Fast Speed | (55.98,61.25) | (31.86,34.10) | (1.31,1.44) | (51.46,56.67) | (13.66,18.32) | (-1.30,0.39) | |
| Mian et. Al6 Old | 20 | 74.0±3.4 | 26.06 | 1.25±0.32 | 63.2±3.1 | - | - |
| Preferred Speed | - | - | - | - | - | - | |
| Mian et. al6 Young | 12 | 26.6±3.3 | 25.12 | 1.25±0.32 | 60.9±2.8 | - | - |
| Preferred Speed | - | - | - | - | - | - | |
| Healthy Controls | 13 | 49.77±7.46 | 26.26±8.67 | 1.38±0.22 | 65.45±3.20 | 10.33±3.58 | -2.47±2.71 |
| Preferred Speed | (45.26,54.28) | (21.02,31.50) | (1.24,1.51) | (63.50,67.40) | (8.17,12.49) | (-4.11,-0.83) | |
|
| |||||||
| p=0.0027* | p<0.0001* | p<0.0001* | p=0.0018* | p=0.0257* | p=0.457 | ||
significant differences: p values represent independent t-test differences between the knee OA preferred speed means and the healthy controls preferred speed means
Values are reported as mean ± sd and (95% confidence intervals)
There was a strong significant negative relationship (p<0.0001, R2=0.656) between COM percent recovery and congruity (Fig. 2b) and no relationship (p=0.502) between recovery and oscillation amplitude difference (Fig. 2a). The majority of variation in energy recovery is explained by the phase relationship of the fluctuations in KE and PE. Recovery and the K/L score (a measure of radiographic severity) of the most affected knee were not significantly correlated (p=0.280). A similarly weak correlation was found for associations of recovery with subject self-reported pain (Table 2).
Fig. 2.
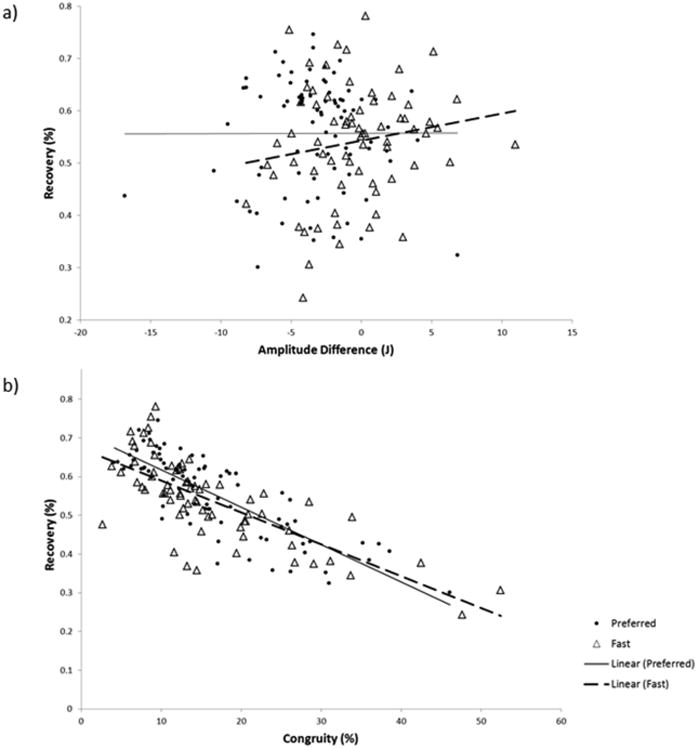
Representation of the percent of variance in the COM recovery that is explained by (a) amplitude oscillation differences and (b) percent congruity.
Fast Walking Velocity
Of the original group of 93 subjects, 70 subjects had datasets complete enough to analyze at the fast speed (see methods for further detail). They exhibited an average walking velocity of 1.38±0.28ms-1, which is similar to the comfortable gait speed of 1.39ms-1 for similarly aged men and women36. When walking at the fast walking speed, subjects had a 54.1±10.9% COM percent recovery, 16.0±9.8% congruity, and fluctuations in PE had on average a higher amplitude than those of KE (-0.46±3.52 J) (Table 2).
As with preferred speeds, there was a strong, significant negative relationship (p<0.001, R2=0.545) between COM percent recovery and congruity (Fig. 2b). The relationship between COM percent recovery and oscillation amplitude difference was not significant (p=0.196) (Fig. 2a). Recovery and the K/L score of the most affected knee were not significantly correlated (p=0.473) (Table 2).
Preferred vs. Fast Walking Velocity
Of the original 93 subjects, 84 provided data at preferred speeds and 70 at fast speeds. There was an overlap of 59 subjects who provided data at both speeds and were available for direct comparison across self-selected preferred and fast walking speeds (see methods for further detail). Subjects who exhibited a slower self-selected preferred speed were found to increase their COM percent recovery when walking at a faster speed, while subjects who exhibited a self-selected preferred walking speed closer to that expected for an unaffected person of the same leg length were found to decrease their COM percent recovery when engaging in fast walking (R2=0.275) (Fig. 3).
Fig. 3.
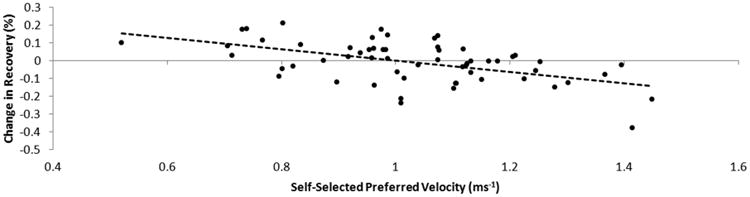
The relationship between the change in the energy recovery of each subject (fast-preferred) during walking. Subjects with slower self-selected preferred walking speeds were more likely to increase their recovery by speeding up, while those with faster self-selected speeds were more likely to decrease their recovery by speeding up.
It is worth noting that several of the subjects with a KL value of 3 or 4 showed show minimum recovery values lower than any exhibited by subjects with KL values of 0 through 2. However, ranges for all groups overlapped considerably and the subjects with KL values of 1 and 2 did not have a higher upper range than those with values of 3 or 4. The few extremely low recovery values seen in patients with low KL values in at least one knee were not enough to drive any statistical trend (Table 2). Similarly, KL score did not statistically explain variation in speed in this sample. However, subjects with KL values of 4 in at least one knee never achieved walking speeds higher than 1.4 m/s when asked to use their fastest comfortable walking speed. Once again, the high variation and overlap in walking speed did not allow for a significant difference across groups or correlation of speed with KL value.
Healthy Controls
The symptomatic knee OA subjects were significantly different from our healthy controls in all variables except energy amplitude difference (Table 2). Of note, the controls exhibited higher percent recovery values and faster self-selected walking velocities (p=0.0018 and p<0.0001, respectively).
Discussion
Subjects affected with symptomatic knee osteoarthritis demonstrate relatively lower energy recovery values at all speeds compared to our own sample of healthy adults. In order to place the results for our OA subjects and asymptomatic controls in context of an older asymptomatic population, we compared our values to that of Mian et al.6 (Table 2, Fig. 4). This comparison demonstrates that our values are lower than those of a healthy sample of similarly aged subjects from this study. The values reported here can also be compared with the one other study of energy recovery in OA15. Our values are not quite as low as those reported by Detrembleur et al.15 Taken together, our data confirm the findings of Detrembleur et al.15 and fail to reject the hypothesis that subjects with knee OA will experience lower values when estimating the exchange of PE and KE when compared with asymptomatic subjects of similar age and body mass.
Fig. 4.
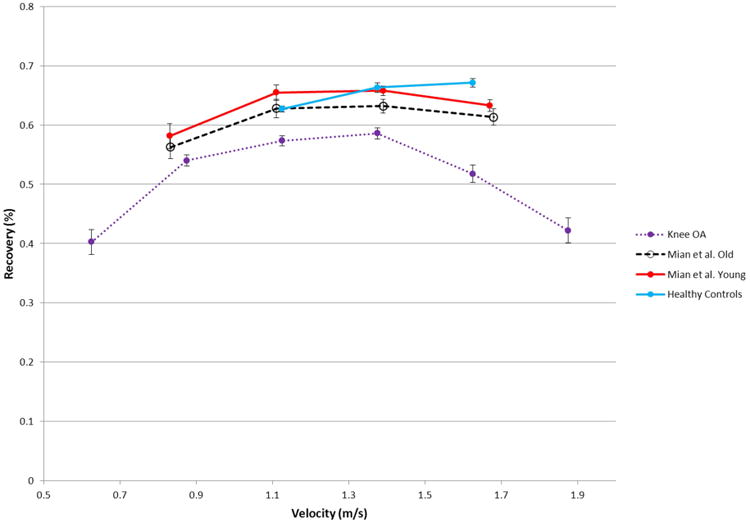
The relationship between recovery and speed. Points indicate the average recovery for 0.25ms-1 increments of velocity and lines indicate the standard deviation. The recovery of knee OA subjects exhibits the standard parabolic shape across speeds but at a lower value than both old and young healthy subjects tested by Mian et. al6 and our own controls.
The source of the relatively low energy exchange in subjects with OA is multifactorial. The lower recovery values reported for this population at a self-selected speed could have been due to the fact that subjects with OA walked at slower speeds than those typically exhibited by unaffected subjects. When subjects increased walking speed into a range that was consistent with unaffected subjects, the recovery values increased (Fig. 4). These results support our second hypothesis that the value of recovery varies in a predictable way with speed. Therefore, subjects who increase speed to the normal speed exhibited by healthy subjects of their stature may experience improvements in energy recovery values. In order understand the effect of walking speed on recovery we restricted the analysis to the area of speed overlap and compared recovery. A highly significant difference (P<0.001) between recoveries of asymptomatic subjects (65%) and OA subjects (56%) in the same speed range existed (Fig 4), suggesting that walking velocity is an important factor in limiting energy recovery in this population, but not the only factor driving this difference.
When walking velocity was accounted for in this population, most of the variation in COM energy recovery was explained by phase differences in the oscillations of KE and PE. Overall, in this population, the KE and PE fluctuations were moving in the same direction (congruence) for 16% of the walking cycle, and this congruity accounted for over 60% of the variance in recovery. In contrast, the amplitude difference between the KE and PE peaks was found to be less important in knee OA recovery. However, amplitude did play a marginally larger role at the faster speed, explaining 3% of the variation compared to approximately zero percent of the variation at the self-selected speed. Although not a direct measure of metabolic costs, our recovery results demonstrate inefficiency in the walking dynamics of patients with symptomatic knee OA, and therefore may also be indirectly representative of higher metabolic costs.
Low energy recoveries appear to be a consistent characteristic of subjects with knee OA regardless of disease severity. In this regard, our third hypothesis-- that recovery would be negatively correlated to radiographic severity of knee OA and self-reported measures of pain--was rejected. Neither K/L score nor any self-report of pain was significantly correlated to energy recovery (Table 2). Previous work by Nebel et al29 did find that AIMS physical disability and WOMAC function scores explained a significant proportion of the variation in gait parameters in this same population; however, the center of mass movements reported here are distinct from the measures of speed, range of motion, and peak force reported by Nebel et al 29 and our focus was primarily on the relationship between pain and COM mechanics.
This study is a first and relatively novel step in examining COM mechanics in subjects with OA. The study grew from a separate study and thus has limitations that deserve to be acknowledged. The subjects walked across multiple force plates rather than a single large surface, which required a more complicated analysis. In addition, although all subjects had been referred to the study by a physician not all had significant levels of radiographically-diagnosed OA. All subjects in the OA pool were also mild or moderately overweight and represented an older age group. Finally we have not yet established a precise control group that includes age-matched people with high BMI values and no OA and low BMI values with OA, both of which are hard to find in a population over fifty years of age. Despite these limitations, the results are consistent with the few other studies of energy recovery in older people6 and those with OA15. We also provide new analysis on congruity and speed and the consequences of gait changes on ambulatory function and mobility.
One of the goals for patient care and treatment for those persons with osteoarthritis is to establish gait patterns that allow normal activities to be conducted with minimal pain and fatigue. Returning energy recovery to values closer to those in unaffected populations may reduce muscular effort during walking and reduce both pain and fatigue. The data presented here shows that people affected by knee OA consistently exhibit gait patterns that yield low levels of energy exchange. Therefore, future research can focus on the kinematics driving recovery in affected populations and develop appropriate interventions.
Table 3.
Parametric correlations of each variable to energy recovery at the two speeds obtained. Values in bold are significant.
| Velocity | Congruity | Amplitude | BMI | K/L | AIMS Pain | WOMAC Pain | VAS | |
|---|---|---|---|---|---|---|---|---|
| Preferred | 0.494 | -0.8097 | 0.0025 | 0.0109 | 0.052 | -0.126 | -0.0683 | 0.0419 |
| Fast | -0.2653 | -0.7379 | 0.168 | 0.059 | 0.0053 | -0.0459 | 0.1152 | 0.0532 |
Acknowledgments
The authors thank Dr. Ershela Sims for her significant contribution to the initial data collection and analysis, Justin DeBiasio for data processing, and Dr. Virginia Kraus for her insight and knowledge in regards to osteoarthritis.
Role of the Funding Source: This study was supported by the National Institutes of Health (grant no. AR50245). The funding source had no role in the study design, collection, analysis and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Author Contributions: All authors collectively worked on the study conception and design:
The original design of the initial study: FJK, FG, DS
Collection of self-reported data: FJK
Collection of biomechanical data: DS, RMQ, TJS
Development of analysis technique: TLS, DS, CEM
Analysis and interpretation of the data: DS, RMQ, CEM, TLS
Drafting of the article: TLS,DS
All authors revised the article and provided final approval of the submitted version.
DS takes responsibility for the integrity of the work as a whole, from inception to finished article.
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tawnee L. Sparling, Email: tlsparling@me.com.
Charlotte E. Miller, Email: charlotte.miller@duke.edu.
Farshid Guilak, Email: farshid.guilak@duke.edu.
Tamara J. Somers, Email: tamara.somers@dm.duke.edu.
Francis J. Keefe, Email: francis.keefe@dm.duke.edu.
References
- 1.Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1977;233:R243–R261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- 2.Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. The Journal of Physiology. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo AD. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Human Movement Science. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Kuo AD, Donelan JM, Ruina A. Energetic Consequences of Walking Like an Inverted Pendulum: Step-to-Step Transitions. Exercise and Sport Sciences Reviews. 2005;33:88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cavagna GA, Thys H, Zamboni A. The sources of external work in level walking and running. The Journal of Physiology. 1976;262:639–657. doi: 10.1113/jphysiol.1976.sp011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mian OS, Thom JM, Ardigò LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiologica. 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 7.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. Journal of Applied Physiology. 2005;98:579–583. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- 8.Duff-Raffaele M, Kerrigan DC, Corcoran PJ, Saini M. The Proportional Work of Lifting the Center of Mass During Walking1. American Journal of Physical Medicine & Rehabilitation. 1996;75:375–379. doi: 10.1097/00002060-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: the energetic cost of raising the body's center-of-mass is significant. Journal of Biomechanics. 2004;37:817–825. doi: 10.1016/j.jbiomech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Bennett BC, Abel MF, Wolovick A, Franklin T, Allaire PE, Kerrigan DC. Center of Mass Movement and Energy Transfer During Walking in Children With Cerebral Palsy. Archives of Physical Medicine and Rehabilitation. 2005;86:2189–2194. doi: 10.1016/j.apmr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Detrembleur C, Dierick F, Stoquart G, Chantraine F, Lejeune T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait & Posture. 2003;18:47–55. doi: 10.1016/s0966-6362(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 12.Tesio L, Civaschi P, Tessari L. Motion of the Center of Gravity of the Body in Clinical Evaluation of Gait. American Journal of Physical Medicine & Rehabilitation. 1985;64:57–70. [PubMed] [Google Scholar]
- 13.Iida H, Yamamuro T. Kinetic analysis of the center of gravity of the human body in normal and pathological gaits. Journal of Biomechanics. 1987;20:987–995. doi: 10.1016/0021-9290(87)90328-9. [DOI] [PubMed] [Google Scholar]
- 14.Cavagna GA, Tesio L, Fuchimoto T, Heglund NC. Ergometric evaluation of pathological gait. Journal of Applied Physiology. 1983;55:606–613. doi: 10.1152/jappl.1983.55.2.606. [DOI] [PubMed] [Google Scholar]
- 15.Detrembleur C, De Nayer J, van den Hecke A. Celecoxib improves the efficiency of the locomotor mechanism in patients with knee osteoarthritis. A randomised, placebo, double-blind and cross-over trial. Osteoarthritis Cartilage. 2005;13:206–210. doi: 10.1016/j.joca.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis & Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Annals of the Rheumatic Diseases. 1991;50:8–13. doi: 10.1136/ard.50.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schepens SL, Kratz AL, Murphy SL. Fatigability in Osteoarthritis: Effects of an Activity Bout on Subsequent Symptoms and Activity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67:1114–1120. doi: 10.1093/gerona/gls076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazis LE, Meenan RF, Anderson JJ. Pain in the rheumatic diseases. Arthritis & Rheumatism. 1983;26:1017–1022. doi: 10.1002/art.1780260811. [DOI] [PubMed] [Google Scholar]
- 20.Mündermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: Reduced walking speed. Arthritis & Rheumatism. 2004;50:1172–1178. doi: 10.1002/art.20132. [DOI] [PubMed] [Google Scholar]
- 21.Stauffer RN, Chao EY, Gyory AN. Biomechanical gait analysis of the diseased knee joint. Clin Orthop Relat Res. 1977;126:246–255. [PubMed] [Google Scholar]
- 22.Fisher NM, White SC, Yack HJ, Smolinski RJ, Pendergast DR. Muscle function and gait in patients with knee osteoarthritis before and after muscle rehabilitation. Disability and Rehabilitation. 1997;19:47–55. doi: 10.3109/09638289709166827. [DOI] [PubMed] [Google Scholar]
- 23.Bernardi M, Macaluso A, Sproviero E, Castellano V, Coratella D, Felici F, et al. Cost of walking and locomotor impairment. Journal of Electromyography and Kinesiology. 1999;9:149–157. doi: 10.1016/s1050-6411(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 24.Waters RL, Perry J, Conaty P, Lunsford B, O'Meara P. The Energy Cost of Walking with Arthritis of the Hip and Knee. Clinical Orthopaedics and Related Research. 1987;214:278–284. [PubMed] [Google Scholar]
- 25.Ahn AN, Furrow E, Biewener AA. Walking and running in the red-legged running frog, Kassina maculata. Journal of Experimental Biology. 2004;207:399–410. doi: 10.1242/jeb.00761. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill MC, Schmitt D. The gaits of primates: center of mass mechanics in walking, cantering and galloping ring-tailed lemurs, Lemur catta. The Journal of Experimental Biology. 2012;215:1728–1739. doi: 10.1242/jeb.052340. [DOI] [PubMed] [Google Scholar]
- 27.Stauffer RN, Chao EY, Gyory AN. Biomechanical gait analysis of the diseased knee joint. Clin Orthop Relat Res. 1977:246–255. [PubMed] [Google Scholar]
- 28.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. Journal of Orthopaedic Research. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 29.Nebel MB, Sims EL, Keefe FJ, Kraus VB, Guilak F, Caldwell DS, et al. The Relationship of Self-Reported Pain and Functional Impairment to Gait Mechanics in Overweight and Obese Persons With Knee Osteoarthritis. Archives of Physical Medicine and Rehabilitation. 2009;90:1874–1879. doi: 10.1016/j.apmr.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: A randomized controlled study. PAIN. 2012;153:1199–1209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sims EL, Keefe FJ, Kraus VB, Guilak F, Queen RM, Schmitt D. Racial differences in gait mechanics associated with knee osteoarthritis. Aging Clinical and Experimental Research. 2009;21:463–469. doi: 10.1007/bf03327442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims EL, Carland JM, Keefe FJ, Kraus VB, Guilak F, Schmitt D. Sex Differences in Biomechanics Associated with Knee Osteoarthritis. Journal of Women & Aging. 2009;21:159–170. doi: 10.1080/08952840903054856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellgren J, Lawrence J. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation-Study of Womac - a Health-Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug-Therapy in Patients with Osteo-Arthritis of the Hip or Knee. Journal of Rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 35.Queen RM, Vap A, Nunley JA, Bolognesi MP, Butler RJ. Biomechanics differences between end-stage hip, knee, and ankle OA when compared with healthy controls. In Review. [Google Scholar]
- 36.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age and Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]


