Abstract
Each skeletal muscle contains a fixed ratio of fast and slow myofibers that are distributed in a stereotyped pattern to achieve a specific motor function. How myofibers are specified during development and regeneration is poorly understood. Here we address this question using transgenic reporter mice that indelibly mark the myofiber lineages based on activation of fast or slow myosin. Lineage tracing indicates that during development all muscles have activated the fast myosin gene Myl1, but not the slow myosin gene Myh7, which is activated in all slow but a subset of fast myofibers. Similarly, most nascent myofibers do not activate Myh7 during fast muscle regeneration, but the ratio and pattern of fast and slow myofibers are restored at the completion of regeneration. At the single myofiber level, most mature fast myofibers are heterogeneous in nuclear composition, manifested by mosaic activation of Myh7. Strikingly, Myh7 is activated in a subpopulation of proliferating myoblasts that co-express the myogenic progenitor marker Pax7. When induced to differentiate, the Myh7-activated myoblasts differentiate more readily than the non-activated myoblasts, and have a higher tendency, but not restricted, to become slow myotubes. Together, our data reveal significant nuclear heterogeneity within a single myofiber, and challenge the conventional view that myosin genes are only expressed after myogenic differentiation. These results provide novel insights into the regulation of muscle fiber type specification.
Keywords: Myogenesis, Muscle fiber type, Cre/LoxP, Regeneration, Satellite cells
Introduction
The skeletal muscle comprises ~40% of the body mass and functions to empower body movements and regulate systemic energy metabolism (Karagounis and Hawley, 2010; Zierath and Hawley, 2004). The skeletal muscle is mainly composed of mature muscle cells called myofibers, and myofiber composition in each muscle is heterogeneous, containing a mixture of slow and fast type myofibers (Wang et al., 2004). Based on the relative abundance of slow and fast myofibers, a muscle can either be classified as a slow or a fast muscle. For example, the soleus (SOL) muscle is a slow muscle as it contains mostly slow (or type I) myofibers; and the extensor digitorum longus (EDL) muscle is a fast muscle because its myofibers are predominantly fast (type II) (Waddell et al., 2010). The slow and fast myofibers are different in biochemical, structural and physiological properties (Bassel-Duby and Olson, 2006). One main criterion to classify myofiber types is based on the myosin heavy chain (Myh) isoform expression. In this scenario, fast myofibers can be further divided into type IIA, IIX and IIB based on the expression of Myh2, Myh1 and Myh4 genes, respectively (Chakkalakal et al., 2012). By contrast, type I slow myofibers uniquely express the Myh7 gene, encoding the β-myosin heavy chain protein that is also expressed in cardiac muscles (Chen and Wang, 2012). The contractile speed of these myofibers ranks in the order of IIB > IIX > IIA > I. Based on energy utilization, myofibers can also be classified as oxidative (I, IIA) and glycolytic (IIX, IIB) myofibers.
When skeletal muscles are damaged, they regenerate to reestablish the preexisting myofiber types (Feldman and Stockdale, 1991). Satellite cells, a population of muscle resident stem cells, are responsible for the regeneration of injured muscles (Relaix and Zammit, 2012). Satellite cells are quiescent in non-injured adult muscles. In response to muscle injury, they are activated and reenter the cell cycle to proliferate, then differentiate and fuse with the damaged myofibers to repair the damages (Kuang and Rudnicki, 2008). Meanwhile, a subpopulation of the proliferating myoblasts undergoes self-renewal to replenish the quiescent satellite cell pool. The self-renewal, proliferation and differentiation status of satellite cells can be distinguished by the expression of Pax7 and MyoD, manifested as Pax7+/MyoD−, Pax7+/MyoD+ and Pax7−/MyoD+, respectively (Olguin and Olwin, 2004; Zammit et al., 2004).
A long-standing unresolved question is whether myofiber specification occurs at the progenitor cell level (i. e. subpopulations of progenitor cells give rise to fast and slow myofibers, respectively) or at the post-differentiation level (i. e. fast and slow myofibers originate from a common progenitor population). Nevertheless, it has been widely accepted that Myh genes are only expressed in post-differentiation muscle cells. In the present study, we conducted genetic lineage analyses to determine the specification of fast and slow myofibers during development and regeneration. As Myh7 and Myl1 genes are unique markers of mature slow (type I) and fast (type II) myofibers, respectively, we used Myh7-Cre and Myl1-Cre mice, in combination of fluorescent reporter mice, to delineate if mature fast and slow myofibers originate from progenitors expressing the corresponding myosin genes. Using this strategy, we show that Myl1 gene is activated in all fast and slow myofibers, but Myh7 is only activated in a subset of fast myofibers in addition to slow myofibers. Surprisingly, we found that Myh7 is activated in a subset of actively proliferating myoblasts, thus challenging the previous view that Myh genes are only activated in differentiated cells. When induced to differentiate, the Myh7-activated myoblasts were not limited to become slow myotubes, though they had a higher tendency to do so. These results provide mechanistic insights into muscle fiber type specification.
Materials and methods
Animals
All procedures involving the use of animals were performed under the guideline of Purdue University’s Animal Care and Use Committee. Mice were housed in animal facility with free access to water and standard rodent chow. The reporter mice were purchased from Jackson Laboratory (Bar Harbor, ME) under that stock numbers: Rosa26-EYFP (Madisen et al., 2010), 007903; Rosa26-tdTomato (Madisen et al., 2010), 007905; and Rosa26-mTmG (Muzumdar et al., 2007), 007576. The Myh7-Cre transgenic mouse (Parsons et al., 2004) was provided by Katherine Yutzey (Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio). The Myl1-Cre transgenic mouse (Bothe et al., 2000) was provided by Steven Burden (New York University Medical Center, NY). The PCR genotyping was done using protocols described by the supplier.
Cardiotoxin (CTX) injection
CTX (Sigma) was used to induce muscle regeneration. Mice were anesthetized by IP injection of 0.1 ml ketamine cocktail per 10 g body weight and TA/EDL muscles were injected with 50 µl of 10 µM CTX. Muscles were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek) and fresh-frozen in isopentane chilled on dry ice. Frozen samples were cut into 10 µm thickness sections on a CM1850 cryostat (Leica). Muscle sections were collected on Superfrost Plus glass slides (Electron Microscopy Sciences).
Immunostaining and imaging
Cyosections were thawed at room temperature and washed with PBS to wash out the OCT. Samples were then blocked in PBS containing 5% goat serum, 2% BSA, 0.2% triton X-100 and 0.1% sodium azide. Primary antibodies used were anti-Pax7, anti- MYHC I, anti-MYHC IIA, anti-MYHC IIB (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), anti-MyoD (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-Laminin (Sigma-Aldrich, St. Louis, MO, USA). Samples were incubated in primary antibodies diluted in blocking buffer for overnight at 4 °C, followed by incubating with secondary antibodies and 4’, 6-diamidino-2-phenylindole (DAPI) diluted in PBS for 30 minutes at room temperature. Cultured cells were fixed by 4% PFA, and then quenched by 100nM Glycine, followed by similar staining steps. Stained samples were mounted with Dako fluorescent mounting media (DAKO corp., Carpinteria, CA, USA). Fluorescent images were captured with a Coolsnap HQ CCD camera (Photometrics, USA) driven by IP Lab software (Scanalytics Inc, USA) using Leica DMI6000B fluorescent microscope (Leica Microsystems, Mannheim, Germany) with 1.25×, 10×, or 20× objectives.
Primary myoblast culture
Hindlimb muscles were collected and trimmed free of connective tissues and tendons, minced with scissors and digested in collagenase/dispase in 6-cm perti dishes at 37 °C for 24 minutes. The digestion solution contained 1% collagenase B (Roche, Indianapolis, IN, USA) and 2.4 U/ml dispase (Roche). The digestion was stopped by adding 3× volume of Ham’s F-10 medium (Multicell) with 5% FBS and triturated, then centrifuged at 250×g for 5 minutes using an IEC CENTRA CL2 centrifuge (Thermo Electron Corporation). After centrifugation, the pellet was resuspended and filtered through 70 µm cell strainer to remove debris, and cells were pelleted and grown in 10 cm collagen-coated dishes with 10 ml of Ham’s F-10 medium containing 20% FBS, 1% penicillin/streptomycin (P/S) and 4 ng/ml bFGF at 37 °C and 5% CO2. The initial culture was refreshed with 5 ml growth medium every 24 h and passaged to a new collagen-coated dish after 72 h. During passages, cells were treated with 0.25% trypsin and closely monitored. To remove fibroblasts, the trypsinization was stopped immediately when most myoblasts are detached from the plate but before fibroblasts detach. Cells were fed with fresh medium every two days. Upon confluence, cells were induced to differentiate in DMEM (Sigma) containing 2% horse serum and 1% P/S.
Isolation and culture of single myofibers
Intact EDL and SOL muscles were dissected out by cutting both tendons and digested in 0.2% type I collagenase (Sigma) in DMEM at 37 °C with agitation for 30 minutes (EDL muscle) or 70 minutes (SOL muscle) until fibers began to detach from the muscle. After digestion, muscles were triturated with polished glass Pasteur pipettes to liberate myofibers from the collagenase loosened EDL and SOL muscles. All pipettes and petri dishes were pre-rinsed with horse serum to prevent myofiber attachment. Single myofibers were either directly fixed or cultured in DMEM containing 20% FBS, 1% P/S, 0.5% Chicken Embryo Extract (CEE).
Statistical analysis
All data are presented as mean ± standard deviation (SD). The “n” in the results represents biological replicates unless specified. At least three technical replicates were usually conducted within each biological sample and the averaged value was used. For the significance analysis, two-tailed student’s t-test was used to calculate the P values. If the P value is less than 0.05, then data are considered as statistically significant.
Results
Activation of Myh7 and Myl1 genes during muscle development
To investigate the progeny of Myh7-lineage myofibers in “fast” and “slow” muscles of adult mice, we established Myh7-Cre/Rosa26-EYFP(or TdT) reporter mice in which myofibers that have once activated the Myh7 promoter were indelibly labeled by EYFP or TdTomato (a red fluorescent protein). To evaluate the specificity of the Myh7-Cre, we examined developing somites and limbbuds of Myh7-Cre/Rosa26-TdT mice. All cells labeled by the Myh7 monoclonal antibody were also TdT+, and 71% of TdT+ cells were Myh7+(Supplemental Fig. S1). This result suggests that Myh7-Cre sufficiently marks all cells that concurrently express Myh7 but additionally marks some cells that have undetectable amounts of Myh7 protein. Additional analysis later in this study confirms that the Myh7-Cre marked myoblasts expressed higher levels of Myh7 mRNA (with undetectable protein), thus confirming the fidelity of the Myh7-Cre mouse.
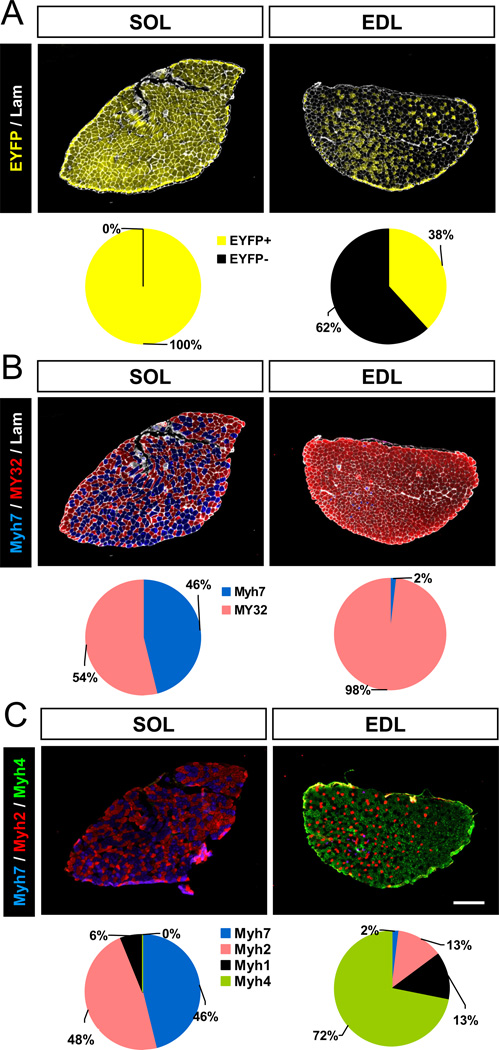
At two months old, all myofibers in the SOL muscle of Myh7-Cre/Rosa26-EYFP mice were EYFP+, but only 38% myofibers in the EDL muscle were EYFP+ (Fig. 1A). To confirm the concurrent expression of myosin in SOL and EDL muscles, we stained serial sections from the same muscles with Myh7 and MY32 antibodies that react with slow and fast Myh, respectively. There were 46% Myh7+ myofibers in the SOL muscles, but only 2% Myh7+ myofibers in the EDL muscles, the rest being MY32+ (Fig. 1B). These results suggest that many mature fast myofibers have expressed Myh7 during development. To further address which subtype of fast myofibers have historically activated Myh7, cohort of serial muscle sections were stained with monoclonal antibodies specifically recognize type I, type IIA and type IIB Myh, and the unlabeled myofibers were presumably type IIX. In the SOL muscle, there were 46% type I, 48% type IIA and 6% type IIX (and 0% IIB) myofibers (Fig. 1C). By contrast, EDL muscles had 72% type IIB, 13% type IIX, 13% type IIA and 2% type I myofibers (Fig. 1C). Co-localization with EYFP signal indicates that 100% type I, 100% type IIA, 97% type IIX and 14% type IIB myofiber have activated the Myh7 (Supplemental Fig. S2). Similar activation pattern of Myh7 was observed in TA muscles (data not shown). These results indicate that mature type I and a subset of type II (primarily IIA and IIX) myofibers had activated Myh7 gene expression during development.
Figure 1.
Myh7-lineage tracing in EDL and SOL muscles from Myh7-Cre/Rosa26-EYFP mice. (A) Muscle cross sections staining with EYFP and laminin (Lam). N = 3. (B) SOL and EDL muscle sections stained with Myh7, MY32, and laminin (Lam) antibodies showing distribution of slow and fast fiber types. (C) SOL and EDL muscle sections stained with monoclonal antibodies reacting with Myh7, Myh2, and Myh4 showing type I, IIA, and IIB myofibers, respectively. Scale bar: 300 µm. N = 3 for quantitative results in pie charts.
In parallel, we established Myl1-Cre/Rosa26-TdTomato mice to specifically label myofibers that have activated Myl1 in red fluorescence (TdT). All myofibers examined in various muscles were tdTomato+ (Supplemental Fig. S3). This observation suggests that even though Myl1 is only concurrently expressed by fast myofibers in adults, it has also been activated in adult slow myofibers during development.
Activation of Myh7 during muscle regeneration
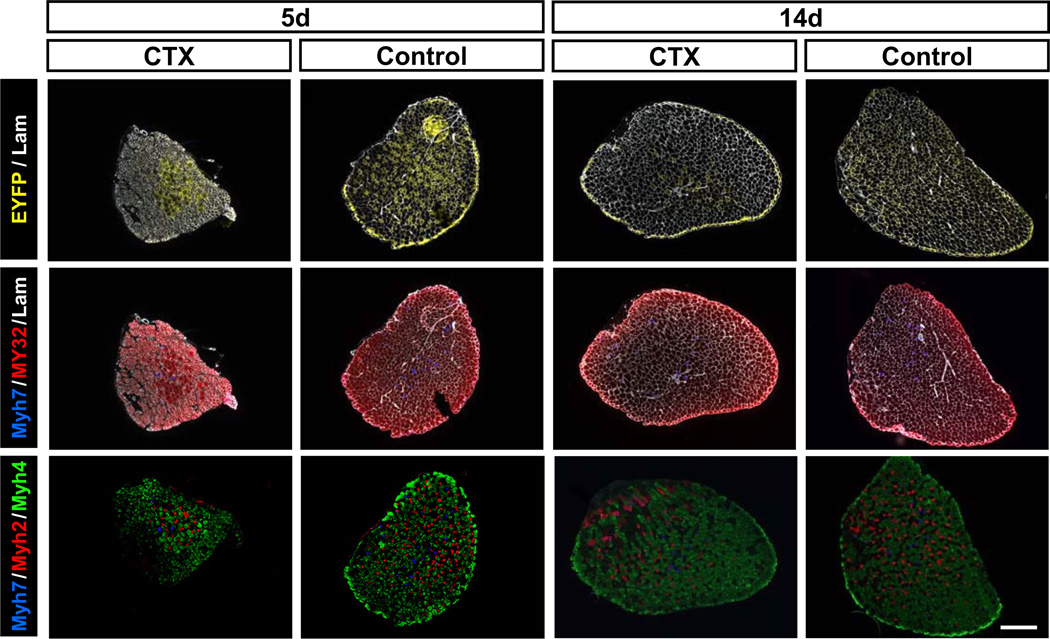
To investigate the activation pattern of Myh7 during regeneration, we analyzed YFP expression and myofiber type distribution after CTX induced muscle regeneration in Myh7-Cre/Rosa26-EYFP mice. EDL muscles were injected 50 µl CTX; the contralateral undamaged EDL muscles were used as control. The CTX treatment caused widespread degeneration of myofibers in the EDL muscles, followed by satellite cell mediated muscle regeneration. EDL muscle samples were collected at 5 and 14 days post CTX injection (dpi). At 5 dpi, there were ~40% EYFP+ myofibers in the non-injured EDL muscles, but there were only ~10% EYFP+ myofibers in the CTX treated EDL muscles (Fig. 2). Interestingly, no EYFP+ myofiber were found in the injured area (left bottom area in the first column of Fig. 2). The regenerating myofibers in the injured area were MY32+ but were Myh4− and Myh7− (Fig. 2, first column), suggesting that these regenerating myofibers express Myh1, Myh2, or Myh8 (perinatal Myh).
Figure 2.
Myh7 activation and fast/slow myosin expression during regeneration of EDL muscles. EDL muscles of Myh7-Cre/Rosa26-EYFP mice were injured with CTX and analyzed at 5 and 14 days post injury. Top panels: muscle sections stained with EYFP and laminin (Lam). Middle panels: muscle sections stained with Myh7, MY32, and Lam antibodies showing the distribution of slow and fast fiber types. Bottom panels: muscle sections stained with Myh7, Myh2, and Myh4 antibodies showing the distribution of type I, IIA and IIB myofibers. Scale bar: 300 µm.
At 14 dpi, there were still only 10% EYFP+ myofibers in the CTX injured muscles, compared to ~40% EYFP+ myofibers in the contralateral non-injured muscles (Fig. 2). Besides type I myofibers, EYFP can only be found in uninjured type II myofibers, which are distinguished from regenerated myofibers by the lack of central nuclei. Surprisingly, very few regenerated type II myofibers, marked by central nuclear and MY32+, had EYFP expression, suggesting that most regenerated type II myofibers have not activated Myh7 at this stage. Interestingly, centronucleated regenerating myofibers coexpressing Myh7 and MY32 were also identified, suggesting that some newly regenerated type II myofibers begun to switch to type I myofiber at this stage (Supplemental Fig. S4). Together, these results demonstrate that Myh7 gene is not activated during muscle regeneration and type I myofibers in regenerated muscles directly emerge from existing type II myofibers.
Heterogeneous origin of myonuclei within a single myofiber
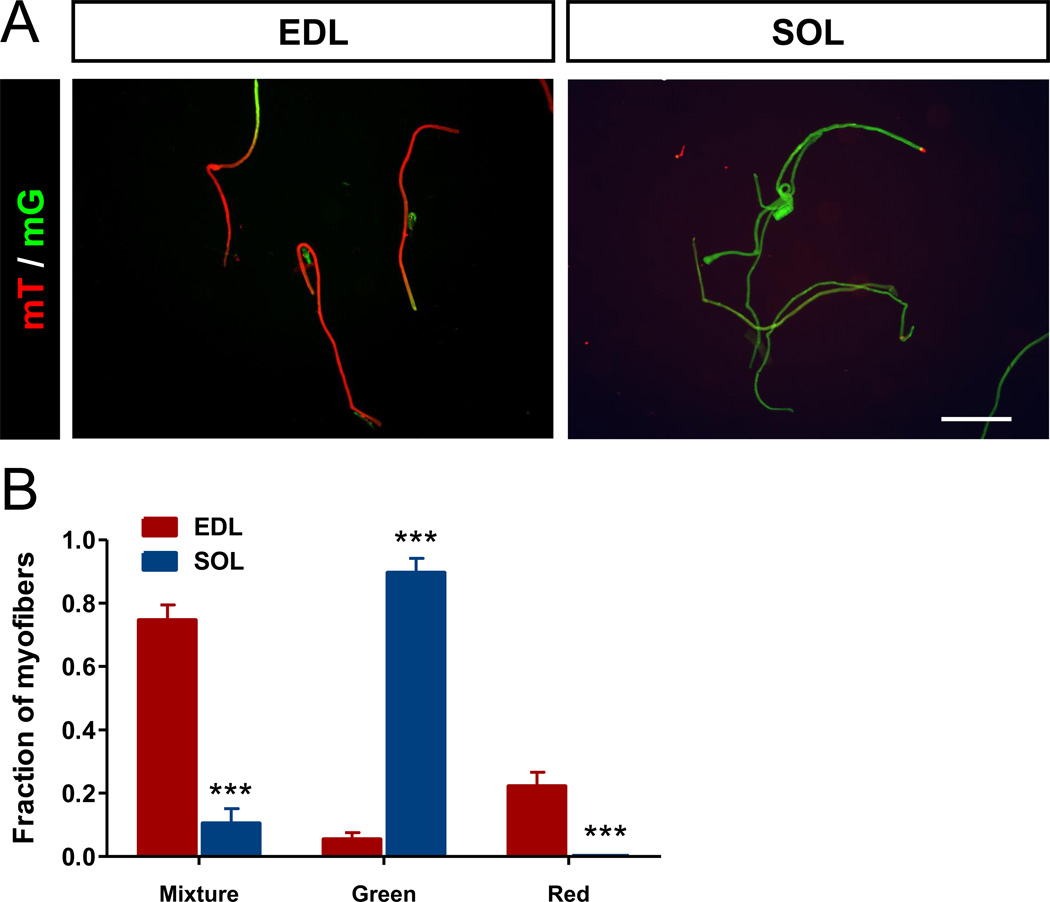
During skeletal muscle development, myoblasts fuse to form multinuclear myotubes, which mature into myofibers (Millay et al., 2013). Each mature myofiber primarily expresses one myosin subtype, but it is unknown if all myonuclei within a myofiber express the same myosin subtype. To address this question, we established the Myh7-Cre/Rosa26-mTmG reporter mice in which cells that have once activated the Myh7 promoter are labeled by membrane-targeted GFP, while other cells express membrane-targeted RFP. The membrane-targeting strategy allows for visualizing nuclear domainal mosaics in multinuclear myofibers. Single myofibers were collected from SOL and EDL muscles and the expression of GFP and RFP were examined. Most single myofibers isolated from SOL muscles were homogeneously green (Fig. 3A), indicating that all myonuclei within these myofibers had activated Myh7. In contrast, many EDL myofibers had mosaic expression of GFP and RFP (Fig. 3A), suggesting heterogeneous contribution of both Myh7 activated and non-activated myonuclei to these myofibers. Notably, most mosaic myofibers predominantly express GFP or RFP, with complementary expression of RFP or GFP at one end of the myofibers (Fig. 3A). Quantitative analysis indicates that SOL myofibers contains approximately 90% of pure GFP+ myofibers and 10% of mosaic GFP+/RFP+ myofibers (Fig. 3B). In contrast, EDL myofibers contains roughly 75% mosaic GFP+/RFP+ myofibers, 20% pure RFP+ myofibers and 5% pure GFP+ myofibers (Fig. 3B). This observation suggest that EDL and SOL myonuclei were apparently different in Myh7 activation, and even myonuclei within a single myofiber can be heterogeneous in developmental origin or myosin gene expression.
Figure 3.
Heterogeneous myonuclei composition within single myofibers. EDL and SOL muscle fibers freshly isolated from Myh7-Cre/Rosa26-mTmG mice were photographed. (A) Fluorescence images showing membrane-targeted green (mT) and red (mG) fluorescent protein signals. Scale bar: 500 µm. (B) Relative composition of myofibers exhibiting mosaic mT/mG fluorescence (Mixture), or pure mG (Green), or mT (Red). N = 3 mice, 50 fibers per muscle were analyzed. ***P < 0.001.
Activation of Myh7 gene in a population of proliferating myoblasts
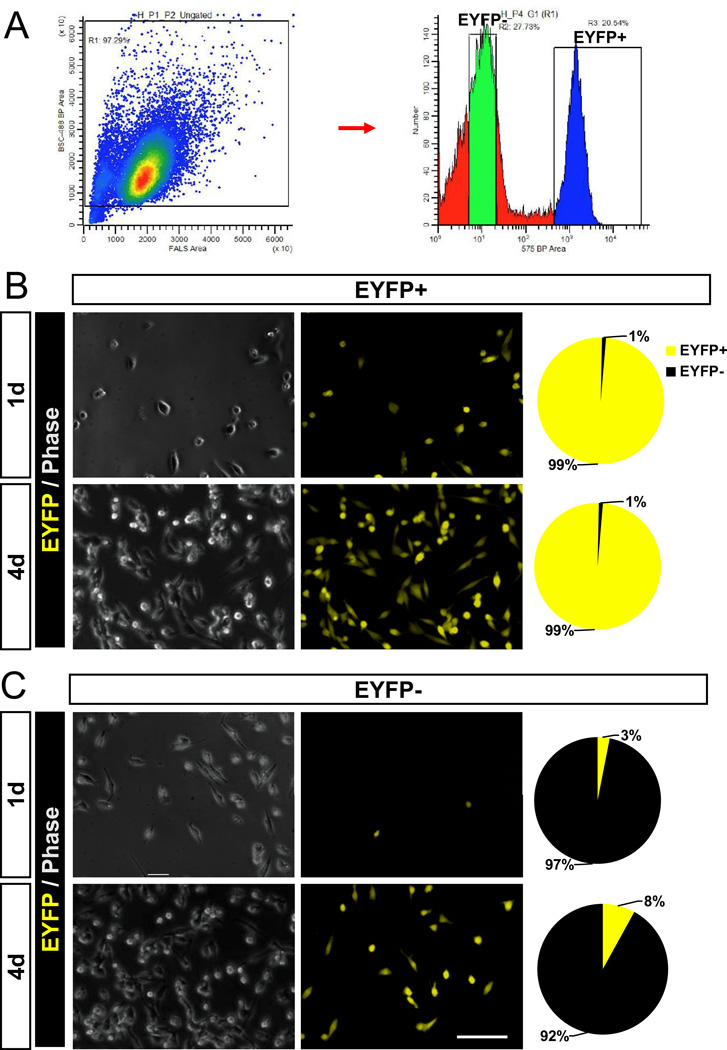
Myosin heavy chain genes are known to be expressed only in differentiated myocytes that have exited cell cycle. Surprisingly, we found that some primary myoblasts derived from Myh7-Cre/Rosa26-mTmG mice exhibited GFP fluorescence under growth conditions, prior to induced differentiation (Supplemental Fig. S5). The GFP+ myoblasts were more abundant in cultures derived from the slow SOL muscles than in cultures derived from the fast EDL muscles (Supplemental Fig. S5). To directly characterize Myh7 activation in myoblasts, we isolated YFP+ and YFP− myoblasts from Myh7-Cre/Rosa26-EYFP mice using fluorescence-activated cell sorting (FACS) (Fig. 4A). About 20% of the myoblasts were gated as YFP+ (R3) and the remainder myoblasts were YFP− (Fig. 4A). After cultured for 1–4 days, the YFP+ and YFP− myoblasts both expanded to similar densities and were morphologically indistinguishable (Fig. 4B–C). The sorted YFP+ myoblasts remained YFP+ during the culture (Fig. 4B). Interestingly, a few EYFP+ cells (3%) emerged in the sorted YFP− myoblasts after cultured for 1 day, and the percentage increased to 8% after 4 days (Fig. 4C), indicating de novo activation of Myh7 during the culture. Importantly, the expansion of sorted YFP+ cells in culture demonstrates that these Myh7-activated myoblasts are proliferative and activation of Myh7 does not necessary mark terminal differentiation.
Figure 4.
Purification and growth analysis of Myh7-activated myoblasts (A) EYFP+ and EYFP− myoblasts were isolated by FACS from Myh7-Cre/Rosa26-EYFP mice. Sorted EYFP+ (B) and EYFP− (C) myoblasts were cultured for 1 day and 4 days. Pie charts show the relative percentage of EYFP+ cells. Scale bar: 50 µm.
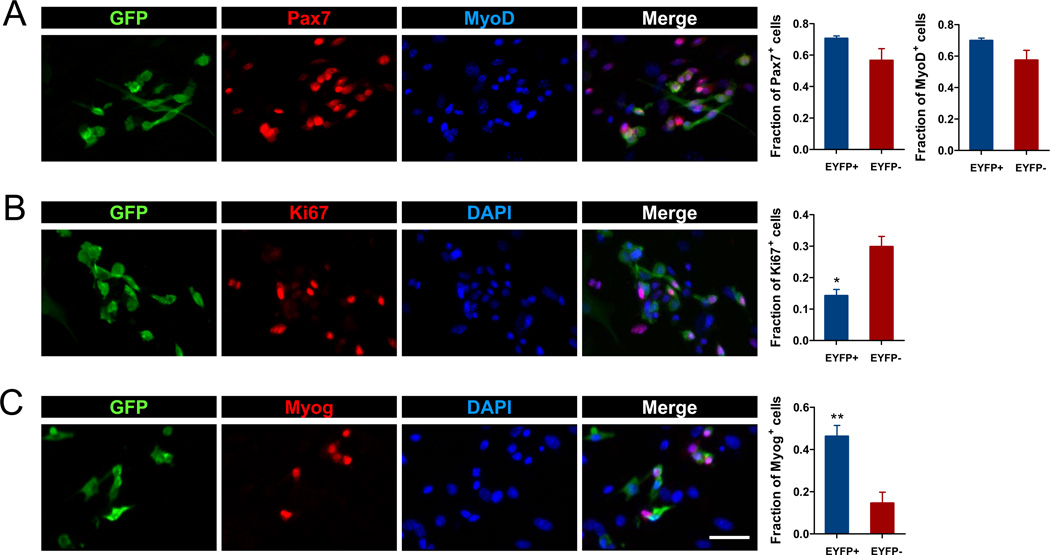
To further characterize myogenic gene expression of the Myh7-activated myoblasts, primary myoblasts from the Myh7-Cre/Rosa26-EYFP mice were stained with Pax7 and MyoD antibodies. Although most of the Pax7+ and MyoD+ myoblasts were YFP−, some Pax7+ and MyoD+ were YFP+ (Fig. 5A). Overall, the relative abundance of Pax7 and MyoD positive cells was not significantly different in the YFP− and YFP+ myoblasts (Fig. 5A). As Pax7/MyoD expression typically marks actively proliferating myoblasts, the co-expression of Pax7 and MyoD in YFP+ cells indicates that these Myh7-activated myoblasts are not differentiated myocytes. To confirm this notion, we next examined expression of the proliferation marker Ki67, and detected Ki67+ cells in both YFP− and YFP+ myoblasts (Fig. 5B). However, there were significantly more Ki67+ cells in YFP− (30%) than in YFP+ (15%) myoblasts (Fig. 5B). Consistently, low-density cultures that allow clonal growth of individual myoblasts revealed that Myh7-activated myblasts formed smaller colonies than the Myh7 non-activated myoblasts (Supplemental Fig. S6). Together, analysis of Pax7, MyoD and Ki67 expression demonstrates that Myh7-activated myoblasts are proliferative, but with reduced proliferative capacity compared to myoblasts that had never activated Myh7.
Figure 5.
Myh7 activation in proliferating and differentiating myoblasts. Myoblasts from Myh7-Cre/Rosa26-EYFP mice were used. (A) Triple staining with GFP, Pax7, and MyoD antibodies. (B) Double staining with GFP, and Ki67 antibodies plus DAPI labeling of nuclei. (C) Double staining with GFP and myogenin (Myog) antibodies plus DAPI labeling of nuclei. N = 3 mice, at least 200 cells/culture were analyzed. *P < 0.05, **P < 0.01 by student t-test. Scale bar: 25 µm.
Myh7 activation promotes but does not restrict differentiation towards slow myotubes
To investigate the differentiation potential of Myh7-activated myoblasts, we first examined expression of the differentiation marker myogenin (Fig. 5C). Interestingly, only a fraction of Myh7-activated myoblasts were myogenin+. Although myogenin expression is detected in both YFP+ and YFP− myoblasts, the percentage of myogenin+/ YFP+ cells was three times as many as the myogenin+/ YFP− cells (Fig. 5C). These results suggest that Myh7-activated myoblasts are more primed for differentiation.
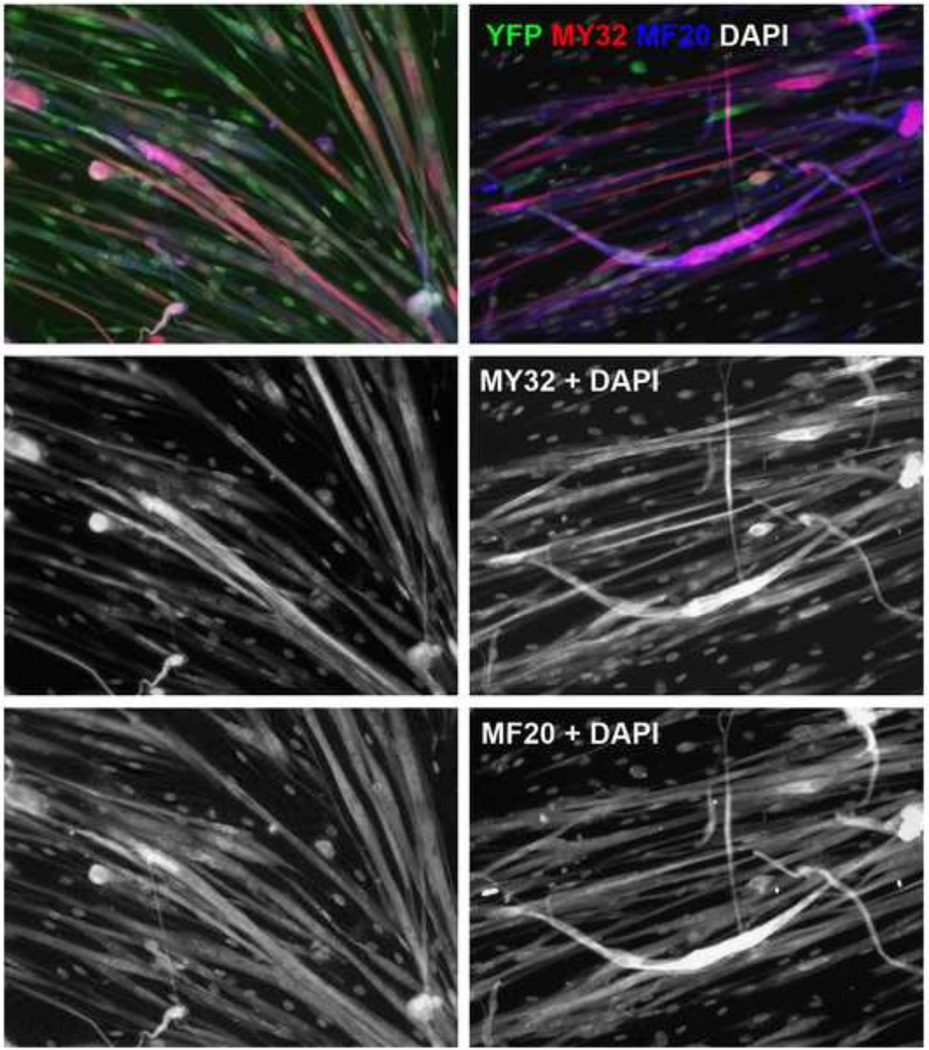
We next examined if activation of Myh7 programs myoblasts to differentiate into corresponding slow myotubes. YFP− and YFP+ myoblasts were sorted from Myh7-Cre/Rosa26-EYFP mice, then induced to differentiate by serum withdrawal. Both YFP− and YFP+ myoblasts fused to form multinuclear myotubes after 3 days of differentiation (Fig. 6). However, the YFP+ myoblasts formed myotubes more robustly than the YFP− myoblasts (Fig. 6), again indicating that Myh7-activated myoblasts differentiate more readily. Furthermore, analysis of myofiber type using MY32 and MF20 antibodies reveal that whereas the YFP− myoblasts predominantly formed MY32+ (fast) myotubes, the YFP+ myoblasts formed both MY32+ (fast) and MY32− (slow) myotubes (Fig. 6). Collectively, activation of Myh7 in myoblasts accelerated their differentiation, though they were not restricted to become either slow or fast myotubes.
Figure 6.
Differentiation of Myh7-activated and non-activated myoblasts. Myoblasts from Myh7-Cre/Rosa26-EYFP mice were sorted based on YFP+ and YFP− and differentiated for 3 days. Differentiated myotubes (MF20+) were then stained with GFP, MY32, and MF20 antibodies. GFP staining was used to enhance YFP signal to confirm Myh7 activation or lack of Myh7 activation in the sorted YFP+ and YFP− cells after differentiation. MY32 signaling indicates expression of fast myosin heavy chain and MF20 signal indicates pan-sarcomeric myosin heavy chain. MY32−/MF20+ myotubes thus express only slow myosin heavy chain. DAPI labels the nuclei. Scale bar: 50 µm.
Discussion
Our studies uncovered several novel features of myosin gene activation and myofiber specification. We show that the fast myosin gene Myl1 is activated in all adult fast and slow myofibers. In contrast, the slow myosin gene Myh7 is only activated in type I, IIA and IIX myofibers, and a small subset of IIB myofibers. Interestingly, even myonuclei within a single myofiber can be highly heterogeneous in the activation of Myh7, suggesting their mosaic origin. Combining lineage labeling and cell culture, we find that Myh7 is activated in a subpopulation of proliferating myoblasts, and Myh7 activation primes, but not restricts, their differentiation towards slow myotubes.
Skeletal muscle myosin is consisted of two heavy (Myh) chains and two light (Myl) chains (Schiaffino and Reggiani, 2011). The essential and regulatory subunits of Myl also contain fast and slow muscle specific isoforms. Myl1 is reported to be only expressed in fast skeletal muscle fibers in the adult (Bicer and Reiser, 2004; Schiaffino and Reggiani, 1996; Timson, 2003), but its activation during development is unknown. The ubiquitous labeling of all adult fast and slow myofibers by the Myl1-Cre knockin mouse demonstrates that Myl1 is activated in all myofibers during development. This result further suggests that the Myl1-Cre mouse may be served as an excellent driver for Cre/LoxP-mediated, skeletal muscle-specific activation or inactivation of target genes. Previously studies have predominantly used MCK-Cre and HSA-Cre for muscle specific gene expression. However, both strains are generated using transgenic promoter constructs, therefore bear risks of non-faithful leaky expression in non-muscle cells. In addition, the MCK-Cre also drives LoxP recombination in cardiac muscle cells (Perdomini et al., 2014), and is thus not skeletal muscle-specific.
The Myh7-Cre transgenic mouse has previously been used to driven gene deletion or expression in cardiac muscles (Colbert et al., 1997; Parsons et al., 2004), but its activation pattern in skeletal muscles is unclear. The observation that all Myh7 immunoreative cells in developing somites are marked by the Myh7-Cre suggests that Myh7-Cre is sufficient to drive slow myoifber specific gene expression. Additionally, ~30% of Myh7-Cre marked cells in the somites did not express Myh7 protein, suggesting that Myh7-Cre transgene activation does not necessarily correspond to detectable levels of Myh7 mRNA and protein. This may be due to microRNA-mediated mRNA degradation or suppression of protein translation (van Rooij et al., 2009). Alternatively, we cannot rule out the possibility that the Myh7-Cre transgene may ectopically activate the Rosa26-EYFP reporter in a small fraction of proliferating myoblasts. Future studies using Myh7Cre knockin allele will distinguish these possibilities.
Using the Rosa26-EYFP reporter, we found that all myofibers in the SOL muscle of adult mice had activated Myh7-Cre but only about half of the myofibers concurrently express Myh7. Similarly, about half of EDL myofibers had activated Myh7, but only few of these myofibers concurrently express the Myh7 protein. Further analysis indicates that activation of Myh7-Cre in the EDL muscle mostly occurs in the type IIA and IIX myofibers. Interestingly, Myh7 is not activated in newly regenerated EDL myofibers, suggesting that Myh7-activation in type IIA and IIX myofibers occurs during myofiber maturation. As myofiber specification during muscle regeneration recapitulate that during embryonic myogenesis (Cerny and Bandman, 1987), our results further suggest that slow myosin gene activation occurs after fast myosin gene activation in a subset of fast myofibers during growth and development.
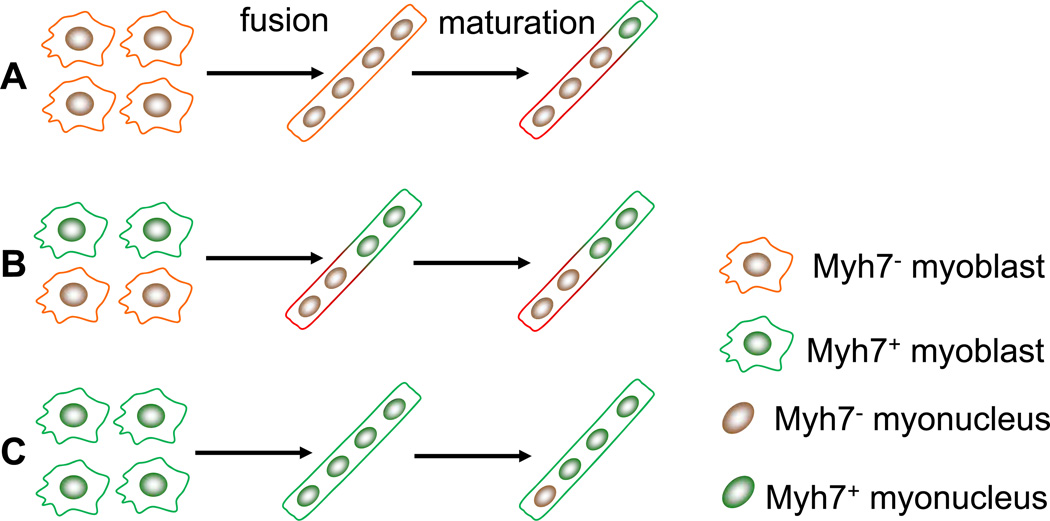
Single fiber analysis using Myh7-Cre/Rosa26-mTmG mice that express membrane-targeted fluorescent proteins (mGFP and mRFP) reveals surprising heterogeneity in nuclear composition with a single myofiber. While SOL myofibers (containing 94% type I and IIA fibers) uniformly exhibit mGFP fluorescence, EDL myofibers (containing 85% type IIX and IIB fibers) mostly exhibit mosaic mGFP and mRFP fluorescence. This could have been resulted from several different possibilities (Fig. 7). It is possible that during development Myh7 non-activated myoblasts fuse to form uniform mRFP+ myotubes, but a subset of the fused myotubes subsequently activate Myh7, therefore turning domains of the corresponding membrane into mGFP+ (Fig. 7A). This notion is supported by our results showing that during EDL muscle regeneration no nascent myotubes have activated the Myh7-Cre transgene and Myh7 protein is subsequently expressed in a subset of fast myofibers during maturation. Alternatively, heterogeneous Myh7-activated and non-activated myoblasts may fuse to form myotubes exhibiting mosaic mRFP/mGFP expression pattern, and the pattern is maintained during maturation (Fig. 7B). The uniform mGFP fluorescence in SOL myofibers suggests that these myofibers are fused from myoblasts that have activated Myh7. However, our model cannot exclude the possibility that a subset of myonuclei subsequently turn off Myh7 expression and turn on fast myosin expression during maturation (Fig. 7C).
Figure 7.
A model depicting how Myh7 activation in undifferentiated and differentiated myoblasts contributes to mosaic mT/mG expression in single myofibers of Myh7-Cre/ Rosa26-mTmG mouse. (A) Myh7 non-activated myoblasts fuse to form a nascent fast myofiber. During maturation, a subset of the fused myonuclei activates Myh7, resulting in mosaic mT/mG fluorescent. (B) Heterogeneous Myh7-activated and non-activated myoblasts fuse to form a myotubes, resulting in mosaic mT/mG expression in the mature myotube (fast or slow). (C) Myh7-activated myoblasts fuse to form a nascent slow myotubes. During maturation, a subset of the fused myonuclei may turn off Myh7 and turn on fast myosin gene without changing the mG fluorescence of the mature myofiber.
Activation of Myh7 in a subset of actively proliferating myoblasts is surprising, as it has been believed that myosin gene expression is only activated in differentiated myocytes (Abmayr and Pavlath, 2012; Pajcini et al., 2010). During the culture of myoblasts from Myh7-Cre/Rosa26-EYFP mice, we observed mononuclear YFP+ cells whose number increases with time. This suggests that either the YFP+ cells were proliferating or spontaneous differentiation of a subset of myoblasts under growth conditions. When the YFP+ cells were purified by FACS, they expanded robustly in culture, suggesting that they are proliferative. This is confirmed by Ki67 expression in the YFP+ cells. The undifferentiated status of the YFP+ cells is confirmed by their expression of the myogenic progenitor marker Pax7. Double staining of Pax7 and Myh7 in developing somites indicates that 99% Pax7+ cells are Myh7− (Supplemental Fig. S7), suggesting that the Myh7-activated proliferating myoblasts do not express significant levels of Myh7 protein. Thus, expression of Myh7 protein, but not activation of Myh7 gene (mRNA), marks myogenic differentiation. Nevertheless, the Myh7-activated myoblasts differentiated more readily and formed both slow and fast myotubes, while the Myh7 non-activated myoblasts differentiated less efficiently and formed predominantly fast myotubes. Collectively, activation of Myh7 in a subpopulation of myoblasts primes, but does not restrict, these cells to become slow myofibers. This observation is consistent with our recent analysis of porcine myoblast differentiation (Zhu et al., 2013).
These results enhance our understanding of myosin gene activation and myofiber specification during development and regeneration. Such knowledge may lead to novel strategies to modify or diversify fiber type composition in the skeletal muscles, and opportunities to improve athletic performance, prevent muscle wasting in muscular dystrophy patients and enhancing meat quality in agriculture.
Supplementary Material
Highlights.
Cre/LoxP strategy was used to label lineage origin of fast/slow myofibers
All skeletal musucles have activated Myl1 during development
All slow myofibers and a subset of fast myofibers have activated Myh7 during development
Myonuclei within a single myofiber exhibit mosaic activation of different myosin genes
Myh7 gene is activated in a subpopulation of proliferating myoblasts
Acknowledgments
The authors thank Katherine Yutzey (Cincinnati Children's Hospital Medical Center, USA) and Steve Burden (New York University, USA) for providing the Myh7-Cre and Myl1-Cre mice, respectively. Jun Wu for technical assistance and maintenance of mouse colony. This study is partially supported by the National Institutes of Health (R01AR060652 to SK) and United States Department of Agriculture (2009-35206-05218 to SK).
Abbreviations
- BSA
Bovine serum albumin
- CTX
Cardiotoxin
- DMEM
Defined minimal essential medium
- EDL
Extensor digitorum longus (muscle)
- EYFP
Enhanced yellow fluorescent protein
- FACS
Fluorescent activated cell sorting
- FBS
Fetal bovine serum
- MYH
Myosin heavy chain
- MYL
Myosin light chain
- Myod
Myogenic differentiation 1
- Pax7
Paired box 7
- RFP
Red fluorescent protein
- SOL
Soleus (muscle)
- TA
Tibialis anterior (muscle)
- tdTomato
Tandem dimer Tomato.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012;139:641–656. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Bicer S, Reiser PJ. Myosin light chain isoform expression among single mammalian skeletal muscle fibers: species variations. J Muscle Res Cell Motil. 2004;25:623–633. doi: 10.1007/s10974-004-5070-9. [DOI] [PubMed] [Google Scholar]
- Bothe GWM, Haspel JA, Smith CL, Wiener HH, Burden SJ. Selective expression of Cre recombinase in skeletal muscle fibers. Genesis. 2000;26:165–166. [PubMed] [Google Scholar]
- Cerny LC, Bandman E. Expression of myosin heavy chain isoforms in regenerating myotubes of innervated and denervated chicken pectoral muscle. Dev Biol. 1987;119:350–362. doi: 10.1016/0012-1606(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Kuang S, Buffelli M, Lichtman JW, Sanes JR. Mouse transgenic lines that selectively label Type I, Type IIA, Types IIX+B skeletal muscle fibers. Genesis. 2012;50:50–58. doi: 10.1002/dvg.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang DZ. microRNAs in cardiovascular development. J Mol Cell Cardiol. 2012;52:949–957. doi: 10.1016/j.yjmcc.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert MC, Hall DG, Kimball TR, Witt SA, Lorenz JN, Kirby ML, Hewett TE, Klevitsky R, Robbins J. Cardiac compartment-specific overexpression of a modified retinoic acid receptor produces dilated cardiomyopathy and congestive heart failure in transgenic mice. The Journal of clinical investigation. 1997;100:1958–1968. doi: 10.1172/JCI119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. Skeletal muscle satellite cell diversity: satellite cells form fibers of different types in cell culture. Dev Biol. 1991;143:320–334. doi: 10.1016/0012-1606(91)90083-f. [DOI] [PubMed] [Google Scholar]
- Karagounis LG, Hawley JA. Skeletal muscle: increasing the size of the locomotor cell. Int J Biochem Cell Biol. 2010;42:1376–1379. doi: 10.1016/j.biocel.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, O'Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R, Olson EN. Myomaker i s a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajcini KV, Corbel SY, Sage J, Pomerantz JH, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell. 2010;7:198–213. doi: 10.1016/j.stem.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem. 2004;279:26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- Perdomini M, Belbellaa B, Monassier L, Reutenauer L, Messaddeq N, Cartier N, Crystal RG, Aubourg P, Puccio H. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat Med. 2014;20:542–547. doi: 10.1038/nm.3510. [DOI] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Timson DJ. Fine tuning the myosin motor: the role of the essential light chain in striated muscle myosin. Biochimie. 2003;85:639–645. doi: 10.1016/s0300-9084(03)00131-7. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Developmental cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell JN, Zhang P, Wen Y, Gupta SK, Yevtodiyenko A, Schmidt JV, Bidwell CA, Kumar A, Kuang S. Dlk1 i s necessary for proper skeletal muscle development and regeneration. PLoS One. 2010;5:e15055. doi: 10.1371/journal.pone.0015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Park S, Scheffler JM, Kuang S, Grant AL, Gerrard DE. Porcine satellite cells are restricted to a phenotype resembling their muscle origin. Journal of Animal Science. 2013;91:4684–4691. doi: 10.2527/jas.2012-5804. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004;2:e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.