Abstract
Evolutionarily unprepared for modern high caloric diets and sedentary lifestyles, humans are now unprecedentedly susceptible to metabolic disorders such as obesity, type 2 diabetes, nonalcoholic fatty liver, and cardiovascular diseases. These metabolic conditions are intertwined, together known as metabolic syndrome, to compromise human life quality as well as lives. Notch signaling, a fundamental signal transduction pathway critical for cell-cell communication and development has recently been recognized as a key player in metabolism. This review summarizes the emerging roles of Notch signaling in regulating metabolism of various cell or tissue types, with emphasis on the underlying molecular mechanisms and potentials of targeting this signal axis to treat metabolic diseases.
Keywords: Notch signaling, obesity, type 2 diabetes, insulin resistance
An overview of Notch signaling
The Notch signaling pathway is an evolutionarily conserved pathway important for cell-cell communication and cell-fate determination during development, and is required for adult tissue homeostasis. It consists of Notch receptors and Notch ligands, as well as intracellular proteins that function to transmit the Notch signal to the cell's nucleus. Notch receptors (Notch1–4) are single-pass transmembrane proteins that are composed of an extracellular (NECD), a transmembrane (TM), and an intracellular (NICD) domain. Notch ligands are also transmembrane proteins and cells expressing Notch ligands must be in close proximity to Notch expressing cells for signaling to occur. Ligands bind to the Notch NECD to induce proteolytic cleavage and release of the NICD, which enters the cell nucleus to modify gene expression. Notch ligands are members of the DSL (Delta/Serrate/LAG-2) family proteins that include Delta-like (Dll1, Dll3, Dll4) and Jagged (Jag1, Jag2) in mammals [1, 2].
Notch signal transduction is initiated upon binding of a Notch receptor to a ligand located on a neighbor cell. Endocytosis of Notch-bound ligand generates a mechanical pulling force, which drives conformational changes of the Notch receptor and facilitates its sequential proteolytic cleavages [3]. The first cleavage, mediated by a disintegrin and metalloproteinase (ADAM) family peptidase, releases the NECD, whereas the second cleavage mediated by γ-secretase releases the NICD [1]. NICD then translocates to the nucleus where it binds with Rbpj (recombination signal binding protein for immunoglobulin kappa j region) and recruits a transcriptional complex to activate the transcription of downstream targets, including Hairy/enhancer-of-split (Hes) and Hes related with YRPW motif protein (Hey) family genes. Simple in design, activation of Notch is tightly orchestrated at multiple levels [1] and the biological output is highly cellular-context dependent. One unique and important feature of Notch signaling is the lack of secondary amplification: NICD is part of Notch receptor as well as the direct activator of Notch targets. Therefore, every single event of Notch activation engages and consumes one Notch receptor. A similar turnover scenario applies to Notch ligands as well. Notch ligand and receptor turnover together establish the oscillating pattern of Notch activation based on the availability of replenished Notch receptors and ligands. Nuclear NICD is eventually targeted for proteasomal degradation mediated by the E3 ubiquitin ligase F-box and WD repeat domain containing 7 (FBW7) [4, 5]. A recent study showed that FBW7 transcription is repressed by the Notch target gene Hes5, thus creating a positive feedback loop that prolongs Notch signaling [6].
Notch signaling is a highly conserved intercellular communication mechanism critical for many cellular processes including survival, proliferation and differentiation, as well as maintaining stem cell quiescence and identity [7]. As such, Notch signaling is widely employed to orchestrate proper development, and perturbation of Notch pathway is linked to a variety of devastating genetic disorders and cancers [8]. In addition, recent studies employing transgenic mouse models of tissue specific manipulation of Notch signaling have begun to reveal the roles of Notch pathway in regulating metabolism of several key metabolic organs.
Notch signaling in diabetic and fatty liver
Notch signaling is involved in embryonic development, postnatal regeneration and carcinogenesis of the liver [9], the central hub for glucose and lipid metabolism. Upon feeding, an increase in blood glucose stimulates the secretion of insulin from the pancreas. Circulating insulin inhibits liver glucose production, including glycogenolysis and gluconeogenesis, and stimulates glucose utilization, including glycolysis and lipogenesis. Recent studies have revealed a key role of Notch signaling in regulating both processes, with abnormal activation of Notch signaling in hepatocytes leading to hyperglycemia and fatty liver disease (Figure 1) [10, 11].
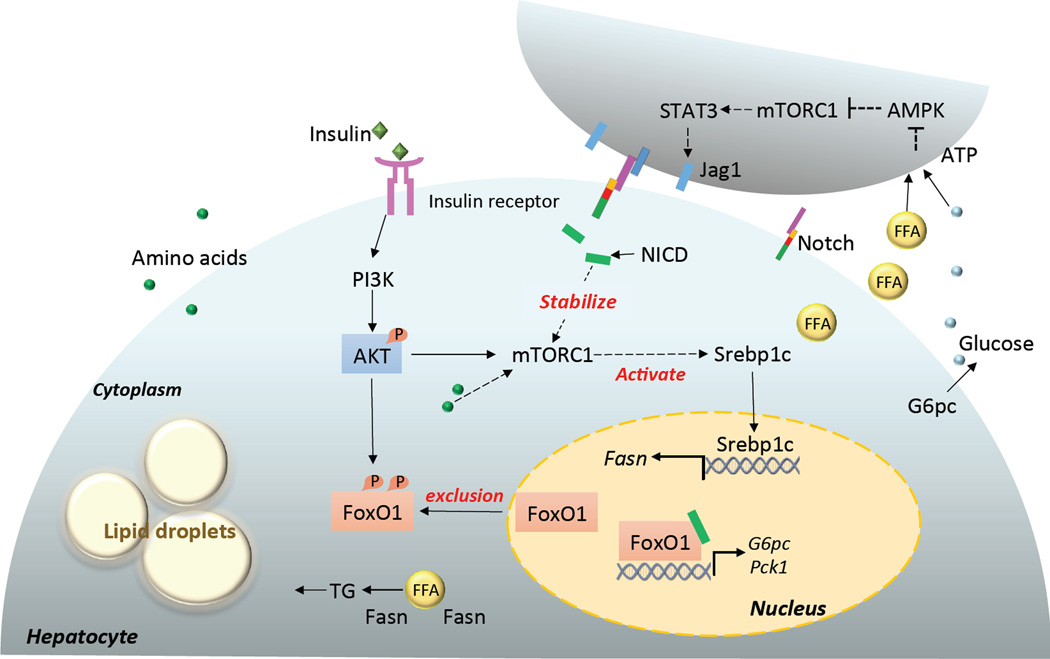
Figure 1. Notch regulates gluconeogenesis and lipogenesis of hepatocytes.
Notch signaling regulates hepatic glucose production through synergy with FoxO1, which directly activates the transcription of G6pc and Pck1, the rate-limiting enzymes in hepatic glycogenolysis and gluconeogenesis, respectively. Transcriptionally active FoxO1 is phosphorylated by AKT and excluded from nucleus. In addition, Notch signaling promotes hepatic lipogenesis through an unknown factor that stabilizes mTORC1, which is normally activated by amino acids, as well as the insulin-PI3K-AKT pathway. mTORC1 in turn activates Srebp1c, a key factor that turns on transcription of Fasn, which encodes a rate limiting enzyme in lipogenesis. In obesity, high levels of glucose and free fatty acids (FFA) activate the AMPK-mTOC1-STAT3 pathway, which eventually upregulates Jag1 and activates Notch signaling in the neighbor hepatocyte. Dotted line indicates indirect effect. G6pc, glucose-6-phosphatase, catalytic subunit; Pck1, phosphoenolpyruvate carboxykinase; AMPK, AMP-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; STAT3, signal transducer and activator of transcription 3; Srebp1c, sterol regulatory element-binding protein 1c; FoxO1, forkhead box protein O1; TG, triglyceride; Fasn, fatty acid synthase; PI3K, phosphatidylinositol 3-kinase.
The effect of Notch signaling on hepatic glucose production is mainly mediated through synergy of NICD with the forkhead transcription factor FoxO1 (Figure 1). FoxO1 directly activates the transcription of the catalytic subunit of glucose-6-phosphatase (G6pc), a rate-limiting enzyme involved in hepatic glycogenolysis and gluconeogenesis [11]. Compound haploinsufficiency of FoxO1 and Notch1 (Foxo1+/−:Notch1+/−) markedly ameliorates insulin resistance in diet-induced obese (DIO) mice [11]. Liver-specific knockout of Rbpj using Albumin-Cre phenocopies FoxO1:Notch1 haploinsufficiency, indicating that Notch signaling is the key driver of hepatic insulin resistance. Consistently, adenovirus-mediated activation of Notch1 in liver induces G6pc expression and exacerbates insulin resistance in a FoxO1-dependent manner [11]. Importantly, pharmacological inhibition of Notch signaling by blocking γ-secretase-mediated cleavage of NICD improves glucose tolerance and insulin sensitivity in DIO mice [11].
Another arm of insulin action in the liver is its stimulatory effect on lipogenesis. In mouse and human diabetic mellitus, hepatic insulin resistance is selective, whereby insulin fails to suppress gluconeogenesis but continues to stimulate lipogenesis, resulting in hyperglycemia and hypertriglyceridemia. In contrast, mice with total hepatic insulin resistance elicited by liver specific deletion of the insulin receptor develop hyperglycemia but not hypertriglyceridemia [12–14]. These results suggest the existence of divergent pathways controlling hepatic gluconeogenesis and lipogenesis. Intriguingly, activation of hepatic Notch signaling leads to a selective insulin resistance phenotype with hyperglycemia and hepatosteatosis (fatty liver) [10].
This result indicates that Notch signaling is a key point in the web of the hepatic insulin paradox, where the two branches of insulin-action converge. Mechanistically, Notch stimulates lipogenesis through an unknown factor that stabilizes mammalian target of rapamycin complex 1 (mTORC1) [10], a central player in lipid metabolism (Figure 1) [15]. Importantly, in both mouse and human, the hepatic Notch signaling is positively correlated with insulin resistance and fatty liver disease [10, 16].
One unanswered yet important question is about the upstream regulator of Notch signaling in hepatocytes. A recent study indicates that the energy sensor AMP-activated protein kinase (AMPK) regulates Notch signaling through mTORC1 under influence of nutrient status [17]. Specifically, excessive amino acids cause insulin resistance in cultured hepatocytes, accompanied by attenuation of AMPK activity and activation of mTORC1-STAT3-Notch1 signaling (Figure 1) [17]. The phenotypes are ameliorated by chronic administration of either the AMPK activator metformin, or the mTORC1 inhibitor rapamycin [17]. Hence, the synergy between Notch and FoxO1, and the positive feedback loop between Notch and mTORC1 in hepatocytes may be targeted to improve liver insulin sensitivity and ameliorate hyperglycemia and hypertriglyceridemia caused by diabetic fatty liver.
Notch signaling regulates adipocyte homeostasis
White adipose tissue (WAT) is the primary sites of long-term energy storage. In response to excess caloric intake, the size of WAT expands through hyperplasia and hypertrophy of adipocytes. Understanding the pathways that regulate adipocyte homeostasis is thus fundamental to the treatment of obesity. Characterization of the role of Notch signaling in adipocyte differentiation by various groups has generated inconsistent results. In 3T3-L1 preadipocytes, the Notch target Hes1 is shown to inhibit adipogenic differentiation by repressing expression of CCAAT/enhancer binding protein alpha (C/EBPα) and peroxisome proliferator-activated receptor gamma (Pparγ) [18]. Paradoxically, knockdown of Hes1 also inhibits adipogenic differentiation of 3T3-L1 cells, accompanied by an increased expression of Delta-like 1 homolog (Dlk1), an inhibitor of adipogenic differentiation [18]. In human primary cell cultures, inhibition of Notch promotes, whereas activation of Notch inhibits, adipogenic differentiation of mesenchymal stem cells and adipose-derived precursor cells [19–21]. However, genetic ablation of several key components of the Notch pathway fails to elicit any obvious deficiencies in adipogenic differentiation of embryonic fibroblasts [22]. The contradictory findings of these cell culture experiments could be attributed to the timing and dosage of Notch intervention, and distinct cell types used. For instance, initiation of adipogenic differentiation of immortalized 3T3-L1 cells requires sequential control of the cell cycle, which is directly affected by Notch signaling [23, 24]. Another confounding factor is the heterogeneity of primary preadipocyte cultures compared with 3T3-L1 cells [25]. It’s possible that adipocytes of different origins (or differentiation stages) employ diverse combinations of Notch ligands and receptors to achieve context-dependent versatility of Notch signaling.
Adipocytes can be classified into white, beige (brite) and brown adipocytes [26]. White adipocytes are the predominant cell type in various depots of subcutaneous and visceral WAT. Brown adipocytes are mainly found in brown adipose tissue (BAT) that are scarcely dispersed along the neck and shoulder of humans [26]. Beige adipocytes are a newly defined type of adipocytes that coexist with white adipocytes in subcutaneous WAT and with brown adipocytes in BAT [26]. While white adipocytes are primarily involved in energy storage, brown and beige adipocytes are highly specialized in energy expenditure due to their higher mitochondria content and abundant expression of uncoupling protein 1 (UCP1), which uncouples electron transport chain from ATP production to generate heat [27]. Of note, UCP1 is activated by fatty acids that are produced by the lipolysis of lipid droplets upon adrenergic stimulation in brown and beige adipocytes (Figure 2) [28]. Functionally, brown adipocytes contain more mitochondria, express higher levels of UCP1 and have stronger thermogenic activity than beige adipocytes [26]. Beige adipocytes can be generated through de novo differentiation of preadipocytes [29, 30], or from direct conversion of mature white adipocytes [31–33]. Several recent lineage-tracing studies have shown that brown, beige and white adipocytes have distinct developmental origins. Specifically, brown adipocytes arise from Myf5+, while white and beige adipocyte from Myf5− lineages [34]. In addition, in WAT, beige and white adipocytes are enriched in Pax3− and Pax3+ cell populations respectively [35].
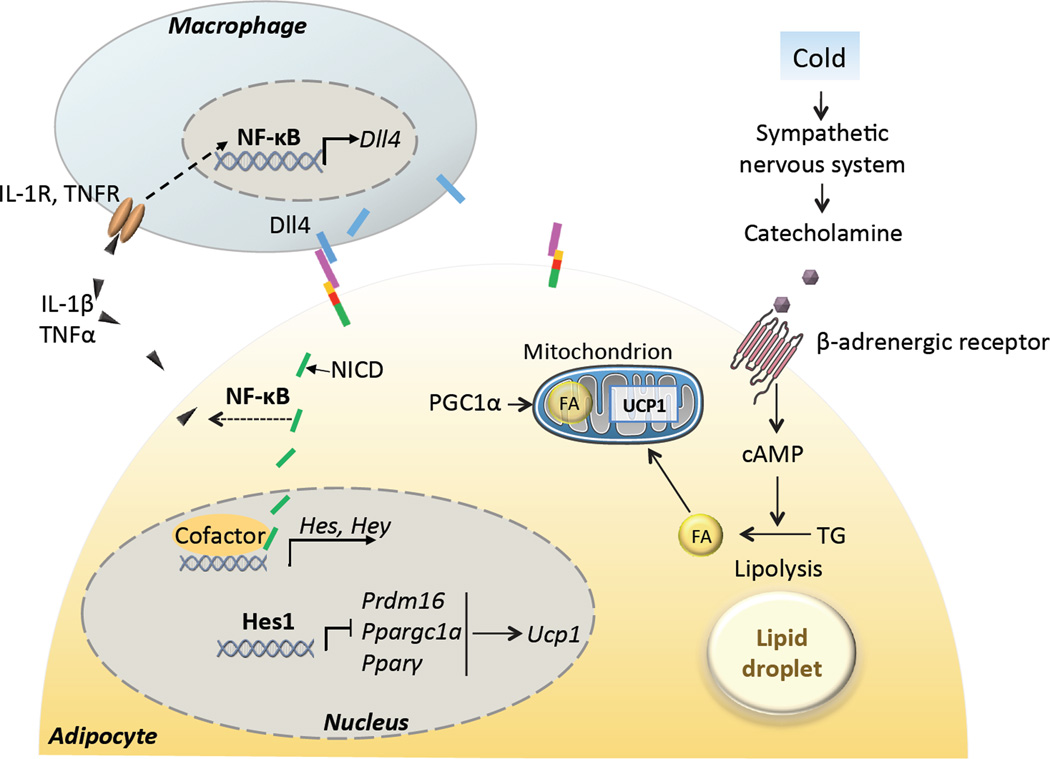
Figure 2. Notch signaling regulates adipocyte thermogenesis.
In response to cold ambient temperature, sympathetic nervous system releases catecholamine, which binds with β-adrenergic receptor and activates lipolysis through the cAMP pathway. Fatty acids (FAs) can directly activate uncoupling protein 1 (UCP1) for heat production. Notch target gene Hes1 directly binds to the promoter region of Prdm16, Ppargc1a and Pparγ, and inhibit their transcription. This leads to reduced mitochondria numbers and expression of UCP1. Notch signaling promotes activation and production of proinflammatory cytokines mediated by NF-κB, which attracts macrophages and together cause low-grade systematic inflammation and exacerbates insulin resistance. In obesity, infiltrated macrophages activate transcription of Notch ligand Dll4 through NF-κB. Dotted line indicates indirect effect. IL-1β, interleukin 1β; TNFα, tumor necrosis factor α; IL-1R, interleukin 1 receptor; TNFR, tumor necrosis factor receptor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Dll4, delta-like 4; Pparγ, peroxisome proliferator-activated receptor gamma; PGC1α, Pparγ coactivator 1-alpha; Prdm16, PR domain containing 16;.
It was recently reported that Notch signaling plays a role in regulating the plasticity (conversion) of white and beige adipocytes in vivo, and consequently affecting body energy metabolism [36]. Adipocyte-specific ablation of Notch1 or Rbpj driven by aP2-Cre decreases the size of various adipose depots and increases the abundance of beige adipocytes in WAT, accompanied by increased metabolic rate, improved glucose tolerance and insulin sensitivity [36]. These phenotypes are associated with elevated expression of beige adipocyte-specific genes in WAT, but not BAT. In addition, mice depleted of Notch1 or Rbpj exhibit accelerated browning (appearance of beige adipocytes within WAT) in response to cold environment. The adipose specific Notch1 mutant mice are also resistant to high fat diet (HFD)-induced obesity. Importantly, intra-peritoneal administration of a γ-secretase inhibitor reduces the adiposity and body weight of obese mice [36]. Currently it is unclear if the reduced size of adipose depots is due to increased energy expenditure (and thus less deposition of fat), or due to a requirement of Notch signaling in normal adipogenesis. Because aP2-Cre is activated in adipose progenitors [37], using a mature adipocyte-specific Cre line to delete Notch1 or Rbpj will distinguish these two possibilities. In addition, as the aP2-Cre mouse has been reported to drive weak Cre expression in the brain and macrophages [38–41], future studies using more stringent adipocyte-restricted Cre driver mice are necessary to confirm these observations. As an initial attempt to confirm the role of Notch signaling in adipose tissues, activation of Notch signaling using a highly adipocyte-specific adiponectin-Cre mouse is shown to inhibit browning of WAT and induce whitening of BAT, manifested by lipid deposition and emergence of white adipocytes in the classic interscapular BAT [36]. Adiponectin-Cre induced Notch activation also renders the mice glucose intolerance and insulin resistance [36]. These phenotypes are in sharp contrast to those observed in the Notch deficient mice. Mechanistically, the Notch target gene Hes1 directly binds to the promoter regions of PR domain containing 16 (Prdm16) and Pparγ coactivator 1 alpha (Ppargc1a) to inhibit the transcription of these two master regulators of mitochondrial biogenesis [42] and beige adipogenesis (Figure 2) [43–45]. Taken together, these results indicate that Notch signaling is a negative regulator of beige adipocyte biogenesis.
Several key questions remain to be answered. Considering the distinct developmental origins of beige and white adipocytes, it’s important to examine whether Notch signaling differentially stimulates the specification, fate determination or differentiation of white versus beige preadipocytes. Alternatively, Notch ligands and receptors may be differentially expressed by beige and white adipocytes to achieve distinct activation pattern or engage different target genes in these two types of adipocytes. Addressing these questions requires the ability to prospectively isolate white preadipocytes and beige progenitors [46], or to definitively identify mature white and beige adipocytes [47].
Notch signaling in skeletal muscle homeostasis
Skeletal muscle utilizes both glucose and free fatty acids as fuel for ATP production during contraction. In addition, skeletal muscle is also a major site of glucose storage, hence coordinately maintaining blood glucose level within the normal range. In particular, the skeletal muscle accounts for 80–90% of postprandial insulin-stimulated glucose uptake [48, 49]. Importantly, skeletal muscle insulin resistance is identified as the primary defect in T2D [50]. Furthermore, muscle exercise elicits strong benefits against metabolic disorders. These benefits are not limited to the direct energy expenditure during the muscle contraction, but also attributed to the muscle secretome: cytokines and peptides produced by and released from skeletal muscle cells (myofibers) to regulate body metabolism [51]. Therefore, maintaining muscle insulin sensitivity and its proper motor and secretory functions are important prerequisites for treating metabolic diseases.
Within the skeletal muscle, a pool of well-defined stem cells called satellite cells are indispensable for the postnatal growth, maintenance and regeneration of myofibers [52]. Notch signaling plays dose-dependent roles in satellite cells [53]. High, intermediate and low Notch activities are essential for the quiescence (self-renewal), activation (cell cycle entry) and differentiation of satellite cells, respectively [54–57]. Specifically, deletion of either Rbpj or Dll1 leads to premature differentiation and depletion of satellite cells, resulting in a loss of postnatal muscle growth and severe muscle hypotrophy [53, 56, 58, 59]. Conversely, constitutive activation of Notch1 promotes the self-renewal but inhibits differentiation of satellite cells, resulting in poor muscle regeneration [57].
In addition to its well-established role in myogenesis, recent studies have pointed to a potential role of Notch signaling in regulating muscle metabolism. In this regard, the interaction between Notch and FoxO1 again appears to be essential. Muscle specific knockout of FoxO1 promotes conversion of oxidative slow-twitch to glycolytic fast-twitch myofibers in the soleus muscle [60]. Using the gold standard euglycemic hyperinsulinemic clamp technique, Pajvani et al found that muscles of Foxo1+/−:Notch1+/− mice have considerably higher rates of glucose uptake compared to those of wild type and Foxo1+/− mice [11], though the fiber type composition in Foxo1+/−:Notch1+/− mice was not characterized [60]. This phenotype can be explained by either muscle specific action of Notch1 and FoxO1, or secondary effect of Notch1:Foxo1 haploinsufficiency in non-muscle organs. It would be interesting to directly examine if myofiber-specific perturbations in Notch signaling affects muscle glucose metabolism and insulin sensitivity in the future.
Notch in the central nervous system (CNS)
CNS plays a key role in orchestrating proper central neuroendocrine function, and regulating systemic glucose and energy metabolism [61]. Of note, obesity is associated with structural and functional impairment of the CNS [62], whose maintenance relies on neurogenesis mediated by adult neural stem cells (NSCs) [63]. Using tamoxifen-inducible conditional Rbpj knockout mice, Imayoshi et al have recently found that deletion of Rbpj in the adult brain caused transient differentiation of NSCs into neurons, leading to a total loss of NSCs and blockage of subsequent neurogenesis [64]. This indicates an indispensable role of Notch signaling in maintaining the quiescence of NSCs in addition to its widely accepted role in inhibiting neuronal differentiation. NSCs in the hypothalamus of DIO mice show impaired survival and neurogenic functions [65]. Mechanistically, HFD feeding activates the IKKβ/NF-κB-Notch signaling axis, which promotes apoptosis and impairs neurogenic differentiation of NSCs [65]. Indeed, hypothalamus specific activation of the pro-inflammatory IKKβ/NF-κB pathway phenocopies the effect of HFD on NSCs, and ultimately leads to the development of obesity and diabetes [65]. Intriguingly, the pro-inflammatory factor NF-κB directly binds to the promoters, and activates expression of Dll4, Notch1 and Notch4 genes in NSCs [65]. Consistently, inhibition of either IKKβ or Notch signaling reverses the differentiation defect of hypothalamic NSCs of DIO mice [65]. Collectively, NF-κB not only mediates systemic low-grade inflammation that is critical for the initiation, development and exacerbation of metabolic syndrome [66], but also impairs neurogenesis through transcriptional upregulation of Notch ligands and receptors during metabolic stress.
Notch signaling also functions as an important NSC niche factor in the CNS. Endothelial cells of cerebral vessels enforce the quiescence of adult NSCs by presenting Jag1 that activates Notch signaling in the neighboring NSCs [67]. Endothelial cell lineage specific ablation of Jag1 results in aberrant activation and depletion of quiescent NSCs [67]. In addition, both HFD and high cholesterol diet increases expression of the Dll4 in the blood vessels of an atherosclerotic mouse model [68]. In summary, Notch signaling must be temporally regulated in NSCs for proper neurogenesis. A low Notch activity facilitates the differentiation of NSCs to immediately supply neurons to repair nerve damage, and a high Notch activity facilitates quiescence and self-renewal of NSCs. Dysregulation of Notch signaling in adult NSCs by obesity associated systemic low-grade inflammation or pro-atherosclerotic vessels represents a novel neurodegenerative mechanism in obese patients.
Notch in metabolic angiogenesis
It has been well-established that Notch signaling regulates the development of embryonic vasculature, and perturbation of Notch pathway genes results in severe vascular defects in mutant mice [69]. During development, Notch signaling specifies arterial fate of endothelial cells [70]. Notch signaling also plays a critical role in controlling the differentiation of vascular smooth muscle cells and senescence of endothelial cells in the postnatal vasculature [71]. Moreover, the Notch pathway actively participates in vascular remodeling by inhibiting the formation and function of endothelial tip cells via the regulation of VEGF signaling [71]. As vascular sprouting is fundamentally important for the growth and expansion of WAT [72], understanding how Notch regulates angiogenesis has important implications in obesity prevention and treatment. Compared to WAT, BAT is hypervasuclarized in order to meet the nutrients and oxygen demands for its active thermogenic metabolism. Additionally, brown adipocytes rely on the blood flow to diffuse the heat throughout the body to defend hypothermia. Investigating whether and how Notch regulates angiogenesis in cold-activated BAT will extend the therapeutic scope of current preclinical trials of Notch-inhibition based intervention of tumor angiogenesis [72].
Of note, vascular dysfunction is not only involved in the development of obesity [72], but also a consequence of metabolic disorders manifested as atherosclerosis and other types of cardiovascular diseases. Strikingly, blockade of Dll4-Notch signaling using neutralizing anti-Dll4 antibody elicited a broad range of benefits: dramatically attenuated the development of atherosclerosis, reduced inflammation, improved insulin resistance, and ameliorated obesity [68].
The vasculature also serves as the main niche factor for both brown and white adipocyte precursors, therefore it may regulate adipose mass and body insulin sensitivity [72]. Currently, it’s unclear how vasculature derived adipose stem cells contribute to the heterogeneity of adipocytes, in terms of their distinct differentiation potential towards white versus beige/brown adipocytes. Future work in characterizing how Notch signaling regulates the fate choice of adipose stem cells in response to physiological (VEGF) and metabolic cues (insulin, energy status) is warranted.
Notch regulates metabolic immunity
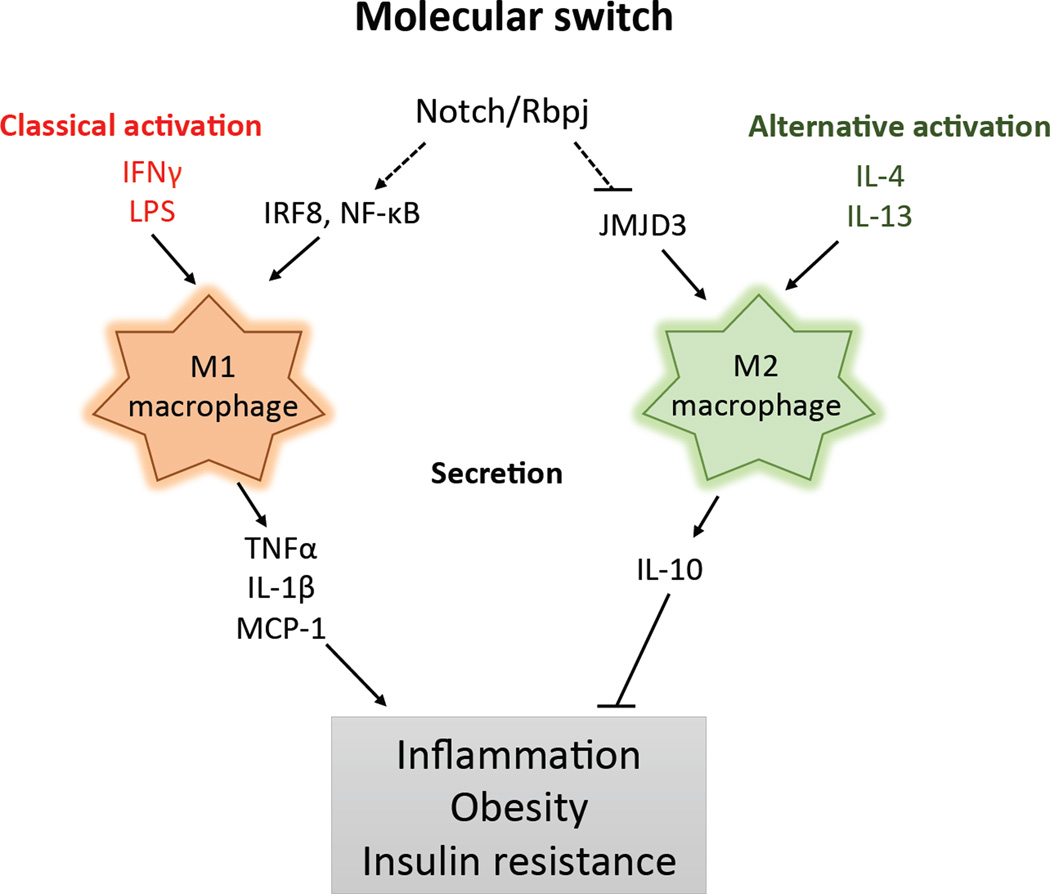
Notch signaling is emerging as an important regulator of both innate and adaptive immune system development and function [73], which have crucial implications in metabolic syndrome [74]. Among various cell types in innate immunity, macrophages are the major players in terms of abundance and functional significance in metabolic disorders. Macrophages can be divided into M1 and M2 macrophages. M1 macrophages are proinflammatory and secret chemokines (e. g. monocyte chemotactic protein 1, MCP-1) and proinflammatory cytokines (e. g. tumor necrosis factor alpha, TNFα) that directly increase inflammation in peripheral tissues and impair local insulin sensitivity (Figure 3) [75]. On the other hand, M2 macrophages ameliorate obesity-induced inflammation and secrete anti-inflammatory cytokines (e. g. interleukin-10, IL-10) that promote insulin sensitivity (Figure 3) [75]. Importantly, Notch signaling regulates M1 versus M2 macrophages specification, through different mechanisms (Figure 3) [76]. First, Rbpj promotes expression of the transcription factor interferon regulatory factor 8 (IRF8) while suppresses expression of the histone H3 Lys 27 (H3K27) demethylase Jumonji domain containing 3 (JMJD3) [77], which are inducers of M1 and M2 polarization respectively [76]. Second, Notch signaling boosts the inflammatory property of M1 macrophages by interacting with NF-κB [78, 79]. Compared to lean animals, DIO mice showed a shift from a M2- to a M1-polarized state [75]. Consistently, Notch signaling in adipose tissue is activated by HFD feeding [36], which in part can be explained by the infiltration of Notch-primed M1 macrophages. It is interesting to determine in the future whether and how inhibition of Notch signaling promotes the transition from M1 to M2 macrophages in obese adipose tissues, and its therapeutic potentials against obesity and other metabolic disorders.
Figure 3. Notch signaling and macrophage polarization.
M1 (classically activated) and M2 (alternatively activated) macrophages are activated by distinct and mutually exclusive activation programs. Notch signaling promotes M1 macrophage polarization through synergy with NF-κB and by upregulating expression of the M1 macrophage regulator IRF8. Notch signaling inhibits M2 macrophage polarization through repressing JMJD3, a M2 macrophage regulator. M1 and M2 macrophages have distinct secretory profiles, which oppositely control inflammation and impact systematic insulin sensitivity. Abbreviation: IFNγ, Interferon gamma; LPS, Lipopolysaccharide; IRF8, Interferon regulatory factor 8; JMJD3, Jumonji domain containing 3; MCP-1, monocyte chemotactic protein 1.
Notch-mediated macrophage polarization contributes to the development of metabolic disorders not only through the above-mentioned inflammatory actions, but also through direct interaction with adipocytes. Notch ligands present on the cell surface of macrophages can potentially activate Notch signaling in the neighboring adipocytes (Figure 2), hepatocytes and myofibers, and consequently exacerbate the insulin resistance of these key peripheral tissues involved in energy metabolism. Indeed, interferon (IFN)-γ, which activates M1 macrophages, induces Jag1 expression rapidly, therefore amplifying Notch signaling in neighbor cells [80].
Macrophage-adipocyte interaction is of significance in obesity and T2D mellitus, where systemic infiltration of macrophages and other immune cells is prevalent. In this scenario, dynamic Notch ligand presentation on these circulating cell types may produce functional diversity in Notch activation, a direction deserving future investigation. The complexity is encoded by the diverse signaling capacity of Notch ligands and receptors [81], and preferential binding of ligands to different Notch receptors [82]. For instance, during angiogenesis, Dll4 has strong, while Jag1 has weak signaling potential, and the relative abundance of these ligands creates distinct outcomes in angiogenesis [83]. Similarly, vasculature may also represent a crucial metabolic niche factor by presenting Notch ligands to fine-tune Notch signaling in peripheral tissues and metabolic disorders: either through regulating stem cells to maintain homeostasis of neurons, myocytes and adipocytes or through directly regulating enzyme expression in hepatocytes. These observations and speculations warrant future investigations into the metabolic phenotypes of mice with macrophage specific deletion of Notch ligand genes. Answers to this question will shed light to the distinct function of different Notch ligands in peripheral tissues, and potentially enable tissue-specific Notch based therapy to treat metabolic disorders.
Concluding remarks and future perspectives
Notch signaling is a key regulator of cell fate and cellular homeostasis in virtually every metabolic organ. In the liver, Notch signaling boosts the gluconeogenesis and lipogenesis programs, which lead to hyperglycemia and fatty liver disease. In the adipose tissue, genetic activation of Notch signaling induces whitening of BAT and insulin resistance, whereas genetic or pharmacological inhibition of Notch signaling promotes browning of white adipocytes and improves insulin sensitivity. In the skeletal muscle and brain, Notch activation actively enforces the quiescence of local adult stem cells, therefore limiting their tissue-repair potentials and subsequently influencing body metabolism. In the immune system, activation of Notch signaling promotes M1 macrophage polarization, producing a systemic low-grade inflammation state that exacerbates insulin resistance in peripheral tissues. Inhibition of Notch signaling in several of these tissues consistently improves glucose tolerance, insulin sensitivity, and ameliorates obesity and atherosclerosis. Several outstanding questions are summarized in Box 1, which merit future investigations With the availability of pharmaceutical grade γ-secretase inhibitors used in various clinical trials to treat Alzheimer’s disease and cancers [84], it would be feasible to examine the effect of these inhibitors in treating diabetes and obesity in humans. Due to the gastrointestinal toxicity and other off-target effects of γ-secretase inhibitors [85–87], however, alternative Notch inhibitors should also be developed. In this regard, antibodies targeting Notch ligands and receptors have recently been used in clinical trials [84]. The anti-obesity and anti-diabetic effect of these promising therapeutic agents should be investigated in the future.
Box 1: Outstanding questions.
What are the ligand presenting cell types that activate Notch signaling in metabolic organs under normal and disease conditions?
Do different Notch receptors have diverse, or redundant, roles in regulating energy metabolism?
How does Notch signaling regulate browning? Is it through determining the fate of adipocyte precursors, or regulating interconversion of mature white and beige adipocytes?
What is the metabolic function of Notch signaling in mature muscle cells (myofibers)?
How do energy-sensing kinases, such as AMPK and mTOR, modulate Notch signaling transduction?
Highlights.
Inhibition of Notch signaling improves liver metabolism and ameliorates steatosis
Genetic or pharmacological inactivation of Notch signaling promotes beige adipogenesis and ameliorates obesity
High fat diet activates Notch signaling in the brain and inhibits neurogenesis of central neuroendocrine cells
Notch signaling favors proinflammatory M1 macrophages over anti-inflammatory M2 macrophages
Acknowledgments
This work was partially supported by a grant from the US National Institutes of Health (R01AR060652).
Glossary
- Atherosclerosis
a type of vascular disease characterized by plaque buildups in arteries resulted from increased cytokines due to metabolic dysfunction, which leads to activation of the innate immune system and chronic inflammation.
- Beige adipocytes
a newly defined type of adipocytes within the white adipose tissue. They are similar to brown adipocytes in that they express UCP1 and have capacity for thermogenesis. They have a distinct gene expression signature from either brown adipocyte or white adipocyte.
- Brown adipocytes
a type of adipocytes that is abundant in rodents and newborn human but less abundant in adult human, and with high capacity for adaptive thermogenesis. Brown adipocytes contain numerous mitochondria expressing UCP1, which uncouples proton gradient from ATP production, to generate heat. Due to their ability to burn lipids (through β-oxidation) to generate heat, brown adipocytes increase energy expenditure and are negatively associated with obesity.
- Delta/Serrate/Lag (DSL) family protein
single-pass transmembrane proteins whose extracellular domain acts as a ligand for Notch receptors on a neighbor cell. In mammals, the family members include Delta-like (Dll1, Dll3, Dll4) and Jagged (Jag1, Jag2).
- Gluconeogenesis
a biochemical process that generates glucose from non-carbohydrate carbon substrates like pyruvate.
- Glycogenolysis
a biochemical process whereby glycogen is broken down to glucose-1-phosphate.
- Glycolysis
a biochemical process that converts glucose to pyruvate, releasing free energy in the form of ATP.
- Hairy/enhancer-of-split related with YRPW motif protein (Hey)
nuclear protein that belongs to the hairy and enhancer of split-related (HESR) family of basic helix-loop-helix (bHLH)-type transcriptional repressors. Hey expression is induced by Notch signaling.
- Hairy and enhancer of split (Hes)
transcription repressor that belongs to the bHLH protein family with important roles in the Notch signaling pathway.
- Lipogenesis
a metabolic pathway that has two separate processes: fatty acid synthesis and triglyceride synthesis.
- M1 and M2 macrophage
also known as classically and alternatively activated macrophages, respectively. M1 macrophages are activated in response to bacterial infections or lipopolysaccharide and interferon-γ, and are highly inflammatory. In contrast, M2 macrophages are activated in response to parasitic infections, or interleukin 4 and -13, and are anti-inflammatory.
- Notch receptors (Notch1–4)
a family of single-pass transmembrane receptors, consisting of an extracellular domain, a transmembrane domain and an intracellular domain. Activation of Notch receptors leads to release of Notch intracellular domain, which then acts as a transcriptional factor to regulate gene expression.
- Recombining binding protein suppressor of hairless (Rbpj)
also known as CBF1 in humans, is a highly conserved DNA-binding protein that mediates canonical Notch signaling.
- White adipocyte
a major type of adipocyte in animals and humans that stores energy in the form of triglycerides.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson ER, et al. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 3.Meloty-Kapella L, et al. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22:1299–1312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neil J, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204:1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry JM, Li L. Self-renewal versus transformation: Fbxw7 deletion leads to stem cell activation and leukemogenesis. Genes Dev. 2008;22:1107–1109. doi: 10.1101/gad.1670708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancho R, et al. Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biol. 2013;11:e1001586. doi: 10.1371/journal.pbio.1001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guruharsha KG, et al. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louvi A, Artavanis-Tsakonas S. Notch and disease: a growing field. Semin Cell Dev Biol. 2012;23:473–480. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol. 2014;60:885–890. doi: 10.1016/j.jhep.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Pajvani UB, et al. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19:1054–1060. doi: 10.1038/nm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pajvani UB, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1- dependent manner. Nat Med. 2011;17:961–967. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael MD, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Biddinger SB, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamming DW, Sabatini DM. A Central role for mTOR in lipid homeostasis. Cell Metab. 2013;18:465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenti L, et al. Hepatic notch signaling correlates with insulin resistance and nonalcoholic fatty liver disease. Diabetes. 2013;62:4052–4062. doi: 10.2337/db13-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, et al. Suppression of the mTORC1/STAT3/Notch1 pathway by activated AMPK prevents hepatic insulin resistance induced by excess amino acids. Am J Physiol Endocrinol Metab. 2014;306:E197–E209. doi: 10.1152/ajpendo.00202.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Ross DA, et al. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol. 2004;24:3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vujovic S, et al. Inhibition of gamma-secretases alters both proliferation and differentiation of mesenchymal stem cells. Cell Prolif. 2007;40:185–195. doi: 10.1111/j.1365-2184.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osathanon T, et al. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biology International. 2012;36:1161–1170. doi: 10.1042/CBI20120288. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, et al. gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-gamma. Cell Prolif. 2010;43:147–156. doi: 10.1111/j.1365-2184.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols AM, et al. Notch pathway is dispensable for adipocyte specification. Genesis. 2004;40:40–44. doi: 10.1002/gene.20061. [DOI] [PubMed] [Google Scholar]
- 23.Lai PY, et al. Active form Notch4 promotes the proliferation and differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2013;430:1132–1139. doi: 10.1016/j.bbrc.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Noda N, et al. Hes1 is required for contact inhibition of cell proliferation in 3T3-L1 preadipocytes. Genes Cells. 2011;16:704–713. doi: 10.1111/j.1365-2443.2011.01518.x. [DOI] [PubMed] [Google Scholar]
- 25.Berry DC, et al. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 27.Matthias A, et al. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem. 2000;275:25073–25081. doi: 10.1074/jbc.M000547200. [DOI] [PubMed] [Google Scholar]
- 28.Fedorenko A, et al. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenwald M, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 32.Ye L, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao L, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci. 2013;126:3527–3532. doi: 10.1242/jcs.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bi P, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan T, et al. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:277–287. doi: 10.1096/fj.12-211516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens K, et al. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett. 2010;584:1054–1058. doi: 10.1016/j.febslet.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 39.Urs S, et al. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 40.Mullican SE, et al. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol. 2013;27:127–134. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KY, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 43.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 44.Cohen P, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seale P, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ussar S, et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med. 2014;6:247ra103. doi: 10.1126/scitranslmed.3008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiebaud D, et al. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31:957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 49.Ferrannini E, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 50.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 52.Bentzinger CF, et al. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mourikis P, Tajbakhsh S. Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev Biol. 2014;14:2. doi: 10.1186/1471-213X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 55.Mourikis P, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 56.Bjornson CR, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen Y, et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasyutina E, et al. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc Natl Acad Sci U S A. 2007;104:4443–4448. doi: 10.1073/pnas.0610647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuster-Gossler K, et al. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci U S A. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitamura T, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandoval DA, et al. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov. 2009;8:386–398. doi: 10.1038/nrd2874. [DOI] [PubMed] [Google Scholar]
- 62.Rusinek H, Convit A. Obesity: Cerebral damage in obesity-associated metabolic syndrome. Nat Rev Endocrinol. 2014;10:642–644. doi: 10.1038/nrendo.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 64.Imayoshi I, et al. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, et al. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker RG, et al. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottone C, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukuda D, et al. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A. 2012;109:E1868–E1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 70.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 71.Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao XC, et al. Inhibition of tumor angiogenesis and tumor growth by the DSL domain of human Delta-like 1 targeted to vascular endothelial cells. Neoplasia. 2013;15:815–825. doi: 10.1593/neo.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radtke F, et al. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–437. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 74.Sell H, et al. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 75.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 76.Xu H, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13:642–650. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Satoh T, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 78.Maniati E, et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121:4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monsalve E, et al. Notch1 upregulates LPS-induced macrophage activation by increasing NFkappaB activity. Eur J Immunol. 2009;39:2556–2570. doi: 10.1002/eji.200838722. [DOI] [PubMed] [Google Scholar]
- 80.Foldi J, et al. Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1. J Immunol. 2010;185:5023–5031. doi: 10.4049/jimmunol.1001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramasamy SK, Lenka N. Notch exhibits ligand bias and maneuvers stage-specific steering of neural differentiation in embryonic stem cells. Mol Cell Biol. 2010;30:1946–1957. doi: 10.1128/MCB.01419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andrawes MB, et al. Intrinsic selectivity of Notch 1 for Delta-like 4 over Delta-like 1. J Biol Chem. 2013;288:25477–25489. doi: 10.1074/jbc.M113.454850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 84.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 85.Doody RS, et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 86.Siemers ER, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 87.Fleisher AS, et al. Phase 2 safety trial targeting amyloid beta production with a gammasecretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]