Abstract
Purpose
To compare the initial biomechanical properties of zone I flexor tendon to bone repairs performed using pull-out and anchor techniques and to investigate the effect of bone quality and suture materials on the strength of anchor repairs.
Methods
Using computed tomography, we measured bone mineral density and cortical thickness of the distal phalanx of 60 cadaver fingers (mean age, 77 years). Flexor digitorum profundus tendons were then transected at their insertion sites and repaired using a 4-strand grasping suture and either pull-out or anchor fixation. For pull-out repair (n = 20), the suture strands (Supramid 3-0; S. Jackson, Inc., Alexandria, VA) were passed through the distal phalanx and tied over a dorsal button. For anchor repair, 2 bone anchors were inserted into the distal phalanx, and tendons were grasped using either Supramid (n = 21), Ethibond (Ethicon, Inc., Somerville, NJ; n = 10), or FiberWire suture (Arthrex Inc., Naples, FL; n = 9) (all 3-0). Mechanical properties of the repaired tendon– bone constructs were determined in linear, load-to-failure loading and correlated with bone characteristics.
Results
The FiberWire-anchor repair group had the best combination of mechanical properties, with ultimate force to failure no different from the pull-out repairs but with greater stiffness and reduced displacement. Pull-out suture repairs had significantly higher ultimate force-to-failure values than did Ethibond-anchor and Supramid-anchor repairs (p < .01). However, pull-out repairs had significantly reduced stiffness and greater displacement at 20 N force than did anchor repairs from all groups (p < .05). Both bone mineral density and cortical thickness correlated significantly with ultimate force (p < .01). Almost all anchors pulled out for bone mineral density below 420 mg/cm3 or cortical thickness less than0.31 mm, which occurred only for specimens aged greater than75 years.
Conclusions
The mechanical properties of the double Mitek bone anchors were sensitive to both suture material and bone quality. FiberWire-anchor repairs provided the best combination of mechanical properties. Pull-out suture repairs had good strength but poor stiffness. Anchor fixation may be contraindicated in patients greater than 75 years because of poor bone quality.
Keywords: Bone quality, flexor tendon repair, Mitek anchor, suture material, zone I
Injury to the FLEXOR DIGITORUM profundus (FDP) at its insertion often requires reattaching the tendon to the distal phalanx. Conventionally, the repair is performed with the pull-out suture technique, originally described by Bunnell.1 However, this technique is complicated and carries the risk of infection secondary to the transcutaneous course of the sutures.2 A newer alternative is to use a bone anchor technique, which makes it possible to reattach tendon to bone relatively easily without the need to have an exposed tie-down mechanism.
Previous cadaver studies have been inconclusive in establishing which repair technique, anchor, or pull out has better initial mechanical properties. Buch et al.3 reported an average failure force of 69 N for repairs performed using a single Mitek Mini (DePuy Mitek, Raynham, MA) anchor. In contrast, Silva et al.4 reported an average failure force of 44 N using the same anchor repair method, which was inferior to the failure force for pull-out repairs (60 N). A notable difference between these 2 studies was the average age of cadaver donors (57 years for Buch et al. vs 74 years for Silva et al.), suggesting that variations in bone quality related to age differences might have contributed to the conflicting findings. More recently, Brustein et al.5 reported no difference between the failure force of pull-out repairs and repairs performed with a single Mitek Mini anchor (average donor age, 69 years) but found both were inferior to repairs performed using 2 Mitek Micro anchors. Notably, measures of the resistance to elongation (eg, stiffness, gap force) have not been reported for the double anchor repair method.
The objective of tendon-bone repair is to re-establish direct tendon–bone contact with adequate initial strength to maintain contact during rehabilitation and healing. The initial mechanical properties of tendon–bone repairs performed using anchors are limited by the strength of the anchor fixation or the suture. Fixation strength, particularly for bone anchors, depends, in large part on bone quality and device design. In a recent cadaver study of rotator cuff reattachment to the proximal humerus, Tingart et al.6 reported a positive correlation between cortical bone mineral density and the failure force of the suture anchor. We are unaware of any reports on the effect of bone quality on flexor tendon–bone repairs performed using 2 bone anchors. Even if good fixation strength is achieved with anchors, the mechanical properties of the repair construct may be inadequate because of suture failure. The relationship between type of suture material and strength of anchor fixation has not been reported for FDP insertion site repairs.
The objectives of this cadaver study were (1) to compare the initial biomechanical properties of zone I flexor tendon to bone repairs performed using either pull-out or anchor techniques, and (2) to investigate the effect of bone quality and suture materials on the strength of anchor repairs. We hypothesized that the mechanical properties of FDP tendon fixation to the distal phalanx are dependent both on bone quality of the distal phalanx and on the strength characteristics of the suture material, and that lower bone mineral density values and lower mechanical strength of sutures negatively affect mechanical properties after bone anchor repair.
MATERIALS AND METHODS
Overview
This study was performed in 2 parts. In both parts, bone quality of the distal phalanx was assessed by measurement of bone mineral density (BMD) using peripheral quantitative computed tomography (pQCT) and cortical thickness using micro–computed tomography (microCT). After computed tomography scanning, tendon– bone repairs were performed and then mechanically tested to determine tensile properties. In part 1, we directly compared the tensile properties of suture pull-out versus anchor repairs performed using the same suture (Supramid 3-0; S. Jackson Inc., Alexandria, VA) and the same grasping technique (modified Becker). Results of this experiment indicated an unexpectedly low force to failure in the anchor repairs with a high proportion of suture ruptures. Therefore, in part 2 we performed anchor repairs using 2 different suture materials (Ethibond, Ethicon Inc., Somerville, NJ; Fiber-Wire, Arthrex Inc., Naples, FL) and compared failure modes and tensile properties between anchor repair groups. Within each part, we used a random assignment scheme. Using the data from the 3 anchor repair groups, we then examined correlations between measures of bone quality and the strength of anchor repairs.
Materials
We obtained 62 index, middle, and ring fingers from 22 hands of 11 adult cadavers (7 men, 4 women) within 24 hours of death. The fingers were stored at −20°C until tested. None of the donors had a known history of musculoskeletal illness, and there were no gross abnormalities of the arms or hands. The ages of patients ranged from 48 to 92 years (77 years ± 15, mean ± SD).
After thawing the digits to room temperature (21°C to 23°C), the insertion sites of the FDP tendons were exposed. After transecting the FDP tendons in the palm, the digits were disarticulated at the proximal interphalangeal joints. The FDP tendons were left attached to the distal phalanges, and specimens were then scanned with computed tomography.
Bone mineral density measurements
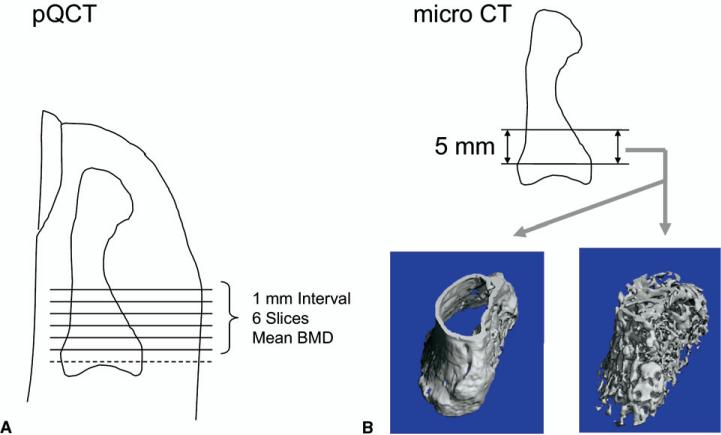
We measured the BMD of the distal phalanx of each finger using pQCT (XCT Research M, Stratec, Pforzheim, Germany). Six transverse slices (0.5-mm thickness; 0.07-mm voxel size) were obtained from 1 to 6 mm distal to the distal interphalangeal joint of each specimen. From these, we determined the average BMD (mg/cm3) using the manufacturer's software and a simple threshold of 280 mg/cm3 as described previously7 (Fig. 1A).
FIGURE 1.
Location of samples from which BMD and morphometric characteristics were measured. A From each finger, 6 transverse slices were obtained from 1 to 6 mm distal to the distal interphalangeal joint. The average BMD (mg/cm3) and bone mineral content (mg) were calculated for the 6 slices. B Cortical thickness, trabecular thickness, and the bone volume/tissue volume (BV/TV) ratio were determined using microCT for a 5-mm segment from the point 1 mm distal to the joint surface.
Cortical thickness measurements
Bone morphometric characteristics were measured using microCT (μCT 40, SCANCO Medical, Brüttisellen, Switzerland). The region of interest was the same 5-mm segment as analyzed by pQCT, from 1 to 6 mm distal to the distal interphalangeal joint (Fig. 1B). We used the manufacturer's software to interactively determine the cortical thickness of the distal phalanx in the region of anchor placement including palmar and dorsal cortex.
Surgical reattachment procedures
After evaluating bone quality, we transected the FDP tendon at its insertion and reattached it with a bone anchor technique or with a pull-out suture technique. In 2 digits (from a 92-year-old donor), the distal phalanx was fractured during anchor insertion; these digits were excluded from further evaluation. In part 1 of the study, we examined 2 repair methods with fixation method as the variable: (1) pull-out suture repair using two 3-0 caprolactam sutures (Supramid Extra II; S. Jackson Inc., Alexandria, VA; n = 20); (2) Mitek Micro anchor repair with Supramid suture (“Supramid-anchor”; n = 21). In part 2 of the study, we examined 2 additional suture materials with anchor repair: (3) Mitek Micro anchor with Ethibond suture (“Ethibond-anchor”; n = 10); (4) Mitek Micro anchor with FiberWire suture (“FiberWire-anchor”; n = 9).
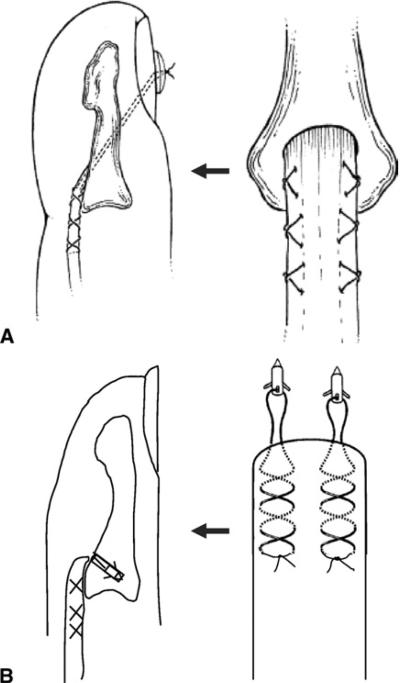
For the pull-out suture repair, we used two 3-0 Supramid sutures for each digit. Each suture grasped the lateral one-fourth of the FDP tendon, in a modified Becker method,8 beginning at a point 15 mm proximal to the cut end of the tendon. After suture placement, 2 Keith needles were drilled antegrade through the palmar surface of the distal phalanx (at the insertion site of the FDP tendon) and exited dorsally through the nail plate. The sutures were passed through the Keith needles, and the needles were then pulled through bone tunnels, exiting dorsally. The tendon was pulled against the palmar surface of the distal phalanx, and the sutures were tied over a button placed on the nail plate, using 3 knots and maximal hand tension (Fig. 2A).
FIGURE 2.
Fixation of the FDP tendon to the distal phalanx. A For pull-out suturing, we placed two Supramid sutures in each finger. Each suture grasped the lateral quarter of the FDP tendon, passed through the palmar surface of the distal phalanx (at the insertion of the FDP tendon), exited dorsally through the nail plate, and was tied over a button placed on the nail plate. B Two Mitek Micro anchors with 3-0 selected sutures (4 strands) were inserted into the distal phalanx, and the suture threads from 2 anchors were woven along both sides of the tendons in a modified Becker method to cross 3 times on each side.
For bone–anchor repair, we used two Mitek Micro anchors (1.3 mm in diameter and 3.7 mm in length) for each digit. The preloaded suture was removed from the anchor eye and replaced with 1 of 3 different suture types to determine the best combination of anchor and suture material: (1) 3-0 cable type nylon (inner nylon fibers enclosed in a smooth nylon outer shell; Supramid Extra II); (2) 3-0 braided polyester (Ethibond); and (3) 3-0 braided polyblend (with an outer covering of ultra-high molecular weight polyethylene [UHMWPE] polyester braided over a UHMWPE core; FiberWire). Two Mitek Micro anchors were inserted into the distal phalanx at both sides of the FDP insertion after predrilling with the 1.3-mm drill bit included in the kit. The 2 suture strands from each anchor (4 strands total) were woven along the sides of the tendons using the modified Becker method8 so that 3 crosses were applied to each side of the tendon.8 The sutures were tied at both sides of the tendon using 3 knots and maximal hand tension (Fig. 2B).
Mechanical testing of the tendon–bone interface
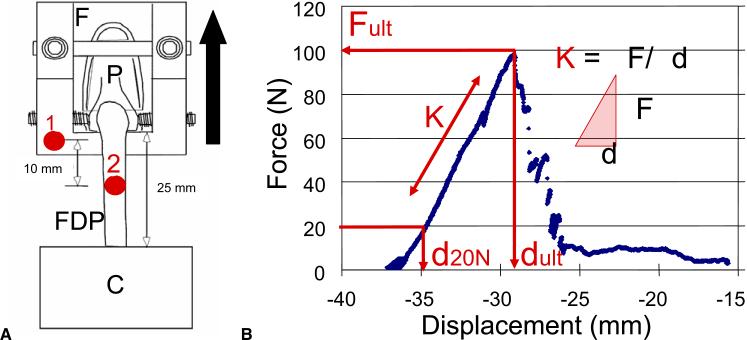
The repaired tendon–bone interfaces were tested to failure at room temperature using a servohydraulic materials testing system (Instron 8500R; Canton, MA, USA) and procedures we have reported previously.4,9 Specimens were prepared just as isolated tendon–bone constructs; the pulley systems were removed and only distal phalanx and FDP tendon remained. The distal phalanx was rigidly held in a custom-made bone clamp (Fig. 3A). Sandpaper was fixed with cyanoacrylate to the proximal tendon stump 2.5 cm from the insertion site, and the proximal tendon stump was then held in a soft tissue clamp. A reflective marker, 2 mm in diameter, was glued to the phalangeal fixture (a custom-made bone clamp that is shown in Fig. 3A) immediately adjacent to the tendon insertion site. A 1-N preload was applied to the tendon, and a second marker was glued to the palmar surface of the tendon, 1 cm proximal to the other marker.
FIGURE 3.
A Setup for biomechanical testing. Here, F is the custom-made fixture, P is the distal phalanx, FDP is the FDP tendon, and C is the proximal tendon clamp that holds the sandpaper attached to the tendon. Number 1 is a 2-mm-diameter reflective marker glued to the phalangeal fixture immediately adjacent to the tendon insertion site, and number 2 is a second marker glued to the palmar surface of the tendon, 1 cm proximal to the other marker. B The force-displacement curve. Here, Fult is the ultimate (maximum) force to failure (N), K is the stiffness of tendon-bone complex (N/mm), d20N is the displacement at 20 N (mm), and dult is the displacement at ultimate force to failure (mm).
The tendon–bone samples were preconditioned (5 cycles, 1 Hz, 1–5 N tension). They were then loaded by an 0.375 mm/s single displacement ramp until the repair failed. The FDP loading direction was tangential to the palmar cortex, and the angle was constant during testing (ie, “linear testing”). Synchronized force and marker data were collected at 60 Hz. Marker positions were determined using a motion analysis system (PC-Reflex; Qualisys, Glastonbury, CT). From the marker positions, we computed repair-site displacement in millimeters. From plots of force versus displacement, we determined the ultimate (maximum) force to failure, repair-site stiffness, displacement at 20 N, and displacement at the moment of failure (Fig. 3B). Failure mode was noted as either suture rupture or anchor pullout.
Video analysis of the failure tests
Before testing, both the distal stump of the FDP tendon and the palmar cortex that was in contact with the distal stump of the tendon were marked with tissue marking dye (TMD-BK; TBS Inc., Durham, NC) to help visualize gap formation. Each tensile test was recorded with a charge-coupled device camera and a digital video recorder. Images were recorded at 60 frames/s, with a time stamp accurate to 0.017 seconds. A light-emitting diode in the testing field was triggered to provide a visual signal of the start of the test. A ruler with 1-mm graduated markings was placed in the video field, parallel and coplanar with the tendon, as a length reference. The video image was captured in the computer and analyzed with video-editing software. The development of the repair site gap on the video image was analyzed frame by frame. The time from the start of the tensile test to each of the gap events was then used to determine the force (N) being applied at each event. From the analysis of the video recordings, we determined the force at 1-, 2-, and 3-mm gaps. The 1-mm gap was defined as the point when the gap length first reached a length of 1 mm or more across the complete width of the tendon. In a similar manner 2- and 3-mm gap was defined. This measurement was done by single a observer (H.M.). Because of technical error in data collection, video analysis could not be performed in 8 specimens (3 pull-out suture, 2 Supramid-anchor, one Ethibond-anchor, and 2 FiberWire-anchor).
Statistical methods
The biomechanical measurements of each repair group were analyzed with analysis of variance (ANOVA), and pairwise differences between groups were tested with Fischer's protected least-significant-difference method (Statview 5.0; SAS Institute Inc, Cary, NC). Correlations between bone quality (BMD and cortical thickness) and tensile strength were assessed with Pearson's correlation coefficient. Alpha was set at 0.05, and all tests were 2-tailed.
RESULTS
Comparison of repair techniques using supramid suture: pull-out suture versus anchor repair
All pull-out suture repairs (21 of 21) failed by suture rupture at the button. Thirteen of 21 Supramid-anchor repairs failed by suture rupture at the anchor, and 8 of 21 failed by anchor pullout from the distal phalanx.
Pull-out repairs had significantly greater strength (ultimate force to failure) compared with Supramid-anchor repairs (p < .01; Table 1). By contrast, pull-out repairs had significantly lower stiffness and greater displacement at 20 N and at failure than did Supramid-anchor repairs (p < .05), indicating inferior resistance to elongation in the pull-out repairs.
TABLE 1.
Tensile Properties for 4 Methods of Zone I Flexor Tendon-Bone Reattachment on 60 Cadaver Fingers*
| Property | Pull-Out Sutures (n = 20) Mean (SD) | Supramid-Anchor (n = 21) Mean (SD) | Ethibond-Anchor (n = 10) Mean (SD) | FiberWire-Anchor (n = 9) Mean (SD) |
|---|---|---|---|---|
| Ultimate force (N) | 75 (11) | 43 (11)†,‡ | 47 (11)†,‡ | 66 (27) |
| Stiffness (N/mm) | 9(2) | 11 (3)†,‡ | 12 (3)† | 14 (4)† |
| Displacement (mm) | ||||
| At 20 N | 3.43 (0.63) | 2.64 (0.60)†,‡ | 2.05 (1.11)† | 1.62 (1.21)† |
| At failure | 9.34 (1.28) | 5.23 (2.15)† | 4.27 (1.81)† | 4.82 (2.02)† |
| Gap force (N) | ||||
| At 1 mm | 23 (12) | 23 (8) | 24 (8) | 32 (11) |
| At 2 mm | 34 (15)‡ | 34 (9)‡ | 36 (10) | 47 (15) |
| At 3 mm | 45 (14)‡ | 39 (13)‡ | 42 (14)‡ | 62 (21) |
Post hoc power analysis indicated that the ANOVA was adequately powered to detect differences in ultimate force (p > .99), stiffness (p = .98), displacement at 20 N (p > .99), displacement at failure (p > .99), and force at 3-mm gap (p = .89). Our study design was not adequately powered for detecting differences in gap force at 1 mm (p = .38) or 2 mm (p = .52). In addition, for pairwise comparisons, our study was adequately powered (p = .8) to detect differences of 25% or greater in ultimate force and stiffness between groups.
Significantly different from the pull-out suture group (p < .05).
Significantly different from FiberWire group (p < .05).
Comparison of suture materials in anchor repair: supramid versus Ethibond versus FiberWire
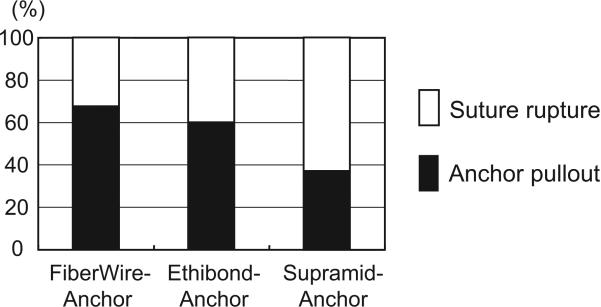
The proportion of anchor repairs that failed by suture rupture was less in the FiberWire-anchor (3 of 9) and the Ethibond-anchor (4 of 10) groups compared with the Supramid-anchor group (13 of 21), and the rest of the anchor repairs all failed by anchor pullout from the distal phalanx (Fig. 4). Thus, use of stronger suture materials shifted the failure mode and resulted in greater loading of the bone anchor.
FIGURE 4.
Causes of failure for the anchor repairs. The cause of failure was anchor pullout in 38% (8 out of 21) of the Supramid group, 60% (6 out of 10) of the Ethibond group, and 67% (6 out of 9) of the FiberWire group, indicating a positive relationship between suture strength and increased incidence of anchor pullout.
The strength (ultimate force to failure) of FiberWire-anchor repairs was significantly greater than that of Supramid-anchor and Ethibond-anchor repairs (p < .01 for each comparison; Table 1). FiberWire-anchor repairs were also significantly stiffer and had less displacement at 20 N than Supramid-anchor repairs (p < .05) and had significantly greater force at 3-mm gap than did Supramid-anchor and Ethibond-anchor groups (p < .01). Thus, use of FiberWire with anchor fixation was clearly superior in resisting pull-out forces to anchor repairs performed with either Supramid or Ethi-bond (which did not differ significantly from each other [p < .05]).
Effect of bone quality on anchor fixation
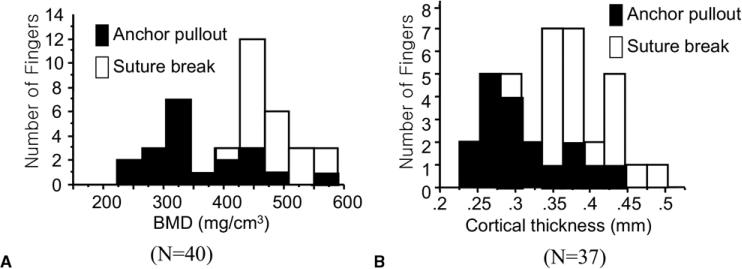
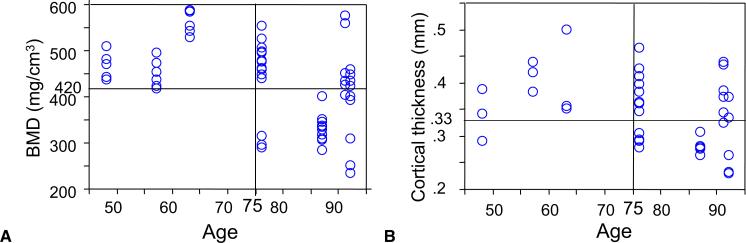
Bone quality affected both ultimate force and failure mode of the anchor repairs. Ultimate force of repairs that failed by anchor pullout correlated positively with BMD of the distal phalanx (r = 0.72, p < .01, n = 20) and with cortical thickness of the distal phalanx (r = 0.65, p < .01, n = 19; Fig. 5). Correlations between stiffness and BMD (r = 0.54, n = 19) and stiffness and cortical thickness (r = 0.53, n = 18) were also significant (p < .05). When BMD was less than 420 mg/cm3 or cortical thickness was less than 0.33 mm, 15 of 16 and 13 of 14 tendon–bone interfaces failed by anchor pullout, respectively (Fig. 6). On comparing bone age and bone quality, we noted that BMD had a moderate correlation with donor age (r = 0.44, p < .01, n = 60), whereas the correlation between cortical thickness and donor age did not reach significance (r = 0.31, p = .058, n = 37). Seventy-five years was a “threshold” age for bone quality (Fig. 7). In specimens from donors younger than 75 years (n = 18), all but 1 had a BMD value greater than 420 mg/cm3 and cortical thickness greater than 0.33 mm. In contrast, in specimens from donors older than 75 years (n = 43), almost half of the specimens had BMD values less than 420 mg/cm3 and cortical thickness values less than 0.33 mm.
FIGURE 5.
Correlation between bone quality and the strength of anchor fixation. A Scatterplot of total BMD by ultimate force (r = 0.77, p < .01). B. Scatterplot of cortical thickness by maximal force (r = 0.70, p < .01). The correlation was analyzed with Pearson's correlation coefficient.
FIGURE 6.
Effect of bone quality on the cause of anchor fixation failure. A Distribution of BMD by the cause of failure. B Distribution of cortical thickness by the cause of failure. A BMD of 420 mg/cm3 or a cortical thickness of 0.33 mm were critical values below, which almost all tendon-bone interfaces failed by suture break.
FIGURE 7.
Correlation between age and bone quality. A Scatterplot of patients’ ages by BMD (r = 0.44, p < .001). B Scatterplot of patients’ ages by cortical thickness (r = 0.31, p = .059). The correlation was analyzed with Pearson's correlation coefficient.
DISCUSSION
The pull-out suture technique has been considered the standard surgical method for reattaching the FDP tendon to the distal phalanx. The method has several limitations, however. Sutures have to be passed through the distal phalanx, tied over a button or over the nail, and kept in place for 6 weeks or more. The technique also carries a risk of infection, facilitated by suture tracks,2 as well as irritation of soft tissues by the button. Moreover, initial fixation strength and stiffness with pull-out suturing may be insufficient to permit early controlled passive rehabilitation when modern place and hold techniques are used.9,10 Therefore, an alternative technique of repairing tendon to the distal phalanx with use of bone anchors has been developed.3,5,11
In our study, the pull-out suture method performed well in resisting pull-out yet had inferior stiffness compared with the 3 anchor repair groups. These findings are not entirely consistent with an earlier report that compared button fixation versus anchor fixation using a single Mitek Mini G2 anchor.4 In that study, button fixation had superior values for both ultimate force and stiffness compared with anchor fixation. Taken together, the results of the 2 studies indicate that use of 2 Mitek Micro anchors provides superior stiffness compared with use of the earlier single anchor technique and with the classic pull-out suture method of repair. In the current study, the displacement at 20 N (a force level that approximates force levels generated by active motion without resistance12) was 3.4 mm for the pull-out technique, indicating that the repair site is at risk of excessive elongation during active digital flexion. In contrast, anchor fixation using the same suture (Supramid 3-0) resulted in a displacement at 20 N of 2.6 mm. The superior stiffness of the anchor fixation groups we attribute to the reduced length of suture between the tendon stump and the point of fixation.
The failure mode and strength of the tendon-bone interface repaired with anchor fixation varied greatly, depending on the suture material used. Nearly two thirds of anchor repairs done using Supramid suture failed by suture rupture, indicating that suture strength was often a limiting factor for this type of repair. As the strength of the suture material increased (from Supramid to Ethibond to FiberWire),13–15 ultimate force to failure increased and the mechanism of failure shifted from suture breakage to anchor pullout from bone. Mitek anchors with FiberWire sutures had a similar ultimate force to failure and significantly higher stiffness values compared with that of pull-out suturing using Supramid (p < .01), indicating that this construct had the best combination of properties among the groups that were tested here and may be appropriate for use with early active mobilization.
Our study provides novel data on the thickness of the cortical shell of the distal phalanx and the effects of bone quality and age on anchor fixation. Notably, the distal phalanx had a cortical thickness of only 0.2 to 0.5 mm at the tendon insertion site, consistent with the thin cortex at other anatomic sites where trabecular bone predominates, such as the vertebral body.16 Notably, bone quality often showed considerable variation within the same patient or within the same hand. Bone mineral density and cortical thickness differed by as much as 24% and 21%, respectively, between right and left digits from the same patient and differed by as much as 24% and 30%, respectively, between index, middle, and ring fingers in the same hand. Ultimate force was positively correlated with both BMD and cortical thickness, indicating that the strength of the tendon–bone repair with anchors depended strongly on the bone quality of the distal phalanx. In the fingers in which BMD was less than 420 mg/cm3 or in which cortical thickness was less than 0.33 mm, greater than 90% of repairs failed by anchor pullout. In such fingers, severe osteopenia prevented strong anchor– bone fixation. The relationship between donor age and bone quality in our samples indicate that age 75 was the threshold for poor bone quality, an age above which anchor fixation may be contraindicated.
There are several limitations to our study. Our original study design (part 1) included only a single suture material (Supramid) and compared anchor versus pull-out repair methods. When we expanded the study (part 2) to include additional suture materials, we did not repeat the 2 original groups. Ideally, the 4 groups would have been defined a priori and equal numbers of specimens assigned at random to each. Nonetheless, we do not believe the order of how we did the different repairs influenced our conclusions. Within each part, we used a random assignment scheme, and each of the outcome measures we used is based on quantitative, objective analysis. A second limitation is that we examined only 1 suture material for the pull-out suture method. It is likely that use of a stiffer, stronger suture such as FiberWire with the pull-out technique would lead to increased stiffness and failure load compared with pullout with Supramid. Additional studies will be needed to evaluate the effect of suture materials on pull-out repair. A third limitation is that our method of linear tension testing of the isolated tendon– bone construct may not replicate the kinematics of the finger during physiologic loading as well as curvilinear testing methods.17 However, one advantage of our method is that we can visualize the failure of the repair site and thus monitor repair-site elongation and gap formation, which are important prefailure properties.
When assessing the clinical implications of our study, it may be useful to consider that the average age of the digits, 77 years, constituted a much older cohort than that seen typically for patients who require tendonto-bone fixation.18 Based on the average age at clinical presentation, we believe that most patients requiring FDP insertion-site repair will have adequate bone quality for anchor fixation.
Acknowledgments
Funding for this study was provided by the United States National Institutes of Health (AR33097). Anchors were provided as a gift from DePuy Mitek (USA), and Fiber Wires were also provided as a gift from Arthrex Inc. (USA).
REFERENCES
- 1.Bunnell S. Surgery of the hand. Lippincott; Philadelphia: 1948. [Google Scholar]
- 2.Guinard D, Montanier F, Thomas D, Corcella D, Moutet F. The Mantero flexor tendon repair in zone I. J Hand Surg. 1999;24B:148–151. doi: 10.1054/jhsb.1998.0173. [DOI] [PubMed] [Google Scholar]
- 3.Buch BD, Innis P, McClinton MA, Kotani Y. The Mitek mini G2 Suture Anchor: biomechanical analysis of use in the hand. J Hand Surg. 1995;20A:877–881. doi: 10.1016/S0363-5023(05)80448-4. [DOI] [PubMed] [Google Scholar]
- 4.Silva MJ, Hollstein SB, Fayazi AH, Adler P, Gelberman RH, Boyer MI. The effects of multiple-strand suture techniques on the tensile properties of repair of the flexor digitorum profundus tendon to bone. J Bone Joint Surg. 1998;80A:1507–1514. doi: 10.2106/00004623-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Brustein M, Pelligrini J, Chuoeka J, Heminger H, Mass D. Bone suture anchor versus the pullout button for repair of distal profundus tendon injuries: a comparison of strength in human cadaveric hands. J Hand Surg. 2001;26A:489–496. doi: 10.1053/jhsu.2001.24135. [DOI] [PubMed] [Google Scholar]
- 6.Tingart MJ, Apreleva M, Lehtinen J, Zurakowski D, Warner JJ. Anchor design and bone mineral density affect the pull-out strength of suture anchors in rotator cuff repair: which anchors are best to use in patients with low bone quality? Am J Sports Med. 2004;32:1466–1473. doi: 10.1177/0363546503262644. [DOI] [PubMed] [Google Scholar]
- 7.Ditsios K, Boyer MI, Kusano N, Gelberman RH, Silva MJ. Bone loss following tendon laceration, repair and passive mobilization. J Orthop Res. 2003;21:990–996. doi: 10.1016/S0736-0266(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 8.Boyer MI, Ditsios K, Gelberman RH, Leversedge F, Silva M. Repair of flexor digitorum profundus tendon avulsions from bone: an ex vivo biomechanical analysis. J Hand Surg. 2002;27A:594–598. doi: 10.1053/jhsu.2002.33708. [DOI] [PubMed] [Google Scholar]
- 9.Dovan TT, Gelberman RH, Kusano N, Calcaterra M, Silva MJ. Zone I flexor digitorum profundus repair: an ex vivo biomechanical analysis of tendon to bone repair in cadavera. J Hand Surg. 2005;30A:258–266. doi: 10.1016/j.jhsa.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Kusano N, Zaegel MA, Placzek JD, Gelberman RH, Silva MJ. Supplementary core sutures increase resistance to gapping for flexor digitorum profundus tendon to bone surface repair: an in vitro biomechanical analysis. J Hand Surg. 2005;30B:288–293. doi: 10.1016/j.jhsb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33:211–213. doi: 10.1097/00000637-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Schuind F, Garcia-Elias M, Cooney WP, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg. 1992;17A:291–298. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg. 2005;30A:836–841. doi: 10.1016/j.jhsa.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Croog A, Goldstein R, Nasser P, Lee SK. Comparative biomechanic performances of locked cruciate four-strand flexor tendon repairs in an ex vivo porcine model. J Hand Surg. 2007;32A:225–232. doi: 10.1016/j.jhsa.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Miller B, Dodds SD, deMars A, Zagoreas N, Waitayawinyu T, Trumble TE. Flexor tendon repairs: the impact of fiberwire on grasping and locking core sutures. J Hand Surg. 2007;32A:591–596. doi: 10.1016/j.jhsa.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Silva MJ, Wang C, Keaveny TM, Hayes WC. Direct and computed tomography thickness measurements of the human, lumbar vertebral shell and endplate. Bone. 1994;15:409–414. doi: 10.1016/8756-3282(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 17.Choueka J, Heminger H, Mass DP. Cyclical testing of zone II flexor tendon repairs. J Hand Surg. 2000;25A:1127–1134. doi: 10.1053/jhsu.2000.20155. [DOI] [PubMed] [Google Scholar]
- 18.Leddy JP. Avulsions of the flexor digitorum profundus. Hand Clin. 1985;1:77–83. [PubMed] [Google Scholar]