Abstract
IMPORTANCE
Psychotic disorders (including schizophrenia, schizoaffective disorder, and psychotic bipolar disorder) are devastating illnesses characterized by breakdown in the integration of information processing. Recent advances in neuroimaging allow for the estimation of brain networks on the basis of intrinsic functional connectivity, but the specific network abnormalities in psychotic disorders are poorly understood.
OBJECTIVE
To compare intrinsic functional connectivity across the cerebral cortex in patients with schizophrenia spectrum disorders or psychotic bipolar disorder and healthy controls.
DESIGN, SETTING, AND PARTICIPANTS
We studied 100 patients from an academic psychiatric hospital (28 patients with schizophrenia, 32 patients with schizoaffective disorder, and 40 patients with bipolar disorder with psychosis) and 100 healthy controls matched for age, sex, race, handedness, and scan quality from December 2009 to October 2011.
MAIN OUTCOMES AND MEASURES
Functional connectivity profiles across 122 regions that covered the entire cerebral cortex.
RESULTS
Relative to the healthy controls, individuals with a psychotic illness had disruption across several brain networks, with preferential reductions in functional connectivity within the frontoparietal control network (P < .05, corrected for family-wise error rate). This functionally defined network includes portions of the dorsolateral prefrontal cortex, posteromedial prefrontal cortex, lateral parietal cortex, and posterior temporal cortex. This effect was seen across diagnoses and persisted after matching patients and controls on the basis of scan quality.
CONCLUSIONS AND RELEVANCE
Our study results support the view that cortical information processing is disrupted in psychosis and provides new evidence that disruptions within the frontoparietal control network may be a shared feature across both schizophrenia and affective psychosis.
Delusions, hallucinations, and formal thought disorder, as seen in a range of psychotic disorders (including schizophrenia, schizoaffective disorder, and psychotic bipolar disorder), are widely assumed to result from breakdown in information processing across large-scale distributed brain networks. Multiple brain networks have been implicated in the functional deficits observed in schizophrenia and bipolar disorder. Recent attention has focused on abnormalities in the default network, which is implicated in processing internal stimuli and representations of the self. Default network abnormalities have been discovered in a range of neuropsychiatric conditions, raising the possibility that this dysfunction could represent a common substrate for mental illness.1,2 However, other systems are also affected in schizophrenia, with abnormalities in the dorsolateral prefrontal cortex perhaps the best characterized,3–8 supporting the notion that multiple brain networks are functioning abnormally in psychotic disorders.
Despite much progress, it remains unclear whether a single organizing principle underlies network abnormalities in psychotic disorders. For example, does brain network dysfunction occur piecemeal in schizophrenia and bipolar disorder because of a shared but broadly distributed mechanism, or is it better explained by damage to key control systems?9,10 The frontoparietal control network spans portions of the dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, lateral parietal cortex, and posterior temporal cortex and corresponding portions of the striatum11 and cerebellum.12 Situated between the default and dorsal attention networks,13,14 the frontoparietal control network is believed to play a crucial role in goal-directed planning14 and the application of complex, nested rules.15,16 One hypothesis is that this network serves as a bridge between the 2 distinct modes of information processing subserved by the default network (ie, memory and other self-oriented processing) and the dorsal attention network (ie, spatial and other externally oriented stimuli). As a result, disruption of the frontoparietal control network might result in widespread changes to cortical information processing reflected across multiple brain networks.2
Magnetic resonance imaging (MRI)–based techniques in humans suggest broad breakdowns in cortical functional organization, reduced local network connectivity, reduced modular structure, and increased global network robustness in schizophrenia.17–20 Although consistent with some degree of widespread network dysfunction, other studies21 highlight preferential breakdown in certain cortical territories. For instance, Fornito et al21 probed network function in schizophrenia in the context of a working memory task. They found evidence of task-by-disease interaction effects that overlap substantially with frontoparietal control network regions. Moreover, the schizophrenia imaging literature has classically characterized the hypofrontality of psychosis on the basis of positron emission tomographic abnormalities that are most pronounced in the lateral prefrontal cortex.7,22,23 We explored network dysfunction using a novel approach that examined functional connectivity profiles at rest across the entire cerebral cortex. First, we studied a large sample of patients with a psychotic disorder. Second, we examined shifts in the strength and nature of interactions between functionally defined brain regions rather than comparing brain activation patterns or relying on anatomically defined cortical territories. Third, our study sample was largely recruited from inpatient psychiatric units where participants displayed particularly acute disease (most of which remained acute at scan time) and took several steps to rule out potential head movement confounders that can complicate the interpretation of studies using samples of patients with acute disease. Our analyses revealed disruption across several brain networks in patients with a psychotic disorder relative to healthy controls, with evidence of highly significant disruption of the frontoparietal control network. Frontoparietal network disruption was evident transdiagnostically and was independent of data quality, supporting the hypothesis that disruption to key control structures may represent a common biological substrate central to the pathophysiology of psychosis.
Methods
Functional MRI (fMRI) data were collected from 100 patients (28 with schizophrenia, 32 with schizoaffective disorder, and 40 with bipolar disorder with psychosis) and 100 controls at rest with eyes open from December 2009 to October 2011. Analyses were designed to identify differences between patients and controls in the functional connectivity profiles across the cerebral cortex without imposing prior assumptions about the network-specific location of effects.
Participants
Participants’ demographic and clinical characteristics are summarized in eTable 1 in the Supplement. See eMaterials in the Supplement for details of participant recruitment and characterization.
Image Acquisition
All imaging data were collected on 3-T Tim Trio scanners (Siemens) with a 12-channel phased-array head coil, using VB17 as the console version. Functional data were acquired using a gradient-echo echoplanar imaging sequence sensitive to blood oxygenation level–dependent contrast. Participants were instructed to remain still, stay awake, and keep their eyes open. No fixation image was used, but patients were monitored via eye tracking video to ensure that eyes remained open during functional scans. The echoplanar imaging parameters were as follows: repetition time, 3000 milliseconds; echo time, 30 milliseconds; flip angle, 85°; 3 × 3 × 3-mm voxels; field of view, 216; and 47 axial sections collected with interleaved acquisition and no gap. Each functional run lasted 6.2 minutes (124 time points). A total of 1 to 2 runs were acquired per participant (mean of 1.39 for controls and 1.40 for patients). Whole-brain coverage was achieved with sections aligned to the anterior commissure–posterior commissure plane using an automated alignment procedure, ensuring consistency among participants.24 Structural data included a high-resolution, multiecho, T1-weighted, magnetization-prepared, gradient-echo image,25 which allows increased contrast through weighted averaging of the 4 derived images.
Image Preprocessing and Functional-Structural Data Alignment
See eMaterials in the Supplement for details.
Region Definition and Comparison Between Groups
Primary analyses began with a highly reliable cortical parcellation derived from the fMRI data of 1000 healthy controls.26 In this approach, the cortical mesh is parcelated into k sets of vertices on the basis of similar functional connectivity profiles across 1175 vertices spaced approximately 16 mm apart and uniformly distributed across each cerebral hemisphere. We then defined a set of 122 cortical regions composed of 61 roughly symmetric territories in the left and right hemispheres by selecting vertices on the sphere with respect to network boundaries, sulcal patterns, and confidence maps.26
We computed the Pearson correlation coefficient between each regional fMRI time course, averaged across all vertices within the region, and the mean fMRI time course for every other region (matrix plots, Figure 1, Figure 2A, and Figure 3C; eFigure 1 in the Supplement). We also computed the Pearson correlation coefficient between the mean fMRI time course of a region and the time courses of all other vertices across the cortical mantle (surface plots, Figure 3A and B). To compare regional correlation in the 2 groups, correlation values were z-transformed to increase normality of the correlation distribution and then compared using an analysis of variance after linear regression of age, sex, race, and handedness. All tests survived correction for multiple comparisons using a family-wise error rate (FWER; Bonferroni procedure) of P < .05 or false discovery rate (FDR) of q < 0.05.
Figure 1. Functional Connectivity Correlation Matrices in Patients and Controls.
Each 61 × 61 grid shows the Pearson correlation between resting blood oxygenation level–dependent activity in intrahemispheric regional pairs for controls (A) and patients (B). Regions are ordered based on their network groupings adapted from Yeo et al.26 Diagonal white lines represent network boundaries. DorsAttn indicates dorsal attention; L, left hemisphere; R, right hemisphere; Sal, salience; SomMot, somatomotor; and VentAttn, ventral attention.
Figure 2. Functional Connectivity Differences Between Patients and Controls.
A, The 61 × 61 grid shows the differences in resting blood oxygenation level–dependent correlation between controls and patients for each intrahemispheric regional pair. Differences were obtained by an analysis of variance of z-transformed Pearson correlation values after linear regression of the effects of age, sex, race, and handedness. Regions are ordered based on their network groupings adapted from Yeo et al.26 Diagonal white lines represent network boundaries. B, Manhattan plot showing associated network-wide P values of psychosis-related differences in functional connectivity. The y-axis shows the −log10 P values of 240 within-network regional pairs, and the x-axis shows their network positions. The horizontal red line represents the threshold of P = 1.37 × 10−5 for Bonferroni-corrected significance; the horizontal blue line represents the threshold of P = 7.8 × 10−3 that corresponds to the false discovery rate (q < 0.05). See Figure 1 legend for explanation of abbreviations.
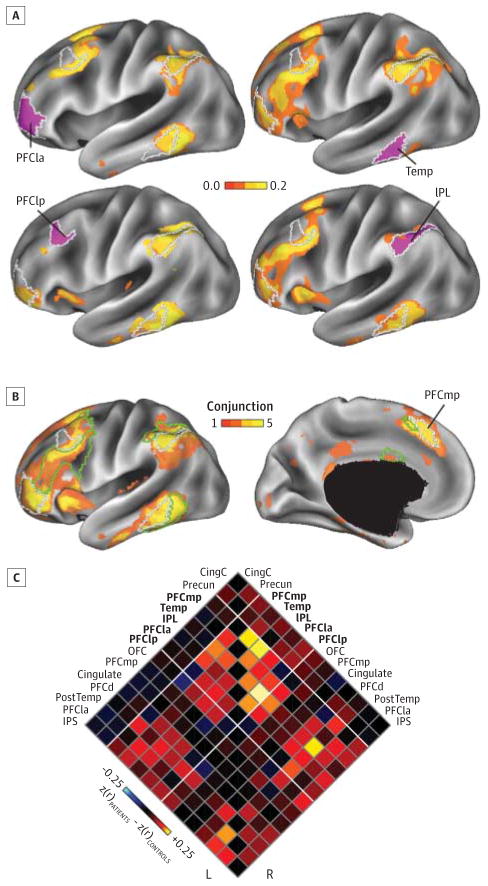
Figure 3. Network Disruptions in the Distributed Regions of the Frontoparietal Control Network.
A, Functional connectivity differences for the 4 lateral regions in the B component of the frontoparietal network, shown using a conventional seed-based approach. Maps are color-coded based on group differences in z-transformed Pearson correlation (controls-patients), computed between each mean regional time course and the time course at every vertex on the cortical mesh and thresholded for significant differences (P < .01 uncorrected). As above, differences were obtained after linear regression of the effects of age, sex, race, and handedness. B, Conjunction maps showing the degree of overlap in thresholded (P < .01) functional connectivity difference maps for the 5 left hemisphere regions (regions shown in A and PFCmp) within the control B network shown on lateral (left) and medial (right) inflated views of the cerebral cortex. Green and white lines indicate boundaries of control A and control B regions, respectively. C, Functional connectivity matrix for the 14 left and right hemisphere regions of the frontoparietal control network. Bold text indicates control B regions. Diagonal lines represent boundaries among the A, B, and C components of the frontoparietal control network. CingC indicates the C component of the cingulate gyrus; IPL, inferior parietal lobule; IPS, intraparietal sulcus; OFC, orbitofrontal cortex; PFCd, dorsal prefrontal cortex; PFCla, lateral anterior prefrontal cortex; PFClp, lateral posterior prefrontal cortex; PFCmp, medial posterior prefrontal cortex; PostTemp, posterior temporal; Precun, precuneus; and Temp, temporal.
To supplement the primary analyses, leveraging 122 regions defined using healthy control data, we applied a previously described26 clustering approach on the independent patient and control group blood oxygenation level–dependent data. This analysis grouped vertices into 7 nonoverlapping networks based on similarity of fMRI connectivity profiles (see eMaterials in the Supplement for details). In addition to comparing parcellations in full (n = 100) groups of patients and controls, we separated patients and controls into high- and low-quality data groups (n = 50) using a median signal-to-noise ratio split (see eTable 2 in the Supplement) and applied the clustering approach independently to each group.
Results
Functional Connectivity Across the Cerebral Cortex in Psychosis
Functional connectivity matrices derived from 100 patients and 100 controls are depicted in Figure 1, with regional interactions organized into intrinsic left and right hemispheric cortical networks. Controls (Figure 1A) and patients (Figure 1B) had broadly similar network connectivity patterns, with each network showing the expected pattern of high within-network and low between-network correlation.
To examine the effects of a psychotic disorder on functional connectivity, we compared z-transformed Pearson correlation values in the 2 groups for all 3660 (61 × 60) pairwise regional interactions (excluding unity interactions) using a regression model that partialed out the effects of age, sex, race, and handedness. Similar results were obtained when education and data quality (signal-to-noise ratio) were included in the model (data not shown). Residual differences between the 2 groups are displayed in Figure 2A for the full set of intrahemispheric interactions. The pattern of cortical functional connectivity differences revealed psychosis-related reductions in correlation between regions that spanned several functional networks, including the frontoparietal control, default, and ventral attention networks. Interhemispheric correlation differences showed a similar pattern (see eFigure 1 in the Supplement).
Across the set of 3660 possible intrahemispheric connections, 97 (2.7%) had a significant difference between the 2 groups (FWER-corrected P < .05, corresponding to an uncorrected P = 1.4 × 10−5). Of these, psychosis-related reductions in regional correlation (n = 34 of 97) occurred almost exclusively (n = 33 of 34) between regions located within the same network (eg, control B to control B) or immediate hierarchical cluster of networks (eg, control A to control B) (see eMaterials in the Supplement for details on the control and other networks). By contrast, all 63 psychosis-related increases in regional correlation occurred among between-network interactions that are negatively correlated at rest (ie, negative correlations between these regions became less negative in the psychotic sample). This pattern held when using a less stringent statistical criterion (FDR-corrected q < 0.05, corresponding to an uncorrected P = 7.8 × 10−3).
Psychosis-related differences in functional connectivity occurred across several networks but were particularly marked for connections that involved the frontoparietal control network. This effect was evident for within-network reductions in connectivity, as depicted in Figure 2B using a Manhattan plot, which shows the statistical significance of each regional interaction difference for all within-network findings (for Manhattan plot of all interactions, see eFigure 2 in the Supplement).
We next considered the differences for a higher-resolution parcellation of the cerebral cortex into 17 networks (Table, eFigure 3 in the Supplement). This analysis revealed that in the second component of the frontoparietal control network (referred to here as the control B network), 12 of 20 possible interactions had significantly reduced correlation in patients with psychoses (FWER-corrected P < .05; 17 of 20 using FDR correction). Of the other 17-network components, no other network had more than 2 within-network differences significant at the more stringent threshold, and only the ventral somatomotor network had a comparable proportion with 1 significant of a possible 2 interactions (see eFigure 3 in the Supplement for the number of significant differences across all network pairs). When a less conservative statistical threshold (FDR) was used, patients displayed reduced integrity in other networks, including ventral attention, salience, and default (Table). Permutation testing revealed that effect sizes for connections that involved the control B network were greater than those found in ventral attention (P = .001), salience (P < .001), or control A (P = .001), 3 network components that also had a number of significant differences at the less stringent threshold. Compared with control B, effect sizes that involved default network components (B and C) were not found to be significantly different (P > .10); however, the small number of possible connections that involved these components suggests that these tests likely were not adequately powered to detect such a difference.
Table.
Network Interactions Affected Significantly by Psychosisa
| Cortical Network | FWER P < .05 | FDR q < 0.05 | Total | Mean (SD) |
|---|---|---|---|---|
| Visual central | 0 | 0 | 2 | −0.007 (0.004) |
| Visual peripheral | 2 | 2 | 6 | 0.095 (0.105) |
| Somatomotor A | 1 | 2 | 2 | 0.180 (0.004) |
| Somatomotor B | 0 | 2 | 12 | 0.069 (0.039) |
| Dorsal attention A | 0 | 2 | 6 | 0.073 (0.059) |
| Dorsal attention B | 0 | 0 | 12 | 0.028 (0.042) |
| Ventral attention | 1 | 20 | 70 | 0.080 (0.060) |
| Salience | 1 | 9 | 20 | 0.083 (0.065) |
| Limbic | 0 | 0 | 2 | 0.008 (0.004) |
| Control A | 2 | 9 | 42 | 0.065 (0.066) |
| Control B | 12 | 17 | 20 | 0.171 (0.087) |
| Control C | 0 | 0 | 2 | 0.082 (0.013) |
| Default A | 0 | 2 | 30 | 0.064 (0.050) |
| Default B | 1 | 4 | 6 | 0.168 (0.055) |
| Default C | 0 | 1 | 6 | 0.088 (0.024) |
| Default D | 0 | 0 | 2 | −0.012 (0.051) |
Abbreviations: FDR, false discovery rate; FWER, family-wise error rate.
Values represent the number significant of total possible within-network interactions. Means (SDs) reflect the difference in correlation between controls and patients across all regional interactions for that network.
Frontoparietal Control Network Disruption in Psychosis
To further explore the nature and anatomical extent of functional connectivity changes in the frontoparietal control network, we conducted a conventional seed-based analysis for 10 control network regions to visualize correlation differences between patients and controls across the whole cortical surface (Figure 3). We examined 5 bilateral sets of regions that comprised the control B component of the frontoparietal control network26: medial posterior prefrontal cortex, lateral anterior prefrontal cortex, temporal cortex, lateral posterior pre-frontal cortex, and inferior parietal lobule. By examining difference maps derived from these 10 seed regions and their spatial overlap on the cortical surface, we confirmed that the differences in functional connectivity were consistent across multiple regions of the frontoparietal control network and largely confined to reductions in correlation between seed regions and other regions of the control A and control B networks. Results from seed-based analyses for the full set of 122 regions of interest are depicted in a supplemental animation (Video).
Cortical Network Parcellations
We next sought to address 2 potential confounders in our study design that could have accounted for our finding of preferential control network disruption. First, our main analysis relied on a functional parcellation derived exclusively from healthy participants26; therefore, it remained possible that subtle shifts in location or boundaries of selected regions might explain apparent reductions in correlation strength due to spatial misregistration between healthy and patient parcellations. Second, although we explicitly selected control participants on the basis of matched data quality (and found the 2 groups to have moved similar amounts [Table]), subtle differences in the distribution of head motion and/or data quality in 2 comparison groups might still lead to idiosyncratic, geographically distributed patterns of correlation difference.27–29
Therefore, we conducted a second analysis to compute the 7-network cortical network parcellation from patients and controls independently and also examined the solutions for 2 key subgroups of study participants with low- and high-quality data: (1) the 50 control participants with the lowest-quality functional scans and (2) the 50 patients with the highest-quality scans. This analysis was thus an explicit attempt to pit data quality against patient-control status to understand whether our findings persisted even when data quality was higher in the patient sample than in controls. Compared with patients with low signal-to-noise ratio, patients with high SNR had significantly lower positive scores on the Positive and Negative Syndrome Scale (15.8 vs 18.7, P = .04) and lower Youth Mania Rating Scale scores (12.6 vs 18.1, P = .008) but were otherwise similar (see eTable 2 in the Supplement).
eFigure 4 in the Supplement shows the parcellation derived from these 2 key subgroups and, for reference, the parcellation derived from 1000 healthy participants (eFigure 4A in the Supplement, adapted from Yeo et al26). Across both medial and lateral cortical surfaces, the parcellation derived from the low-quality data controls (eFigure 4B in the Supplement) closely matched the reference parcellation (eFigure 4A in the Supplement), whereas the parcellation derived from the high-quality data patients (eFigure 4C in the Supplement) showed a qualitatively different pattern: vertices previously assigned to the frontoparietal control network were assigned to other nearby network clusters. We did not detect a systematic pattern of reassignment of vertices assigned to the frontoparietal control network to a single other network. Because the parcellation procedure specified a priori a solution with 7 networks, the somatomotor network was divided into dorsal and ventral segments.
These results, together with the parcellation findings obtained on the full (n = 100) data sets (data not shown), provide confirmatory evidence of frontoparietal control network disruption that seems to be independent of the selection of control group and data quality. These findings support our interpretation that connectivity among nodes of the frontoparietal control network was altered in the psychotic group and not the result of regional misregistration between patient and control data sets due to subtle changes in region positions or boundaries. We explored this further by visualizing the correlation matrices obtained from patients and controls as spring-loaded graphs, which depict the correlation among nodes spatially (Figure 4). Although a full-graph theoretical analysis is beyond the scope of the present study, this illustration provides an intuitive sense for how network connectivity changed in the patient group: nodes from the control B network are less clustered. Thus, although differences in data quality and indirect influences on effective spatial smoothing always remain a concern in between-group fMRI studies,30 our main findings withstand tests of known confounds.
Figure 4. Spatial Network Model of 3 Cortical Association Networks in Psychosis.
Spring-loaded graphs showing selected nodes of the frontoparietal control network, dorsal attention network, and default network in controls (A) and patients (B). Node size is based on nodal degree; edge connection strength is represented by grayscale value and line thickness. Controls had a more segregated pattern clustering of frontoparietal and default networks (represented with nonoverlapping colored halos); by contrast, patients had less clustering within default and frontoparietal control networks and evidence of extension of frontoparietal nodes into the default cluster (represented with blended red-orange halos). FEF indicates frontal eye fields; InfParOcc, inferior parieto-occipital; IPLa, lateral inferior parietal lobule; IPLp, posterior inferior parietal lobule; PCC, posterior cingulate; pCUN, precuneus; PFCdA, dorsal anterior prefrontal cortex; PFCdB, B component of dorsal prefrontal cortex; PFCl, lateral prefrontal cortex; PFCm, medial prefrontal cortex; PFCmpA, A component of medial posterior prefrontal cortex; PFCmpB, B component of medial posterior prefrontal cortex; PostC, postcentral gyrus; PostTempOcc, posterior temporal occipital; PrCv, ventral precentral gyrus; SupPar, superior parietal lobule; Temp, temporal cortex; TempA, A component of temporal cortex; TempB, B component of temporal cortex. See Figure 3 legend for explanation of other abbreviations.
Relationship to Diagnosis, Active Symptoms, or Treatment
We next examined whether the pattern or extent of frontoparietal control network disruption was related to DSM-IV diagnosis or other clinical variables. Mean correlation between all regional pairs within control A, within control B, and between control A and control B were compared between controls (n = 100), patients with bipolar disorder (n = 40), and patients with schizophrenia or schizoaffective disorder (n = 60). As observed at the psychotic disorders group level, mean correlation among regions of the frontoparietal control B network was significantly greater (P < .01) in healthy participants (r = 0.494) than in patients with bipolar disorder with psychosis (r = 0.405) or patients with schizophrenia or schizoaffective disorder (r = 0.411; Figure 5). The pattern of frontoparietal network disruption displayed consistency across the patient groups (Figure 5; for full grid, see eFigure 5 in the Supplement). We did not discover any significant relationships in a series of demographic and clinical variables and our findings (see eMaterials in the Supplement).
Figure 5. Equivalent Disruption of Frontoparietal Control Network Connectivity in Bipolar Disorder and Schizophrenia.
Functional connectivity difference matrices for the 14 left and right hemisphere regions of the frontoparietal control network shown for schizophrenic patients relative to controls (A), bipolar patients relative to controls (B), and schizophrenic patients relative to bipolar patients (C). Differences significant at false discovery rate q < 0.05 are shown in each panel just to the lower right of the unthresholded matrix. D, Histograms show the mean correlation between components of the frontoparietal control network in controls and patients with bipolar disorder or schizophrenia. Error bars denote SE. See Figure 3 and Figure 4 captions for explanation of other abbreviations.
Discussion
In this study, we examined whether common functional brain network abnormalities were present in patients with schizophrenia and psychotic bipolar disorder. To capture and compare the integrity of regional and network-level interactions across the cerebral cortex, we examined spontaneous resting-state hemodynamic fluctuations in 122 distinct cortical regions comprising 17 networks, the stability of which had been previously established in the functional connectivity profiles from 1000 healthy adults.26 Our analyses identified a pattern of disrupted connectivity consistent with pronounced disruption of the frontoparietal control network and with less marked aberrant connectivity within and across other networks. We confirmed this disruption of the frontoparietal control network in a separate clustering analysis, in which we compared the cortical parcellation in subgroups of patients and controls that explicitly controlled for data quality. Together, these findings indicate that the frontoparietal control network is disrupted in multiple psychotic disorders.
Abnormal connectivity within and among the brain’s associative networks has been a central theme in efforts to understand the etiology of psychosis since Bleuler46 first coined the term schizophrenia in 1911 (Greek for a split mind). A substantial literature indicates disrupted functional connectivity in schizophrenia and bipolar disorder across multiple brain networks,1,19,31–37 which has been reviewed extensively.20,38 Our results suggest that psychosis is associated with a disruption in the control architecture needed to mediate between modes of information processing, possibly resulting in inappropriate activation of other networks, including the default network. This disruption might lead to blurring of the normally strongly defined boundary between internally and externally oriented processing.2,39 Depending on which internal and external processes are currently engaged, control network disruption might in turn result in a heterogeneous pattern of mal-adaptive thoughts and behaviors that varies during the illness and among individuals.1
Our findings are largely compatible with the existing schizophrenia neuroimaging literature, particularly the task-based literature about abnormalities in cognitive control and context processing.40–42 Although previous studies40,42,43 have focused on dysfunction in the dorsolateral prefrontal component of this network, our findings indicate that this network is affected across its frontal, parietal, temporal, and medial pre-frontal components. Notably, the distributed nature of dysfunction is consistent with other studies19 that have reported results from whole-brain analyses of task-based data. Although nodes within this network have previously been implicated in psychotic disorders, our work indicates that the disease-related abnormalities are preferential to the territory of an independently defined functional network.
The present analyses do not indicate a selective frontoparietal cortex disruption in specific psychotic disorders. Rather these data support the view that the frontoparietal control network may support the most diverse set of cognitive demands impaired in multiple disorders. It is possible that selective network disruptions may be evident in more homogeneous patient samples. Along these lines, although it was not the emphasis of this study, we observed a unique pattern of connectivity differences in our schizophrenic and bipolar disorder subgroup analysis, although these differences failed to reach statistical significance (see eFigure 5 in the Supplement). Note that another group has recently reported differences in functional connectivity between schizophrenia and bipolar disorder,38 albeit using different methods for defining and comparing network correlations and using a less acute bipolar patient sample (eg, current psychosis among bipolar patients in that study was 50% vs 78% in our study), which may help to explain the reported differences.
In our findings, there was evidence that a subcomponent of the frontoparietal control network, what we refer to as control B, was particularly affected. Although few studies have probed the specific functions of this network, a review15 of the task-based fMRI literature suggests that higher-order application recruits cortical territories in the frontoparietal network, with the highest-order tasks activating territory most consistent with the control B component of the network.45 The correspondence between these higher-order task activations and the locations of reduced resting connectivity in our psychotic sample suggests dysfunction in a network critical to the kind of nested cognition that is particularly challenging for thought-disordered patients and could give rise to complex delusions, hallucinations, and other misperceptions. In sum, our findings provide a novel and comprehensive characterization of dysfunctional territories in the cerebral cortex of psychotic individuals that matches well with the core deficits observed in these patients.
Although the statistical criterion we used for our primary analysis indicated the most differences outside the frontoparietal network were not statistically significant in our sample, there was evidence of dysfunction in other cortical networks (eg, ventral attention network and default network). The pattern we observed suggests that frontoparietal control disruption may be the most common and reliably observed across psychotic samples because we observed it in a large but heterogeneous sample of psychotic patients but does not rule out the possibility that it may be possible to subgroup patients with particular symptom clusters or diagnoses and show pronounced effects in other networks. In other words, our findings predict that psychosis will be associated with particular difficulty (and altered fMRI responses) on context-dependent tasks but also any tasks that require frontoparietal control network–dependent shifts between internal and external processing modes. This rationale may also explain why we failed to detect any significant relationship between our findings and specific clinical ratings; that is, in a large heterogeneous sample, biological differences shared across the group could manifest differently in different clinical subgroups (eg, due to other disruptions or compensations). An alternative possibility is that our findings indicate a shared vulnerability to thought disorder (ie, a trait marker for psychosis) rather than an indicator of current clinical state. Future work will be necessary to leverage the statistical power afforded by high-throughput imaging approaches to examine subgroup differences in network dysfunction.
To our knowledge, our study is one of the largest resting-state fMRI studies in a sample of individuals with a lifetime history of psychosis, most of whom were inpatients and/or patients with acute psychosis at the time of the scan. We also included data from patients that spanned DSM-IV diagnostic categories within the psychotic disorders. Our analysis included all resting-state networks rather than being restricted to a limited number of a priori regions and networks. We directly addressed the potential confounders of head motion and scan quality by matching control participants on the basis of signal-to-noise properties of the functional scan and confirmed our findings in an analysis in which data quality and patient status were pitted against each other (ie, high-quality patient data vs low-quality control data). We also used a recently developed functional parcellation strategy to compare regional correlation across the entire cortex and then to rule out subtle regional boundary shifts. We computed the functional parcellation separately based solely on patient data.
The study has several limitations. First, combining data from patients with schizophrenia and psychotic bipolar disorder could obscure differences in the biology of psychosis in the 2 disorders38 and therefore might be viewed as a limitation of our study design. Although the 2 disorders have some unique features, the present study was designed to reveal shared features of psychosis. Future studies adequately powered to detect connectivity differences within each diagnosis will address the question of overlapping and unique substrates for psychosis in different diagnostic entities. Second, there were several commonalities among the patient sample other than lifetime history of psychosis differentiating them from controls, including current and past use of centrally active medications, anxiety or distress, and mood disturbances. Thus, we cannot establish definitively whether frontoparietal network disruptions in this group were due to a common neural phenotype or these other factors. Similarly powered studies that examined unaffected relatives, unmedicated patients, or medicated controls would be helpful in ruling out these and other potential confounders. Third, we found a small but significant difference between patients and controls in educational history, with both patients and their parents having a lesser educational background than controls (by a mean of 1.74 years for patients and 0.8 years for their parents). Given the potential relationship between the frontoparietal control network and the types of higher cognitive functions that would accompany scholastic achievement, this difference was potentially significant. However, our results held even after adjusting for educational history, and no correlation was observed between educational variables and frontoparietal control network integrity. Differences in education also could potentially be mitigated by the fact that estimated IQ scores were similar in the 2 groups. Fourth, subcortical structures are known to play a central role in the pathophysiology of psychotic disorders and yet received minimal attention in this study, which focused on disruptions to neocortical networks. This choice was partly methodologic because our goal was to apply a parcellation with which we have high confidence to this patient population. Although our group and others have made advances in applying parcellation strategies to subcortical structures,11,44 we thought that applying them here would be premature and somewhat beyond the scope of our study. We are actively pursuing analyses that include subcortical structures, which will be critical to our understanding of the changes we have observed here, and expect disruptions observed in the neocortex to also be present in other connected subcortical structures.
In conclusion, we identified the frontoparietal control network as preferentially disrupted in psychosis. This finding persisted even after controlling for the effects of data quality and was present in subgroups of patients with schizophrenia and psychotic bipolar disorder. Given this network’s putative role in higher-order cognition, this disruption may underlie the shared vulnerability to thought disorder that characterizes both schizophrenia and affective psychosis.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by grants 1K23MH079982-01A1, 5R01MH094594 from the National Institute of Mental Health (Dr Öngür), the Clinical Investigator Training Fellowship from Harvard/MIT (Dr Öngür), and the Taplin Family Foundation. Dr Baker is supported by a Dupont-Warren Fellowship (HMS), the APA-Pfizer MD/PhD Fellowship, and the Maria Lorenz Pope Fellowship. Dr Holmes is supported by K01MH099232 from the National Institute of Mental Health.
Footnotes
Video at jamapsychiatry.com
Conflict of Interest Disclosures: Dr Buckner is a paid consultant for Pfizer, Inc and Johnson & Johnson. Dr Öngür serves on the advisory board for Lilly.
Additional Contributions: Marisa Hollinshead, BA, Julie McCarthy, BA, and Alissa Cooper, BA, assisted with participant recruitment, clinical assessment, and scanning. Subjects were provided monetary compensation for their participation.
Author Contributions: Dr Baker had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Baker, Buckner, Öngür.
Acquisition of data: Baker, Holmes, Masters, Buckner, Öngür.
Analysis and interpretation of data: Baker, Holmes, Yeo, Krienen, Buckner, Öngür.
Drafting of the manuscript: Baker, Holmes, Masters, Yeo, Öngür.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Baker, Holmes, Yeo, Krienen, Buckner.
Obtained funding: Baker, Buckner, Öngür.
Administrative, technical, and material support: Holmes, Masters, Yeo, Krienen, Buckner, Öngür.
Study supervision: Buckner, Öngür.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 3.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 4.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 1988;45(7):609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 8.Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of Physiology. Vol. 5. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 10.Weinberger DR. Schizophrenia and the frontal lobe. Trends Neurosci. 1988;11(8):367–370. doi: 10.1016/0166-2236(88)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 16.Wendelken C, O’Hare ED, Whitaker KJ, Ferrer E, Bunge SA. Increased functional selectivity over development in rostrolateral prefrontal cortex. J Neurosci. 2011;31(47):17260–17268. doi: 10.1523/JNEUROSCI.1193-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Alexander-Bloch AF, Gogtay N, Meunier D, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 22.Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: search for a pathophysiological mechanism. Int Rev Psychiatry. 2007;19(4):417–427. doi: 10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14(2):157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- 24.van der Kouwe AJ, Benner T, Fischl B, et al. Online automatic slice positioning for brain MR imaging. Neuroimage. 2005;27(1):222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 25.van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 31.McGuire PK, Frith CD. Disordered functional connectivity in schizophrenia. Psychol Med. 1996;26(4):663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Lindenberg A, Poline JB, Kohn PD, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 33.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51(12):1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- 34.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 35.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ongür D, Lundy M, Greenhouse I, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. doi: 10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meda SA, Gill A, Stevens MC, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71(10):881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frith C. The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1505–1512. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- 40.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 41.Barch DM, Carter CS, MacDonald AW, III, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112(1):132–143. [PubMed] [Google Scholar]
- 42.MacDonald AW, III, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 43.Shelton RC, Karson CN, Doran AR, Pickar D, Bigelow LB, Weinberger DR. Cerebral structural pathology in schizophrenia: evidence for a selective prefrontal cortical defect. Am J Psychiatry. 1988;145(2):154–163. doi: 10.1176/ajp.145.2.154. [DOI] [PubMed] [Google Scholar]
- 44.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi EY, Badre D. Resting-state functional connectivity evidence for asymmetric rostro-to-caudal prefrontostriatal connectivity [program No. 390.02]. Paper presented at: Neuroscience; October 15, 2012; New Orleans, LA. 2012. [Google Scholar]
- 46.Bleuler E. Dementia Praecox oder Gruppe der Schizophrenien. Leipzig, Germany: Deuticke; 1911. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.