Abstract
Epidemiologic studies have shown an increased rate of adverse perinatal outcomes, including small for gestational age (SGA) births, in fresh in vitro fertilization (IVF) cycles compared with frozen embryo transfer cycles. This increase is not seen in the donor oocyte population, suggesting that it is the peri-implantation environment created after superovulation that is responsible for these changes. During a fresh IVF cycle, multiple corpora lutea secrete high levels of hormones and other factors that can affect the endometrium and the implanting embryo. In this review, we discuss both animal and human data demonstrating that superovulation has significant effects on the endometrium and embryo. Additionally, potential mechanisms for the adverse effects of gonadotropin stimulation on implantation and placental development are proposed. We think that these data, along with the growing body of epidemiologic evidence, support the proposal that frozen embryo transfer should be considered preferentially, particularly in high responders, as a means to potentially decrease at least some of the adverse perinatal outcomes associated with IVF.
Keywords: In vitro fertilization, frozen embryo transfer, superovulation, implantation, placentation
As success rates following assisted reproductive technologies (ART) have improved, attention has increasingly turned to birth outcomes and the long-term health of children born following ART (1, 2). Although the great majority of children born following ART are healthy, epidemiologic studies suggest that ART is associated with an increased risk of adverse perinatal outcomes, including fetal growth restriction, low birth weight, preterm birth, and preeclampsia, even when controlling for multiple gestations (3–5). These outcomes may be associated not only with neonatal morbidity but also with long-term health outcomes, including an increased incidence of metabolic diseases later in life (6, 7). In addition, several rare genetic and epigenetic diseases have been associated with, as yet incompletely identified, aspects of this therapeutic technology (8, 9). Even though the overarching goal must be to minimize these risks for our patients, controversy exists over what aspects of ART are responsible for the observed outcomes (10). One intervention used ubiquitously during in vitro fertilization (IVF) is superovulation with gonadotropins. Superovulation is an integral part of the IVF process, because controlled ovarian hyperstimulation allows the retrieval of multiple oocytes for fertilization and embryo development. However, superovulation results in supraphysiologic levels of multiple hormones and other factors, including E2, P, and vascular endothelial growth factor (VEGF), both during oocyte development and after embryo transfer. This can have multiple effects, including potential changes to the oocyte, endometrium, and implanting embryo, and the potential contribution of each of these effects on adverse outcomes is not well understood (11).
The negative effects of superovulation may be expressed as a decreased implantation rate, and recent data indeed suggest possible decreased implantation rates in fresh compared with frozen embryo transfers (12–14). However, a large percentage of embryos do implant after fresh IVF transfer. So a question remains: What happens to the developing embryo that does successfully implant in an endometrium exposed to the abnormal hormonal milieu following superovulation? Recent epidemiologic evidence suggests that the peri-implantation environment created by superovulation may be contributing to at least some of the adverse perinatal outcomes following ART, and that these effects can be minimized by transferring embryos in a subsequent frozen embryo transfer cycle (4, 5, 15–23). A large retrospective study by Kalra et al. using the Society for Assisted Reproductive Technologies (SART) database demonstrated a significant increase in low-birthweight (LBW) singleton infants (<2,500 g) born after fresh embryo transfers compared with infants born after frozen embryo transfer cycles (odds ratio [OR] 1.46, 95% confidence interval [CI] 1.34–1.58) (5). This difference was even stronger when analyzing pregnancies within a single individual who conceived children after both fresh and frozen embryo transfers (OR for LBW 2.52, 95% CI 1.59–4.00). Importantly, no differences were observed in the rates of LBW between fresh and frozen embryo transfers in donor egg cycles, eliminating the possibility that the freeze-thaw process was responsible for the observed differences. Other studies have confirmed these results, and a growing body of literature supports the observation that fresh embryo transfer leads to increased rates of LBW, preterm delivery, and other adverse pregnancy outcomes compared with frozen embryo transfers (2, 18, 22, 23). In addition, a recent meta-analysis showed that the incidence of LBW was the same following frozen embryo transfer or natural conception (2).
The cause-effect relationship between the peri-implantation environment and perinatal outcomes is supported by several studies demonstrating that the adverse effects of the peri-implantation environment are most significant in those patients with vigorous responses to ovarian stimulation (24–26). A recent pilot study by Imudia et al. showed that when patients at high risk of ovarian hyperstimulation syndrome (OHSS) chose elective cryopreservation of all embryos, their rates of preeclampsia and SGA, defined as <10% for gestational age, were lower than patients who chose to proceed with a fresh embryo transfer (26). That group has also found higher rates of preeclampsia and SGA in patients with E2 levels greater than the 90th percentile for their institution, suggesting that vigorous response to superovulation may be associated with a greater risk of adverse pregnancy outcomes (OR for SGA 9.40, 95% CI 3.22–27.46; OR for preeclampsia 4.79, 95% CI 1.55–14.84) (27).
Taken together, these studies suggest that the abnormal hormonal milieu following superovulation contributes, directly or indirectly, to the adverse outcomes seen in pregnancies conceived with the use ART. Although there is minimal direct evidence linking the superovulation-related hormonal milieu to adverse perinatal outcomes, in this review we will discuss both human and animal data showing that ovarian hyperstimulation with gonadotropins has effects on the endometrium and early embryo that may affect early implantation and placentation. We think that these data, along with compelling recent epidemiologic observations, support the preferential transfer of cryopreserved embryos in a more physiologic hormonal milieu over the transfer of fresh embryos immediately following ovarian hyperstimulation.
EFFECTS ON THE ENDOMETRIUM
Endometrial Receptivity
Evidence from human and animal studies suggests that superovulation leads to histologic changes in the endometrium at the time of implantation. In animal models, superovulation has been shown to affect the depth of the surface epithelium, the number and length of microvilli, and the mitotic activity in the surface epithelium and stromal cells (28, 29). Both human and animal studies have found that superovulation lowers the expression of specific integrins associated with the window of implantation (30, 31). Evidence also suggests that superovulation may affect the timing of the “window of receptivity,” the time period during which the endometrium is receptive to embryo implantation. In humans, implantation normally occurs 8–10 days after ovulation (32). Histologically, this is represented by glandular changes in the endometrium, which exhibits subnuclear vacuoles, as well as the appearance of pinopodes on the luminal surface of the epithelium (33). In superovulated cycles, these cellular changes occur earlier than in nonsuperovulated cycles. Studies of endometrial biopsies taken on the day of oocyte retrieval in IVF cycles show endometrial advancement in a majority of samples, with a more significant increase in this advancement in younger patients and those who had a larger number of oocytes retrieved (34, 35). The histologic advancement seen with superovulation has also been confirmed with an earlier appearance of endometrial nucleolar channel systems, a marker of endometrial maturation, after superovulation (36). This shift in the window of endometrial receptivity can affect implantation; endometrial advancement of >3 days has been associated with failed implantation (35). The shift in the window of implantation may also affect the development of an embryo once it successfully implants; mouse studies suggest that embryos that implant beyond the normal window of receptivity are more likely to show defects in placental formation and fetal growth (37).
Endometrial receptivity may be most affected in those patients with an exaggerated response to ovarian stimulation. This may be due to the fact that these patients have the greatest rise in both estrogen and progesterone during and after superovulation. Clinical studies indicate that higher E2 levels are correlated with earlier rises in P, even before administration of the hCG ovulation trigger (38–41). Elevated P levels (particularly >1.5 ng/mL) have been associated with histologic endometrial advancement and decreased pregnancy rates after fresh IVF transfers (42). In a retrospective study of 4,032 fresh IVF/intracytoplasmic sperm injection cycles performed by Bosch et al., patients who had a P level >1.5 ng/mL on the day of hCG ovulation trigger had an ongoing pregnancy rate of 19% compared with 31% in patients with a P<1.5 ng/mL (P<.001; OR 0.53, 95% CI 0.38–0.72) (38). Pregnancy rates were not decreased when these embryos were transferred in a subsequent frozen cycle, demonstrating that the detrimental effect of the elevated P is on the endometrium, not the embryo (43, 44).
The effects of superovulation on endometrial receptivity may therefore affect both the rate and the quality of implantation, including effects on early placentation. Within the context of superovulation, further research is needed to elucidate the impact of impaired endometrial receptivity on placentation and fetal growth.
Endometrial Gene Expression
Superovulation has been shown to change gene expression profiles in the endometrium in studies of both animal models and humans. In humans, multiple studies have shown differences in endometrial gene expression between superovulated and natural cycles (45–50) (Table 1). A study of endometrial biopsies from women after a vigorous response to superovulation compared with biopsies from control subjects showed significant differences in gene expression between the two groups. The genes identified are involved in numerous pathways thought to be involved in implantation, including the Wnt-signaling pathway, which is known to be important in estrogen-mediated uterine growth and implantation in the mouse, and STC1, which has been shown to be important in angiogenesis in the mouse (49). A recent unpublished study by our laboratory examined gene expression in endometrial biopsies obtained from oocyte donors after stimulation or in natural cycles. We found a difference in gene expression in >150 genes, including genes regulating angiogenesis and early implantation. Another study comparing endometrial biopsies in stimulated and natural cycles found changes in endometrial gene expression consistent with a 2–4 day acceleration in maturation in superovulated compared with natural cycles, which is consistent with a shift in the window of receptivity due to superovulation (48). These changes may be directly related to the altered estrogen and progesterone levels following superovulation. A study of endometrial biopsies after ovarian stimulation and oocyte retrieval showed differences in endometrial gene expression between patients with elevated P on the day of hCG trigger compared with patients with normal P levels (51). These studies suggest that superovulation may be detrimental to implantation by altering genes crucial for the endometrium-embryo interaction.
TABLE 1.
Gene expression profiles of simulated and nonstimulated human endometrium during the window of embryo implantation.
| Study | No. of samples | Fold change considered to be significant | Number of genes | |
|---|---|---|---|---|
| Up | Down | |||
| Mirkin et al. (45) | 13 | ≥1.2 | 5–6a | 1–6a |
| Horcajadas et al. (46) | 19 | ≥3 | 281 | 277 |
| Simon et al. (47) | 28 | ≥2 | 22–88a | 24–100a |
| Horcajadas et al. (48) | 49 | – | 69 | 73 |
| Liu et al. (49) | 13 | ≥2 | 5–244a | 2–159a |
| Haouzi et al. (50) | 84 | ≥2 | 321–657a | 0–4a |
Ranges represent variation seen between different stimulation protocols.
There is some evidence in both mice and humans to support differential endometrial gene expression based on the stimulation protocol used for superovulation, and this may influence placentation and growth (52, 53). Although changes in stimulation protocols may favorably alter endometrial gene expression, there is insufficient evidence currently to recommend an optimal regimen to maximize favorable endometrial gene expression profiles. In the absence of definitive research, frozen embryo transfer cycles minimize any potential differences in protocols by transferring all embryos in a physiologic nonsuperovulated environment.
Immunologic Changes to the Endometrium
Recent studies suggest that the immune environment of the endometrium plays an important role in implantation. Natural killer (NK) cells, in particular, have been associated with endometrial receptivity (54). NK cells are recruited to the endometrium during the menstrual cycle, with increasing concentrations seen at the mid-secretory phase and early pregnancy, at which time they represent 70% of uterine leukocytes (55). As implantation occurs, NK cells secrete interleukin-15, which transforms NK cells into decidual NK cells. These decidual NK cells secrete multiple factors that may be important for implantation, including angiogenic factors such as VEGF and cytokines and growth factors such as leukemia inhibiting factor (LIF) (54). Studies of patients with recurrent pregnancy loss or recurrent implantation failure have shown changes in NK cell concentration and subtypes compared to fertile patients (40, 56). Superovulation has been shown to alter the immune environment of the endometrium. Superovulated mice have been found to have lower concentrations of NK cells in the endometrium compared to nonsuperovulated controls (57). Human data also confirms this finding: in a study of endometrial biopsies obtained from oocyte donors during natural and stimulated cycles, endometrial NK cells were significantly reduced following superovulation compared to the natural cycles (58). Changes in NK cell number and subtype may impair or adversely affect implantation through their roles as regulators of implantation (59).
EFFECT OF SUPEROVULATION ON THE ENDOMETRIAL-EMBRYO INTERACTION
Effect on Embryonic Development and Fetal Growth
Multiple aspects of the IVF procedure have been shown to affect embryo development and growth. Mouse studies have shown that superovulation, IVF, and embryo culture can all lead to altered fetal and placental development (10, 60, 61). Epidemiologic evidence has isolated the abnormal hormonal environment following superovulation as an independent factor adversely affecting placentation and fetal growth (27, 62). The mechanisms behind these changes, however, are poorly understood. To further elucidate the effect of the superovulated environment on the developing embryo, our group recently performed a series of mouse experiments to isolate the effect of the peri-implantation environment on fetal and placental growth (63). Naturally conceived blastocysts were flushed and nonsurgically transferred to pseudopregnant females after either natural mating (control) or gonadotropin administration and mating (experimental). Pregnant mice were then sacrificed near term (embryonic day 19) and the resultant placentas and fetuses isolated. The pups isolated from the superovulated females weighed ~25% less than those from the control mice. The placentas from the superovulated female mice were also significantly smaller than their control counterparts. Histologically, the placentas differed significantly between the two groups, with differences seen in the labyrinth zone, the area responsible for nutrient transfer in the mouse placenta (Fig. 1). The differences seen in placental histology suggest that the environment may be affecting early trophoblast differentiation. In addition, placental gene expression was noted to be different between the two groups. Grb10, an imprinted gene known to be a regulator of fetal growth, was expressed in significantly higher amounts in the superovulated group compared with control (63).
FIGURE 1.
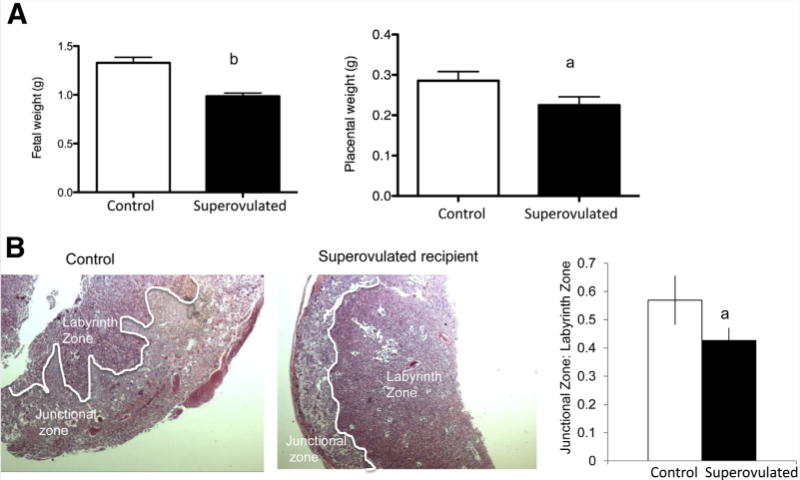
Effect of the peri-implantation environment on mouse placentation and fetal growth. (A) Fetal and placental weights of embryos at embryonic day 19 resulting from the transfer of naturally conceived blastocysts to control and superovulated recipient mice. Fetal and placental weights were significantly smaller in the superovulated group. Data are expressed as mean ± SEM. (B) Placental histology differed significantly in the offspring of control compared with superovulated recipients. Placentas from superovulated recipients showed attenuated branching with limited invasion of the junctional zone by the labyrinth zone with a decrease in the junctional:labyrinth zone ratio. aP<.05 vs. natural mating. bP<.001 vs. natural mating. Adapted from Mainigi et al. (63).
Our study suggests that the superovulated environment affects fetal growth through an effect on the implanting embryo that results in impaired trophoblast differentiation and, therefore, placental development. It is one of the first studies to isolate the impact of the peri-implantation environment following superovulation from the numerous other effects of superovulation on the oocyte or preimplantation embryo. We are currently using this model to further investigate the mechanisms responsible for the growth restriction seen following alteration in the peri-implantation hormonal milieu.
Epigenetic Changes to the Embryo
Changes in growth may be due to epigenetic effects of superovulation on the embryo. The time of implantation is a very important period of environmental influence; changes in the environment may induce epigenetic changes in the embryo that are maintained throughout the life of the resulting offspring. Epigenetic regulation of gene expression is accomplished through several mechanisms, including selective methylation of imprinted genes (64). The process of methylation begins during gamete development, as oocytes and sperm each obtain sex-specific methylation patterns. The precise timing of methylation is different in sperm and oocytes and is gene specific (9, 64). After fertilization, the preimplantation embryo undergoes genome-wide demethylation; however, sex-specific methylation patterns must be maintained for a select group of imprinted genes (65). During and after implantation, the embryo must maintain methylation of imprinted genes as well as acquire new methylation patterns for the remainder of the genome (66). Studies of mouse oocytes have shown that superovulation has an effect not only on the methylation of the developing oocyte, but also on the developing embryo, with loss of methylation in select imprinted select genes seen after superovulation (67). Specifically, methylation of certain paternally imprinted genes can be affected by superovulation, suggesting that ovarian stimulation affects the post-fertilization embryo (67). Loss of methylation owing to a nonphysiologic uterine environment may have effects on placentation and fetal growth, as defects in methylation have been correlated to impaired fetal growth (68, 69). The peri-implantation environment following superovulation may, therefore, lead to epigenetic alterations that affect fetal growth and long-term health of the offspring.
POTENTIAL MEDIATORS OF THESE EFFECTS
Understanding the mechanism by which superovulation results in these changes on the endometrium and resulting embryo implantation is critical both for our understanding of early implantation and for determining how best to modify the adverse effects. Ovarian stimulation results in multiple corpora lutea which secrete several factors that may be playing a role in the changes seen. Here we present several potential mediators that may play a role in the effect of superovulation on both the endometrium and the implanting embryo.
Estradiol
Ovarian hyperstimulation results in the production of multiple follicles, each secreting E2 into the circulation. E2 levels are, therefore, significantly higher following superovulation, owing to the presence of multiple follicles compared with the single dominant follicle produced during a natural cycle. Average E2 levels in a natural cycle are 200–300 pg/mL, whereas in IVF it is not uncommon to have E2 levels as high as 1,500–5,000 pg/mL (25). There is a growing body of literature to support the clinical observation that elevated peak E2 levels are associated with decreased success after IVF as well as increased adverse outcomes, including SGA and preeclampsia (24, 25, 27, 70). Preliminary data suggest that cryopreservation with subsequent transfer in an unstimulated cycle decreases these risks (26).
In mouse studies, administration of high doses of E2 in the form of injection induces a refractory state in the endometrium. In contrast, low doses of E2 support a receptive endometrium (71). However, the endometrium of the mouse is different from humans in its response to estrogen and progesterone, so it is difficult to extrapolate directly to clinical IVF (72). Nevertheless, high doses of E2 do appear to affect human endometrial tissue as well. In one in vitro study, mouse embryos were placed in culture with human endometrial tissue obtained from fertile women. Exposure of the embryos to high levels of E2 resulted in impaired embryo development and decreased adhesion (73).
Estradiol has also been shown to impact the proliferation of human embryonic stem cells in vitro. In one study, human embryonic stem cells were cultured with varying levels of E2. Physiologic levels of E2 induced proliferation of the stem cells, whereas supraphysiologic levels impaired cell proliferation, possibly due to down-regulation of the gene HDAC1 (histone deacetylase), which is known to be important in embryo development, histone acetylation, and gene expression (74). E2 may also have an effect on trophoblast differentiation and invasion, which are key steps in establishing a well functioning placenta. In multiple studies of nonhuman primates, Albrecht et al. have shown that exogenous estrogen given during early pregnancy impairs extravillous trophoblast invasion of the uterine spiral arteries (75, 76). That group has also found that premature elevation of estrogen in early pregnancy reduces uterine vessel remodeling and leads to abnormal uteroplacental blood flow dynamics (77).
Progesterone
Progesterone is known to be crucial for human endometrial receptivity, inducing changes in endometrial histology and gene expression that are critical for embryo implantation (37). Subtle elevations of P during superovulation, therefore, may affect embryo implantation owing to changes in endometrial receptivity and gene expression, including shifting the time period that the endometrium is receptive to the implanting embryo (42, 51). Clinical studies have confirmed that elevated P before hCG administration is associated with decreased pregnancy rates in IVF, suggesting that an elevated P level does, in fact, shift the window of receptivity and reduce normal embryo implantation (38, 42). Supplementing P during the luteal phase alters endometrial gene expression, and luteal phase supplementation may be able to overcome some of the changes in endometrial receptivity induced by other aspects of superovulation (53, 78, 79). Although supplemental P is given to patients during both fresh and frozen embryo transfer cycles, the timing of P exposure differs in a fresh cycle, because the premature elevations in P often seen during stimulation in a fresh cycle do not occur in a frozen cycle. Progesterone may also be directly involved in the vascular proliferation necessary for implantation, as reported in a study in mice, which showed that P stimulated endothelial cell proliferation (80). Additionally, a recent study suggests that P may be further involved in embryo implantation by regulating VEGF, an angiogenic factor important in human implantation (81).
Vascular Endothelial Growth Factor
VEGF appears to have an essential role in the process of normal implantation. VEGF is known to be secreted by the corpus luteum, the preimplantation embryo, and the endometrium (82). VEGF stimulates angiogenesis and is thought to be critical for trophoblast invasion and spiral artery remodeling. It is tightly regulated in the uterine endometrium, and disturbances in VEGF may be related to both impaired and excessive trophoblast invasion, which can eventually lead to placental insufficiency syndromes, such as preeclampsia (83). The soluble form of the VEGF receptor 1, s-FLT1, has been specifically associated with preeclampsia: Elevated serum levels of s-FLT1 can predict preeclampsia even before the onset of clinical signs or symptoms (83).
Superovulation is known to have an effect on VEGF levels at the level of both the corpus luteum and the endometrium. After luteinization, granulosa-lutein cells produce VEGF, and this production increases in the presence of hCG (84). Serum VEGF levels are significantly elevated after superovulation compared with natural mating in both humans and mice (63, 85). Superovulation also results in increased endometrial expression of VEGF; a study of biopsies from fertile oocyte donors 5–8 days after ovulation in natural and stimulated cycles showed increased levels of VEGF in the endometrium of stimulated cycles compared with natural cycles (58).
Although no direct link has yet been made between altered VEGF levels and adverse pregnancy outcomes, data from studies of ovarian hyperstimulation syndrome (OHSS) suggest an association of VEGF with defects in placentation and fetal growth. Pregnancies complicated by OHSS have been shown to be associated with higher rates of adverse perinatal outcomes, including preterm delivery, LBW, and preeclampsia (3, 86). One of these studies found a threefold increase in LBW associated with the development of OHSS (3). Although the precise mechanism behind these adverse outcomes in OHSS is not known, human and other animal studies have confirmed the role of VEGF in the pathogenesis of OHSS (87, 88). In a rat model of OHSS, blocking activation of VEGF-R2, one of the main VEGF receptors, resulted in the resolution of the pathology, including vascular permeability (87). A study of IVF patients with OHSS compared with those who did not experience OHSS found higher levels of free VEGF in the serum of women with OHSS (88).
Additional support for a causal relationship between VEGF and adverse perinatal outcomes comes from nonhuman animal studies that establish the role of VEGF in both placental vasculogenesis and uterine angiogenesis. A study of implantation sites in the mouse showed that VEGF was a key regulator of decidual angiogenesis, specifically finding that VEGF affected both the amount and the spacial distribution of angiogenesis in early pregnancy (81). In the nonhuman primate model, decreasing VEGF levels reverses estrogen-induced changes in endothelial cell proliferation, suggesting that VEGF is the primary mediator of early angiogenesis (76).
It is likely that multiple factors play a role in mediating the effects of superovulation on implantation and embryo and placental development. These factors may regulate each other, as studies suggest that estrogen and progesterone both play a role in regulating VEGF (76, 81). Further research will help elucidate the mechanisms by which superovulation impairs placentation and fetal growth, including the roles of both hormonal and angiogenic factors.
CONCLUSION
ART has progressed to the point that we can now offer high success rates to many of our patients. Our goals must now shift beyond early pregnancy to offer patients the safest pregnancies and the best possible outcomes. Minimizing the number of multiple pregnancies should be foremost among our goals in decreasing adverse perinatal outcomes, and single-embryo transfer should be considered whenever possible. In addition, it is critical that we identify modifiable elements in our protocols to minimize the risks to our patients and their offspring. A growing body of clinical evidence has recently emerged that demonstrates that the peri-implantation environment following superovulation increases the risk of abnormal placentation, leading to increased rates of LBW and preeclampsia (22, 23, 26, 62, 70). In this review, we have presented human and animal data that demonstrate the potential changes in the implantation process that may occur following superovulation, and we have introduced mechanisms that potentially mediate these changes. We believe these data demonstrate that the peri-implantation environment following gonadotropin exposure affects implantation and fetal growth, and that these effects can be prevented by avoiding the transfer of embryos into the nonphysiologic hormonal environment induced by the process of superovulation.
However, there are clearly reasons for hesitation before outright recommendation, at this juncture, of the uniform cryopreservation of all fresh autologous embryos with subsequent transfer during programmed “physiologic” cycles. Vitrification, although yielding excellent thaw rates and clinical results, is a new technology whose ramifications are not yet fully understood. There is some evidence from mice to suggest that vitrification itself may affect methylation of the early embryo (89, 90). Additionally, retrospective studies showing an increased rate of large for gestational age babies after frozen embryo transfer cycles require further investigation (22, 91). Comparisons with donor oocyte cycles may help elucidate some of the effects of the cryopreservation process, but the donor model has limitations as well, including differences in donor compared with infertile populations and differences in the immunologic environment that may play a role in the process (92). Finally, the optimal regimen for preparing the endometrium in a frozen embryo transfer cycle needs to be further elucidated, particularly if the hormonal environment is indeed playing a detrimental role.
As the mechanisms for the effects of superovulation on implantation and placentation become further elucidated, ART practice can be modified to optimize the peri-implantation environment. Whether modifications, such as low-dose superovulation or the use of VEGF-modifying agents, such as cabergoline, after ovulation trigger, have an effect on placentation and perinatal outcome will not be known until further research, both clinical and translational, is performed. Until then, preferentially cryopreserving embryos and transferring them into a more physiologic environment can reduce at least some of the risks associated with ART. Although we recognize that frozen embryo transfers may not be feasible or necessary for all patients, this approach appears to be an efficient way to decrease morbidity for patients at high risk for adverse outcomes, particularly those with high E2 levels, early elevated P levels, and high risk for OHSS.
Footnotes
R.W. has nothing to disclose. M.M. has nothing to disclose.
Discuss: You can discuss this article with its authors and other ASRM members at http://fertstertforum.com/weinermanr-frozen-fresh-embryos-translational-rationale/
References
- 1.Kalra SK, Barnhart KT. In vitro fertilization and adverse childhood outcomes: what we know, where we are going, and how we will get there. A glimpse into what lies behind and beckons ahead. Fertil Steril. 2011;95:1887–9. doi: 10.1016/j.fertnstert.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soder-strom-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19:87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 3.Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, et al. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril. 2006;86:1634–41. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95:959–63. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 5.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol. 2011;118:863–71. doi: 10.1097/AOG.0b013e31822be65f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovi P, Andersson S, Eriksson JG, Jarvenpaa AL, Strang-Karlsson S, Makitie O, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–63. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 7.Kajantie E, Hovi P. Is very preterm birth a risk factor for adult cardiometabolic disease? Semin Fetal Neonat Med. 2014;19:112–7. doi: 10.1016/j.siny.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Eroglu A, Layman LC. Role of ART in imprinting disorders. Semin Reprod Med. 2012;30:92–104. doi: 10.1055/s-0032-1307417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denomme MM, Mann MR. Genomic imprints as a model for the analysis of epigenetic stability during assisted reproductive technologies. Reproduction. 2012;144:393–409. doi: 10.1530/REP-12-0237. [DOI] [PubMed] [Google Scholar]
- 10.Feuer SK, Camarano L, Rinaudo PF. ART and health: clinical outcomes and insights on molecular mechanisms from rodent studies. Mol Hum Reprod. 2013;19:189–204. doi: 10.1093/molehr/gas066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99:389–92. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–62. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95:548–53. doi: 10.1016/j.fertnstert.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, et al. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu SY, Teng B, Fu J, Li X, Zheng Y, Sun XX. Obstetric and neonatal outcomes after transfer of vitrified early cleavage embryos. Hum Reprod. 2013;28:2093–100. doi: 10.1093/humrep/det104. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368–77.e1–9. doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari AM, Hyden-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod. 2010;25:914–23. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 20.Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995–2006. Fertil Steril. 2010;94:1320–7. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 21.Wikland M, Hardarson T, Hillensjo T, Westin C, Westlander G, Wood M, et al. Obstetric outcomes after transfer of vitrified blastocysts. Hum Reprod. 2010;25:1699–707. doi: 10.1093/humrep/deq117. [DOI] [PubMed] [Google Scholar]
- 22.Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CONARTAS group. Hum Reprod. 2013;28:2545–53. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128–33. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online. 2010;21:331–7. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93:442–6. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 26.Imudia AN, Awonuga AO, Kaimal AJ, Wright DL, Styer AK, Toth TL. Elective cryopreservation of all embryos with subsequent cryothaw embryo transfer in patients at risk for ovarian hyperstimulation syndrome reduces the risk of adverse obstetric outcomes: a preliminary study. Fertil Steril. 2013;99:168–73. doi: 10.1016/j.fertnstert.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 27.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97:1374–9. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Dursun A, Sendag F, Terek MC, Yilmaz H, Oztekin K, Baka M, et al. Morphometric changes in the endometrium and serum leptin levels during the implantation period of the embryo in the rat in response to exogenous ovarian stimulation. Fertil Steril. 2004;82(Suppl 3):1121–6. doi: 10.1016/j.fertnstert.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Kramer B, Stein BA, van der Walt LA. Exogenous gonadotropins—serum oestrogen and progesterone and the effect on endometrial morphology in the rat. J Anat. 1990;173:177–86. [PMC free article] [PubMed] [Google Scholar]
- 30.Sendag F, Akdogan A, Ozbilgin K, Giray G, Oztekin K. Effect of ovarian stimulation with human menopausal gonadotropin and recombinant follicle stimulating hormone on the expression of integrins alpha3, beta1 in the rat endometrium during the implantation period. Eur J Obstet Gynecol Reprod Biol. 2010;150:57–60. doi: 10.1016/j.ejogrb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Meyer WR, Novotny DB, Fritz MA, Beyler SA, Wolf LJ, Lessey BA. Effect of exogenous gonadotropins on endometrial maturation in oocyte donors. Fertil Steril. 1999;71:109–14. doi: 10.1016/s0015-0282(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 33.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 34.Lass A, Peat D, Avery S, Brinsden P. Histological evaluation of endometrium on the day of oocyte retrieval after gonadotrophin-releasing hormone agonist-follicle stimulating hormone ovulation induction for in-vitro fertilization. Hum Reprod. 1998;13:3203–5. doi: 10.1093/humrep/13.11.3203. [DOI] [PubMed] [Google Scholar]
- 35.Ubaldi F, Bourgain C, Tournaye H, Smitz J, van Steirteghem A, Devroey P. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril. 1997;67:521–6. doi: 10.1016/s0015-0282(97)80080-5. [DOI] [PubMed] [Google Scholar]
- 36.Zapantis G, Szmyga MJ, Rybak EA, Meier UT. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod. 2013;28:3292–300. doi: 10.1093/humrep/det358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 39.Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharmaceut Biotechnol. 2012;13:464–70. doi: 10.2174/138920112799361927. [DOI] [PubMed] [Google Scholar]
- 40.Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online. 2013;27:530–8. doi: 10.1016/j.rbmo.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, van Landuyt L, et al. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24:2902–9. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- 42.Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, van Vaerenbergh I, Devroey P, et al. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online. 2012;24:381–8. doi: 10.1016/j.rbmo.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril. 2010;93:636–41. doi: 10.1016/j.fertnstert.2009.01.134. [DOI] [PubMed] [Google Scholar]
- 44.Polotsky AJ, Daif JL, Jindal S, Lieman HJ, Santoro N, Pal L. Serum progesterone on the day of human chorionic gonadotropin administration predicts clinical pregnancy of sibling frozen embryos. Fertil Steril. 2009;92:1880–5. doi: 10.1016/j.fertnstert.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirkin S, Nikas G, Hsiu JG, Diaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. 2004;89:5742–52. doi: 10.1210/jc.2004-0605. [DOI] [PubMed] [Google Scholar]
- 46.Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 47.Simon C, Oberye J, Bellver J, Vidal C, Bosch E, Horcajadas JA, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–27. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 48.Horcajadas JA, Minguez P, Dopazo J, Esteban FJ, Dominguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–10. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril. 2008;90:2152–64. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Haouzi D, Assou S, Mahmoud K, Tondeur S, Reme T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–45. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29. doi: 10.1186/1477-7827-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong M, Zhang H, Jin L, Ai J, Huang Z, Zhu G. Association of controlled ovarian hyperstimulation treatment with down-regulation of key regulators involved in embryonic implantation in mice. J Huazhong Univ Sci Technolog Med Sci. 2011;31:535–42. doi: 10.1007/s11596-011-0486-0. [DOI] [PubMed] [Google Scholar]
- 53.Humaidan P, van Vaerenbergh I, Bourgain C, Alsbjerg B, Blockeel C, Schuit F, et al. Endometrial gene expression in the early luteal phase is impacted by mode of triggering final oocyte maturation in recFSH stimulated and GnRH antagonist co-treated IVF cycles. Hum Reprod. 2012;27:3259–72. doi: 10.1093/humrep/des279. [DOI] [PubMed] [Google Scholar]
- 54.Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clin Exp Reprod Med. 2011;38:119–25. doi: 10.5653/cerm.2011.38.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from nonpregnant human endometrium. Am J Reprod Immunol. 2000;43:209–17. doi: 10.1111/j.8755-8920.2000.430405.x. [DOI] [PubMed] [Google Scholar]
- 56.Mariee N, Li TC, Laird SM. Expression of leukaemia inhibitory factor and interleukin 15 in endometrium of women with recurrent implantation failure after IVF; correlation with the number of endometrial natural killer cells. Hum Reprod. 2012;27:1946–54. doi: 10.1093/humrep/des134. [DOI] [PubMed] [Google Scholar]
- 57.Dorfeshan P, Salehnia M, Moazzeni SM. Ovarian stimulation affects the population of mouse uterine NK cells at early pregnancy. Biomed Res Int. 2013;2013:182531. doi: 10.1155/2013/182531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junovich G, Mayer Y, Azpiroz A, Daher S, Iglesias A, Zylverstein C, et al. Ovarian stimulation affects the levels of regulatory endometrial NK cells and angiogenic cytokine VEGF. Am J Reprod Immunol. 2011;65:146–53. doi: 10.1111/j.1600-0897.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 59.Chaouat G. Inflammation, NK cells and implantation: friend and foe (the good, the bad and the ugly?): replacing placental viviparity in an evolutionary perspective. J Reprod Immunol. 2013;97:2–13. doi: 10.1016/j.jri.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 60.delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, et al. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–46. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloise E, Lin W, Liu X, Simbulan R, Kolahi KS, Petraglia F, et al. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153:3457–67. doi: 10.1210/en.2011-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalra SK. Adverse perinatal outcome and in vitro fertilization singleton pregnancies: what lies beneath? Further evidence to support an underlying role of the modifiable hormonal milieu in in vitro fertilization stimulation. Fertil Steril. 2012;97:1295–6. doi: 10.1016/j.fertnstert.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 63.Mainigi MA, Olalere D, Burd I, Sapienza C, Bartolomei M, Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth. Biol Reprod. 2014;90:26. doi: 10.1095/biolreprod.113.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucifero D, Mann MR, Bartolomei MS, Trasler JM. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet. 2004;13:839–49. doi: 10.1093/hmg/ddh104. [DOI] [PubMed] [Google Scholar]
- 65.Denomme MM, Mann MR. Maternal control of genomic imprint maintenance. Reprod Biomed Online. 2013;27:629–36. doi: 10.1016/j.rbmo.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Cedar H, Bergman Y. Programming of DNA methylation patterns. Annu Rev Biochem. 2012;81:97–117. doi: 10.1146/annurev-biochem-052610-091920. [DOI] [PubMed] [Google Scholar]
- 67.Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19:36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- 68.Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–9. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 69.Turan N, Ghalwash MF, Katari S, Coutifaris C, Obradovic Z, Sapienza C. DNA methylation differences at growth related genes correlate with birth weight: a molecular signature linked to developmental origins of adult disease? BMC Med Genomics. 2012;5:10. doi: 10.1186/1755-8794-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet. 2014;31:65–71. doi: 10.1007/s10815-013-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad U S A. 2003;100:2963–8. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon C, Dominguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab. 2003;14:197–9. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 73.Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–8. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Zhou C, Chen W, Li T, Huang J, Zhuang G. Supraphysiological estrogen levels adversely impact proliferation and histone modification in human embryonic stem cells: possible implications for controlled ovarian hyperstimulation assisted pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;155:58–64. doi: 10.1016/j.ejogrb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta. 2006;27:483–90. doi: 10.1016/j.placenta.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology. 2008;149:5078–87. doi: 10.1210/en.2008-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. Am J Physiol Heart Circ Physiol. 2012;302:H1936–44. doi: 10.1152/ajpheart.00590.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forde N, Carter F, di Francesco S, Mehta JP, Garcia-Herreros M, Gad A, et al. Endometrial response of beef heifers on day 7 following insemination to supraphysiological concentrations of progesterone associated with superovulation. Physiol Genomics. 2012;44:1107–15. doi: 10.1152/physiolgenomics.00092.2012. [DOI] [PubMed] [Google Scholar]
- 79.Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Walter LM, Rogers PA, Girling JE. The role of progesterone in endometrial angiogenesis in pregnant and ovariectomised mice. Reproduction. 2005;129:765–77. doi: 10.1530/rep.1.00625. [DOI] [PubMed] [Google Scholar]
- 81.Kim M, Park HJ, Seol JW, Jang JY, Cho YS, Kim KR, et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med. 2013;5:1415–30. doi: 10.1002/emmm.201302618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–68. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Andraweera PH, Dekker GA, Roberts CT. The vascular endothelial growth factor family in adverse pregnancy outcomes. Hum Reprod Update. 2012;18:436–57. doi: 10.1093/humupd/dms011. [DOI] [PubMed] [Google Scholar]
- 84.Rizk B, Aboulghar M, Smitz J, Ron-El R. The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Hum Reprod Update. 1997;3:255–66. doi: 10.1093/humupd/3.3.255. [DOI] [PubMed] [Google Scholar]
- 85.Licht P, Neuwinger J, Fischer O, Siebzehnrubl E, Wildt L. VEGF plasma pattern in ovulation induction: evidence for an episodic secretion and lack of immediate effect of hCG. Exp Clin Endocrinol Diabetes. 2002;110:130–3. doi: 10.1055/s-2002-29090. [DOI] [PubMed] [Google Scholar]
- 86.Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC, 3rd, Stern JE. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril. 2010;94:1399–404. doi: 10.1016/j.fertnstert.2009.05.092. [DOI] [PubMed] [Google Scholar]
- 87.Gomez R, Soares SR, Busso C, Garcia-Velasco JA, Simon C, Pellicer A. Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med. 2010;28:448–57. doi: 10.1055/s-0030-1265670. [DOI] [PubMed] [Google Scholar]
- 88.Pietrowski D, Szabo L, Sator M, Just A, Egarter C. Ovarian hyperstimulation syndrome is correlated with a reduction of soluble VEGF receptor protein level and a higher amount of VEGF-A. Hum Reprod. 2012;27:196–9. doi: 10.1093/humrep/der349. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z, Xu L, He F. Embryo vitrification affects the methylation of the H19/Igf2 differentially methylated domain and the expression of H19 and Igf2. Fertil Steril. 2010;93:2729–33. doi: 10.1016/j.fertnstert.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 90.Zhao XM, Du WH, Hao HS, Wang D, Qin T, Liu Y, et al. Effect of vitrification on promoter methylation and the expression of pluripotency and differentiation genes in mouse blastocysts. Mol Reprod Devel. 2012;79:445–50. doi: 10.1002/mrd.22052. [DOI] [PubMed] [Google Scholar]
- 91.Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29:618–27. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 92.Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010;93:397–404. doi: 10.1016/j.fertnstert.2008.12.144. [DOI] [PubMed] [Google Scholar]


