Abstract
Objective
The aim of this investigation is to determine the effect of human serum albumin (HSA) and α-acid glycoprotein (AAG) on buprenorphine (BUP) transplacental transfer and distribution.
Methods
The technique of dual perfusion of placental lobule (DPPL) was utilized. Buprenorphine was co-perfused with the marker compound antipyrine (AP). In each experiment, the radiolabeled isotopes [3H]-buprenorphine and [14C]-AP were added to enhance their detection limits. Human plasma proteins, HSA and AAG, were added to both the maternal and fetal circuits separately and in combination at their physiological concentrations in maternal and fetal circulations close to term.
Results
Transplacental transfer of BUP, in absence of plasma proteins, is a 2-step process: the first is its uptake by the syncytiotrophoblast from the maternal circuit, and the second is its transfer/release from the tissue to the fetal circuit. The addition of HSA to the perfusion medium affected only the second step of BUP transfer, but AAG affected both steps. The combined effect of HSA and AAG was not different from that observed in presence of the latter alone.
Conclusions
Binding of BUP to circulating AAG could have an important role in the transfer of the drug from the maternal to fetal circulation.
Keywords: Buprenorphine, Human Serum Albumin, α-Acid Glycoprotein, Transplacental Transfer, Pregnant Addict
Introduction
Opioid dependence during pregnancy remains a problem for public health providers and physicians. Pharmacotherapy of this patient population with methadone results in improving maternal and neonatal outcomes [1,2]. Nevertheless, the newborns of methadone-maintained mothers may suffer from neonatal abstinent syndrome (NAS), which requires extended hospitalization and treatment. Moreover, methadone is the only drug approved for treatment of the pregnant opiate addict during pregnancy in the United States of America.
On the other hand, treatment of the adult opiate addict with BUP followed by abrupt termination caused little or no withdrawal symptoms [3]. Accordingly, it was speculated that BUP could be beneficial for treatment of the pregnant opiate addict. Several reports and limited clinical trails, reviewed by Johnson et al, 2003, demonstrated that treatment of the pregnant opiate addict with buprenoprphine appears to provide the same benefits to the mother as methadone, and NAS associated with buprenorphine is less intense than that with methadone [4].
The results of randomized, double-blind, double-dummy controlled trials on the effects of buprenorphine and methadone on the incidence and intensity of NAS did not reveal significant differences between medication groups on the percentage of neonates treated for NAS [5]. However, the hospitalization duration of neonates exposed to buprenorphine was shorter than that for those exposed to methadone [5]. Moreover, several investigators reported on qualitative and quantitative differences in NAS associated with buprenorphine versus those with methadone [4–7], which prompted speculations of the cause. One of these explanations, offered by a review [4] and of direct relevance to this investigation, is the lower-than-methadone transplacental transfer of buprenorphine to the fetal circuit of the dually perfused human placental lobule and, hence, less opiate in the fetal circulation [8].
The major determinants for drug transfer across human placenta are physicochemical properties of the drug, blood flow in the intervillous space, binding to plasma or placental tissue proteins, biotransformation by placental metabolic enzymes, and efflux by trophoblast transporters. Previously, it was demonstrated that human placental microsomes metabolize BUP by its dealkylation to norbuprenorphine and that CYP19/aromatase is the major enzyme responsible for catalyzing the reaction [9]. However, the Vmax of placental micorosomal CYP19 was 2.9 pmol/mg protein·min [9], ie, only a fraction of that observed for human hepatic microsome (CYP 3A4) of 712 pmol/mg protein·min [10]. Accordingly, placental metabolism of BUP should not have a significant effect on transplacental transfer of BUP.
In addition, utilizing the ex vivo technique of dual perfusion of placental lobule, it was demonstrated that the transfer of BUP from the maternal to fetal circuit was not affected by the addition of the P-glycoprotein inhibitor GF-120918. Therefore, it was concluded that the BUP is not a substrate of this efflux transporter and consequently does not contribute to the transplacental transfer of the opiate [11].
Another important factor that influences drug disposition by the placenta is its binding to proteins in the plasma and placental tissue. BUP binds to human serum albumin, gamma-globulin, and alpha-acid glycoprotein, and their binding ratios are 65.6%, 41.5%, and 84.9%, respectively [12]. However, the total plasma protein concentration declines during pregnancy, predominantly due to a decrease in albumin concentration, from a mean of 43 mg/mL in non-pregnant women to 29 mg/mL in pregnant [13, 14]. Moreover, maternal and fetal plasma concentrations of albumin and AAG undergo changes throughout gestation. Thus, close to term, the fetal/maternal serum concentration of albumin and AAG are approximately 1.16 and 0.37, respectively [15–17].
Therefore, the goal of this investigation is to determine the effect of HSA and AAG on transplacental transfer and distribution of BUP across the dually perfused term human placenta.
Materials and methods
Chemicals
Buprenorphine and its [3H]-isotope (specific activity, 35.8 Ci/mmol) were a generous gift from the National Institute on Drug Abuse (NIDA) drug supply unit. All other chemicals including radioactive [14C]-antipyrine (specific activity, 6.5 mCi/mmol) were purchased from Sigma-Aldrich (St. Louis, Mo).
Clinical Material
Term human placentas (n=70) were obtained immediately after vaginal or abdominal deliveries from the labor and delivery ward of the John Sealy Hospital at the University of Texas Medical Branch, Galveston, according to a protocol approved by the Institutional Review Board. Placentas from women with evidence of maternal infection, drug, or alcohol abuse during pregnancy were excluded. Data from 35%–40% of the perfused placentas were included in this investigation. The remainder were excluded for the reasons enumerated below.
Dual Perfusion of Term Human Placental Lobule
The technique of DPPL was used according to the method of Miller et al [18] and as described in an earlier report from our laboratory [8]. Briefly, an intact peripheral cotyledon was perfused with tissue culture medium M199, supplemented with dextran, gentamicin sulfate, heparin, and bactrim. The flow rates were 2.8 mL/min (fetal circuit) and 12 mL/min (maternal circuit). The pH in the maternal and fetal circuits was adjusted and maintained at 7.4 and 7.35, respectively, by sodium bicarbonate. Maternal perfusate was gassed with 95%O2 and 5%CO2, the fetal perfusate with 95%N2 and 5%CO2, and both were maintained at 37°C.
Each placenta was perfused for an initial control period of 1 hour in the absence of BUP to allow placental tissue to equilibrate and stabilize in its postpartum environment and to determine the vascular and metabolic integrity of perfused tissue as well as the degree of perfusion overlap. The experiment was terminated if one or more of the following was observed: fetal artery pressure in excess of 50 mmHg, a loss of > 2 mL/hr, or a difference between the fetal vein and artery < 60 mmHg (inadequate perfusion overlap between the 2 circuits). Following the control period, the maternal perfusate was replaced with fresh medium containing 10ng/mL of BUP and 1.5μCi of its [3H]-isotope to increase the detection limit. This concentration of BUP is equal to its peak serum level in patients receiving an 8mg dose [19, 20]. In each experiment, the freely diffusible marker compound, antipyrine (AP, 20μg/mL), and its [14C]-isotope (1.5μCi) were cotransfused to account for interplacental variations and to normalize the transfer of BUP. The transplacental transfer and distributon of BUP in the absence of plasma proteins (control group) was compared to its transfer in the presence of either HSA or AAG as well as their combination. Plasma proteins HSA and AAG, alone or in combination, were added to the perfusion medium of both circuits in the beginning of the experimental period along with BUP. HSA was added to the perfusion medium of both circuits at a concentration of 30mg/mL [13, 14]. The concentration of AAG in the maternal circuit was 0.6mg/mL and 0.24mg/mL in the fetal circuits [17]. The effect of albumin and AAG on BUP transfer was investigated utilizing the perfusion system in its recirculating configuration.
Aliquots (0.5mL) were taken from the perfusates of the maternal artery and fetal vein at 0, 5, 10, 15, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210, and 240 minutes and were analyzed for their concentration of BUP and AP utilizing a liquid scintillation counter. At the end of each experiment, the perfused area of placental tissue was dissected, weighed, and homogenized. Sodium hydroxide (1mL of 1 M solution) was added to 1mL of the tissue homogenate and incubated overnight at 60°C in the dark to allow for luminescence decay. Scintillation cocktail was then added to each sample, and the concentration of each drug was determined.
Statistical Analysis
The results reported are expressed as mean ± standard deviation. The concentrations of BUP in the tissue, maternal circuit, and fetal circuit in the presence and absence of plasma proteins were compared and the data analyzed by the 2-tailed t-test.
A P value of <.05 was considered statistically significant.
Results
Effect of plasma proteins on transplacental transfer of buprenorphine
The effect of 2 major plasma proteins, HSA and AAG, on transplacental transfer and distribution of BUP was investigated. The concentration of HSA in both the maternal and fetal circuits was 30mg/mL. The concentration of AAG was 0.6 mg/mL in the maternal circuit and 0.24 mg/mL in the fetal. The above concentrations of HSA and AAG in the maternal and fetal circuits were equal to their respective physiological concentrations near term [15–17].
The transfer of AP across the placental lobule is not affected by the presence of HSA, AAG, or their combination in the perfusion medium. Accordingly, the transplacental transfer of BUP, in the presence and absence of plasma proteins, was normalized to the transfer of the marker compound AP (Table I).
Table I.
Effect of HSA and AAG, alone and in combination, on the transfer of antipyrine across dually perfused human placental lobule
| Antipyrine concentration (μg/mL) | ||||
|---|---|---|---|---|
| In absence of plasma proteins (n=7) | In presence of HSA (n= 5) | In presence of AAG (n= 3) | In presence of combination of HSA & AAG (n=5) | |
| Maternal circuit | 10 ± 1.4 | 10.2 ± 0.6 | 9.8 ± 0.5 | 10.7 ± 0.4 |
| Fetal circuit | 8.3 ± 0.9 | 9.3 ± 0.5 | 8.6 ± 0.7 | 8.9 ± 0.6 |
| Fetal/Maternal concentration ratio | 0.84 ± 0.11 | 0.91 ± 0.09 | 0.88 ± 0.11 | 0.83 ± 0.08 |
Each value represents the mean ± standard deviation determined during the last hour of the perfusion experiment.
Effect of HSA on transplacental transfer of buprenorphine
The transfer of BUP from the maternal to the fetal circuit across the dually perfused human placental lobule was investigated in the presence of 30mg/mL of HSA (n=5) and in its absence (n = 9). In all experiments, AP was cotransfused to normalize the data for BUP since the transfer of the marker compound is not affected by the presence of 30mg/mL HSA.
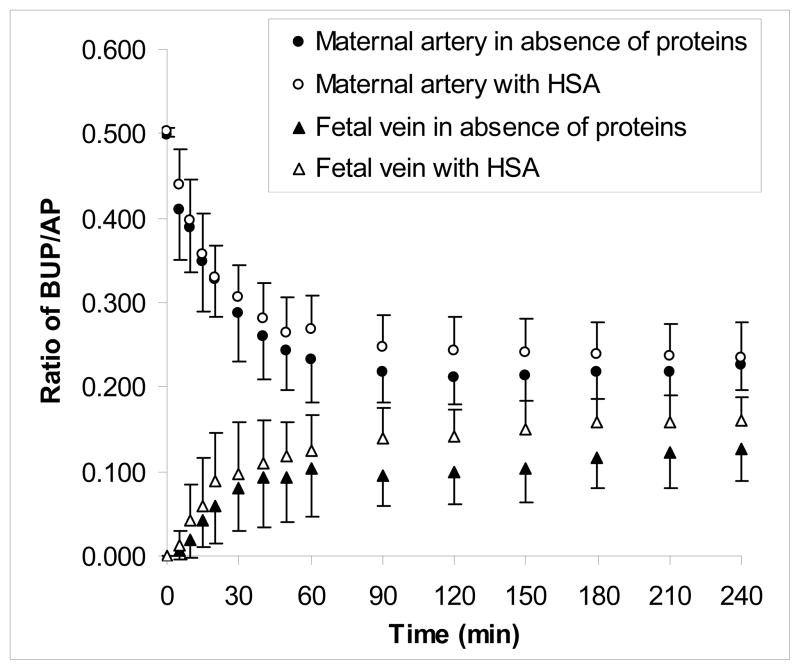
The transplacental transfer of BUP in the recirculating (closed-closed) configuration of the perfusion system in absence of HSA was reported earlier [8]. The decline in BUP concentration in the maternal circuit during perfusion in presence of 30mg/mL of HSA was similar to that in absence of protein (Figure 1). Moreover, the addition of HSA to the maternal circuit did not affect the rate of BUP transfer to the fetal circuit during the initial perfusion period of 60 minutes and was similar to that in the absence of the protein. On the other hand, following 90 minutes of perfusion, the concentration of BUP in the fetal circuit in the presence of HSA was significantly higher (P<.05) than in placentas perfused with the opioid in media devoid of albumin. Therefore, the amount of BUP transferred across the placenta to the fetal circuit in the presence of HSA increased, and consequently, the fetal to maternal concentration ratio of the drug at the end of 4 hours’ perfusion was 0.59 ± 0.06 as compared to 0.43 ± 0.14 (P<.001) in the absence of albumin (Table II).
Figure 1.
Effect of HSA on transplacental transfer of BUP. The transfer of BUP from the maternal to fetal circuit of the dually perfused human placental lobule in the presence (n=5) and absence of HSA (n=9) was determined for a period of 240 minutes. The concentration of HSA in the maternal and fetal circuits was 30 mg/mL. The latter concentration of HSA did not affect the transfer of the marker compound AP, and hence, it was co-transfused with BUP in each experiment. The transfer of BUP in the absence and the presence of HSA was normalized to the transfer of AP and is presented as the concentration ratio of BUP/AP.
Table II.
Effect of HSA and AAG, alone and in combination, on the transfer of buprenorphine across dually perfused human placental lobule
| Buprenorphine concentration (ng/mL) | ||||
|---|---|---|---|---|
| In absence of plasma proteins (n=9) | In presence of HSA (n= 5) | In presence of AAG (n= 4) | In presence of combination of HSA & AAG (n=7) | |
| Maternal circuit | 2.2 ± 0.2 | 2.3 ± 0.5 | 3.1 ± 0.8** | 3.3 ± 0.6** |
| Fetal circuit | 0.9 ± 0.2 | 1.5 ± 0.3* | 1.1 ± 0.2 | 1.5 ± 0.2* |
| Fetal/Maternal concentration ratio | 0.43 ± 0.14 | 0.59 ± 0.06*** | 0.47 ± 0.08 | 0.43 ± 0.12 |
Each value represents the mean ± standard deviation determined during the last hour of the perfusion period.
P< 0.05;
P<.01;
P<.001.
Effect of AAG on transplacental transfer of buprenorphine
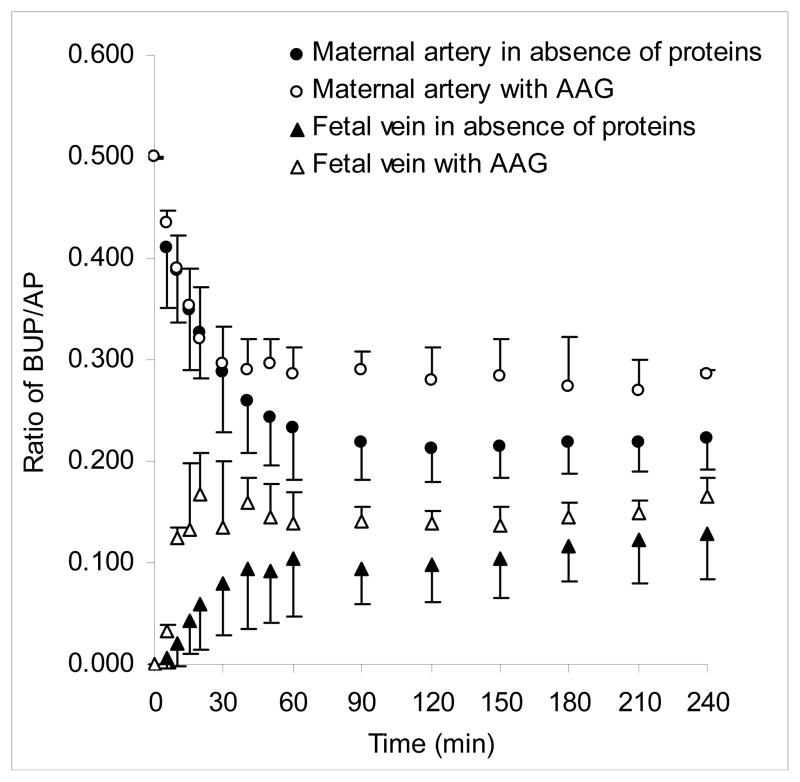
The addition of AAG (0.6 mg/mL) to the perfusion medium of the maternal circuit (n=4) did not affect the initial decline in concentration of BUP from the maternal circuit as compared to its decline in the perfusion experiments in the absence of protein (Figure 2). However, approximately 30 minutes after initiation of perfusion, initial decline in the concentration of BUP from maternal circuit in the presence of AAG slowed down, resulting in more drug remaining in the maternal circuit (P<.01).
Figure 2.
Effect of AAG on transplacental transfer of BUP. The transfer of BUP from the maternal to fetal circuit of the dually perfused human placental lobule in the presence (n=4) and absence of AAG (n=9) was determined for a period of 240 minutes. The concentration of AAG was 0.6 and 0.24 mg/mL in the maternal and fetal circuits, respectively. The addition of AAG to the perfusion medium of both circuits did not affect the transfer of the marker compound AP and was thus co-transfused with BUP in each experiment. Transfer of BUP in the absence and presence of AAG was normalized to the transfer of AP and is presented as the concentration ratio of BUP/AP.
On the contrary, in the fetal circuit, the presence of 0.24 mg/mL of AAG significantly affected only the initial rate of BUP transfer to the fetal circuit, thus resulting in higher BUP concentrations during the first 30 minutes of perfusion (P<.01). However, during the following 210 minutes of perfusion, although concentrations of BUP in the fetal circuit were still higher in perfusions with AAG than in its absence, the observed difference was not statistically significant. Since concentrations of BUP changed simultaneously in maternal and fetal circuits, the F/M concentration ratio in the presence of AAG did not differ from that in its absence: 0.47 ± 0.08 vs 0.43 ± 0.14 (Table II).
The combined effect of HSA and AAG on transplacental transfer of buprenorphine
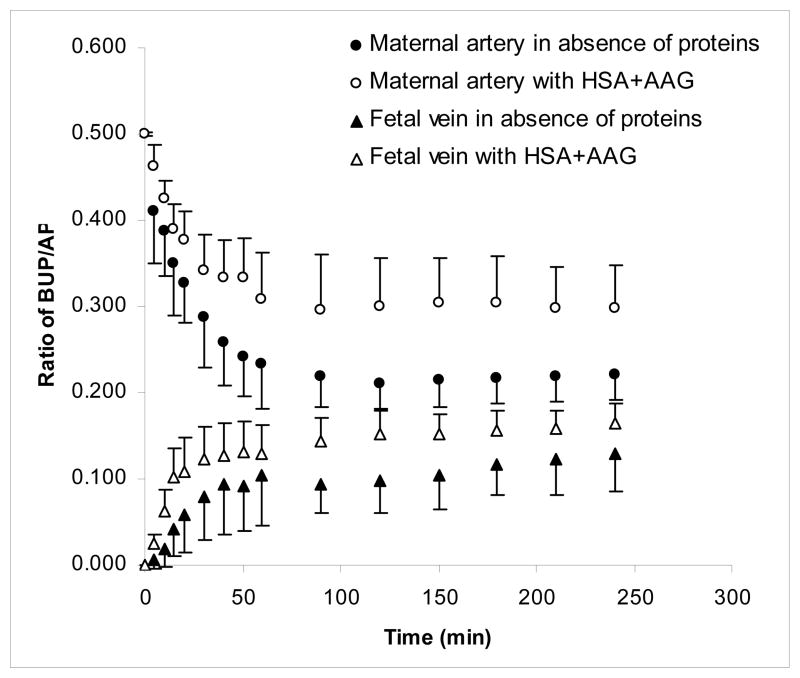
The decline in concentration of BUP in the medium of the maternal circuit in the presence of both HSA and AAG (n=7) was less pronounced than that observed in the absence of the 2 proteins (Figure 3) and as apparent from the concentrations of BUP determined at each time point during 4 hours of perfusion. However, this observed difference between the 2 experimental groups was statistically significant only after 30 minutes of perfusion (P<.01). It should be noted that the concentrations of BUP in the maternal circuit in the presence of both HSA and AAG was not different from the concentrations of the opioid when perfused in the presence of AAG only.
Figure 3.
Effect of the combination of HSA and AAG on transplacental transfer of BUP. The transfer of BUP from the maternal to fetal circuit of dually perfused human placental lobule was determined in the presence (n=7) and absence of the combination of HSA and AAG (n=9) for a period of 240 minutes. The concentration of HSA in both circuits was 30 mg/mL. The concentration of AAG was 0.6 and 0.24 mg/mL in the maternal and fetal circuits, respectively. The combination of HSA and AAG had no effect on the transfer of the marker compound AP, and hence, it was co-transfused with BUP in each experiment. The transfer of BUP in the absence and presence of the combination of HSA and AAG was normalized to the transfer of AP and is presented as the ratio for the concentrations of BUP/AP.
The combined presence of HSA and AAG in the fetal circuit resulted in a higher transfer of BUP from the maternal to the fetal side of the perfusion system (P<.05) than that observed in the absence of the proteins. However, the observed effect of the combination was not different from that observed in the presence of either protein alone. Taken together, these data suggest that transfer of BUP from the maternal to the fetal circuit is affected by both proteins but at different times: during the initial perfusion period of 30 minutes, it is affected by AAG, and during the period between 90 and 240 minutes, by HSA.
It should be noted that since the concentration of BUP in both circuits was higher in the presence of the 2 plasma proteins than in their absence, the fetal to maternal concentration ratio at the end of the experiment remained unchanged, 0.43 ±0.12 and 0.43 ± 0.14, respectively (Table II).
The effect of HSA and AAG on buprenorphine distribution among the tissue, maternal circuit, and fetal circuit
How HSA, AAG, and their combination, added to the perfusion medium of both circuits, affects BUP distribution following its perfusion for 4 hours was investigated. Data on BUP distribution in the presence and absence of the proteins is presented in Figure 4.
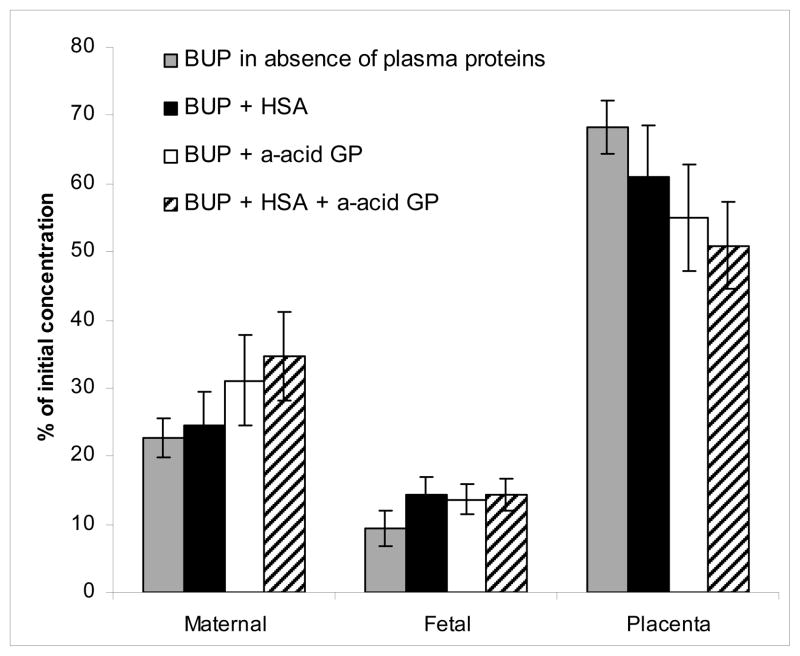
Figure 4.
Effect of HSA and AAG, alone and in combination, on distribution of BUP. The effects of added plasma proteins on the distribution of BUP among placental lobule, maternal circuit, and fetal circuit were determined at the end of the experiment. The concentration of HSA in the maternal and fetal circuits was 30 mg/mL. The concentration of AAG was 0.6 and 0.24 mg/mL in the maternal and fetal circuits, respectively.
The addition of HSA to both circuits decreased the amount of BUP retained by placental tissue from 68 ± 4% to 61 ± 8% (P<0.001) and increased the amount of the opioid in the fetal circuit from 9 ± 3% to 14 ± 3% (P< 0.01). However, the amount of BUP remaining in the maternal circuit was not affected: 25 ± 5% vs 23 ± 3%.
AAG added to both circuits resulted in a significant decrease in the amount of BUP retained by placental tissue from 68 ± 4% to 55 ± 8% (P< 0.001). Moreover, the presence of AAG increased the amount of BUP remaining in the maternal circuit from 23 ± 3% to 31 ± 7% (P< 0.001) and that in the fetal circuit from 9 ± 3% to 14 ± 2% (P< 0.01).
The combination of HSA and AAG further decreased the amount of BUP retained by placental tissue to the lowest observed, 51 ± 6.% (P<.001). This observed decrease was neither additive (ie, for that observed by either protein alone) nor significantly different from that observed in the presence of AAG alone. However, the decrease (in amount of BUP retained by the tissue) in the presence of the 2 proteins was significantly different from the effect of HSA alone (P<.05).
The effect of the 2 proteins on BUP in the maternal circuit was more pronounced than in the fetal. The amount of BUP remaining in the maternal circuit in the presence of both HSA and AAG was significantly higher than in their absence (34 ± 7%, P<0.001) or in the presence of HSA only (P<0.001) but was not different from that in the presence AAG alone. However, there was no apparent difference in the amount of BUP in the fetal circuit in the presence of either protein alone or their combination. Nevertheless, the presence of either protein or their combination resulted in higher amounts of BUP in the fetal circuit than in their absence (P<.001).
Discussion
During the last several years, our investigations focused on better understanding the role of human placenta in the disposition of BUP and methadone. The hypothesis behind our investigations is that the incidence and intensity of NAS associated with both drugs when used for treatment of the pregnant opiate addict should correlate with the concentration of the opiate in the fetal, rather than the maternal, circulation. Accordingly, the concentration of either BUP or methadone in the fetal circulation should depend, in part, on placental disposition of the opiate during gestation. Placental disposition of a drug includes its transfer from the maternal to the fetal circulation, distribution among the 3 (tissue, maternal, and fetal) compartments, and metabolism by the tissue, as well as uptake and efflux by trophoblast transporter proteins. The role of human placental metabolic enzymes [9] and efflux transporter P-gp [11] in the disposition of BUP has been reported earlier and was summarized in the introduction. Therefore, the goal of this investigation was to determine the effect of plasma proteins on placental transfer and distribution of BUP.
The technique of dual perfusion of placental lobule was utilized to determine the effect of HSA and AAG, individually and in combination, on transplacental transfer of BUP. We reported earlier on the transplacental transfer of BUP utilizing the technique of dual perfusion of placental lobule with a medium devoid of plasma proteins [8]. In this and in our previous investigation, we used AP as a marker compound because it freely diffuses across the placenta (by simple diffusion) and, along with oxygen transfer, serves as an indicator for the extent of perfusion overlap between the 2 circuits [21]. This eliminates differences due to the inherent properties of the tissue, such as thickness of the perfused lobule. Moreover, data obtained in this investigation revealed that transplacental transfer of AP is not affected by the presence of HSA or AAG individually or by their combination (Table 1). Therefore, AP was used to normalize the transfer of BUP in the absence and presence of the proteins.
The transplacental transfer of the highly lipophilic compounds is a 2-step process [22]. The first is its uptake by the syncytiotrophoblast from the maternal circuit, and the second is its transfer/release from the tissue to the fetal circuit as has been reported for the transplacental transfer of BUP [8].
Data cited in this report indicate that the addition of equal concentrations of HSA to the perfusion medium of both circuits had no effect on the initial (<60 minutes) transplacental transfer of BUP (Figure 1). However, during the subsequent period, between 90 and 240 minutes, the concentration of BUP in the fetal circuit was significantly higher than in that observed when the medium was devoid of HSA. Moreover, the distribution of BUP in the presence of HSA as compared to its absence, at the end of the 4-hour perfusion period, revealed a significant increase in the drug transferred to the fetal circuit (P<0.01) accompanied by a significant decrease in that retained by the by placental tissue (P<0.001) (Figure 4). However, the amount of BUP remaining in the maternal circuit, in the presence or absence of HSA, was not different. Taken together, these data indicate that HSA did not affect the first step in the transfer process (from the maternal circuit to the placental tissue), but rather the second step, namely, the transfer of BUP from the tissue to the fetal circuit. This could be explained as follows: In the absence of added proteins in the medium, the partition of BUP between the aqueous environment in both circuits and the lipophilic tissue attained its equilibrium. In the presence of HSA, 2 more equilibria should be considered: those for the binding of BUP to HSA in both circuits and, consequently, their effects on the partitioning of the drug between the tissue and the media.
In the presence of AAG in the perfusion medium, the decline in BUP concentration in the maternal circuit was significantly decreased. This decrease indicates that the first step in BUP transfer from the maternal circuit to the tissue was affected (Figure 2). Moreover, the data revealed a higher transfer of BUP from the tissue to the fetal circuit during the initial 30 minutes of perfusion; ie, the presence of AAG affected the second step of the transfer process. As a result of these 2 events, the amount of BUP retained by the perfused lobule at the end of the experiment was lower in the presence of AAG than in its absence (Figure 4). Therefore, it is concluded that the presence of AAG in the perfusion medium affects both transfer steps and results in a higher transfer of BUP from the tissue to the fetal circuit.
The combined effect of both plasma proteins, HSA and AAG, appears to have caused a further decrease in BUP transfer from the maternal circuit to the perfused lobule, and, consequently, more of the opiate remained in the maternal circuit. Although the combined effect of HSA and AAG appears to be more pronounced than that of AAG alone, it did not reach statistical significance, thus suggesting that AAG is likely to be the main plasma protein affecting/limiting the transfer of BUP to the perfused lobule. This assumption is substantiated by the fact that the concentration of AAG in the perfusion medium in the maternal and fetal circuits was 1/50 and 1/125 of HSA, respectively.
Data from this investigation revealed a higher transfer of BUP from the maternal to the fetal circuit in the presence of plasma proteins than in their absence, as previously reported from our laboratory [8]. The experimental conditions used in this investigation closely resemble the in vivo situation, and, consequently, it could be concluded that BUP concentration in the fetal circulation should be higher than what was anticipated on the basis of our earlier investigation [8]. As mentioned above, in media devoid of the proteins, BUP distribution among the tissue, maternal cicuit, and fetal circuit is solely dependent on its partition coefficient. On the other hand, the addition of protein(s) to both circuits should change the partition of BUP by favoring the presence of more of the drug in the 2 circuits. Consequently, the total (free and bound) BUP concentration should be higher in both circuits and lower in the tissue in the presence of the proteins than in their absence. Furthermore, the binding association and dissociation constants of BUP to either HSA or AAG should be the same, irrespective of circuit, and should depend on the law of mass action and the equilibrium constant as well as the partition coefficient of BUP.
An important parameter in the pharmacokinetics of any drug used in treatment of the pregnant patient is its fetal/maternal concentration ratio. This parameter is often used, in the ex vivo model system of dual perfusion of placental lobule to demonstrate the extent of drug transfer from the maternal to the fetal circuit. However, the ratio of the fetal to maternal drug concentration may not, in all cases, reflect the extent of drug transfer from the maternal to the fetal circuit, as was apparent from data obtained in this investigation. The addition of AAG and HSA to both the maternal and fetal circuits resulted in 2 significant changes in BUP concentration as compared to their absence. These 2 changes were: (1) a decrease in BUP transfer from the maternal circuit to the tissue and (2) an increase in BUP transfer from the tissue to the fetal circuit. Accordingly, the fetal to maternal concentration ratio remained approximately the same, namely, 0.43 ± 0.12 in the presence of plasma proteins and 0.43 ±0.14 in the absence of proteins. However, having an equal fetal/maternal concentration ratio of 0.43 for BUP in the absence and presence of plasma proteins, as result of the simultaneous increase in the drug concentration in both circuits, should not be expected to occur for all drugs. For example, in a recent investigation of placental transfer of the hypoglycemic drug glyburide in the presence of HSA, the data revealed that the fetal/maternal concentration ratio of the drug was significantly lower than the ratio in the absence of the protein [23]. This change in the ratio in the presence of HSA was a result of a significant increase in the concentration of the drug in the maternal circuit accompanied by a decrease in the fetal. Therefore, data obtained from the ex vivo perfusion system in the absence of proteins cannot be extrapolated to the more physiologically relevant protein-containing media because the effect of plasma proteins on maternal and fetal concentrations of each drug could be different from BUP and is dependent on its binding properties to the protein.
Alternatively, in vivo, the concentration of BUP in the maternal circulation will depend on the dose administered, its distribution/partition (between tissue and fluids), metabolism by the maternal liver, and excretion (kidney/gut), as well as transplacental transfer to the fetal circulation. Similarly, the concentration of BUP in the fetal circulation will depend on the extent of its transplacental transfer, activity of uptake and/or efflux transporters, and placental and fetal metabolic enzymes, as well as excretion to the amniotic fluid. It is unfortunate that currently there is no data on the concentration of BUP in the fetal circulation and the placenta at the time of delivery. Accordingly, we find it very difficult to extrapolate our ex vivo data and speculate on what it could be in vivo. Nevertheless, it became apparent from data obtained in this investigation that the fetal to maternal concentration ratio of BUP at delivery is an important parameter that should be determined. At that time, the knowledge of BUP concentration in placental tissue, maternal circuit, and fetal circuit at delivery will provide one time point for the pharmacokinetics of BUP during pregnancy. The latter, together with our data on placental disposition of BUP during pregnancy, should lead to a better understanding of the factors leading to the incidence and intensity of NAS.
Acknowledgments
The authors sincerely appreciate the support of the physicians and nurses of the Labor & Delivery Ward of the John Sealy Hospital, the teaching hospital at UTMB, Galveston, Texas, and the Perinatal Research Division of the Department of Obstetrics & Gynecology. The authors greatly appreciate the Publication, Grant, & Media Support area of the Department of Obstetrics & Gynecology. This work was supported by the NIDA to Mahmoud S. Ahmed.
References
- 1.Kandall SR, Albin S, Lowinson J, Berle B, Eidelman AI, Gartner LM. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics. 1976;58:681–685. [PubMed] [Google Scholar]
- 2.Svikis D, Golden A, Huggins G, Pickens RW, McCaul ME, Velez M, Rosendale T, Brooner R, Gazaway P, Stitzer M. Cost-effectivness of comprehensive care for drug abusing pregnant women. Drug Alcohol Depend. 1997;45:105–113. doi: 10.1016/s0376-8716(97)01352-5. [DOI] [PubMed] [Google Scholar]
- 3.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RE, Jones HE, Fisher G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70:S87–S101. doi: 10.1016/s0376-8716(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, Crocetti M, Dudas R, Harrow C, Huestis MA. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinent syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Auriacombe M, Afflelou S, Lavignasse P, Lafitte C, Roux D, Daulouede JP, Tignol J. Pregnancy, abortion, and delivery in a cohort of heroin-dependent patients treated with drug substitution (methadone and buprenorphine) in aquitaine. Presse Med. 1999;28:177. [PubMed] [Google Scholar]
- 7.Rohrmeister K, Bernert G, Langer M, Fisher G, Weninger M, Pollak A. Opiate addiction in gravidity-consequences for the newborn. Results on an interdisciplinary treatment concept. Z Geburtshilfe Neonatol. 2001;205:224–230. doi: 10.1055/s-2001-19054. [DOI] [PubMed] [Google Scholar]
- 8.Nanovskaya TN, Deshmukh S, Brooks M, Ahmed M. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;00:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh SV, Nanovskaya TN, Ahmed MS. Aromatse is the major enzyme metabolizing buprenorphine in human placenta. J Pharmacol Exp Ther. 2003;306:1099–1105. doi: 10.1124/jpet.103.053199. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Yamamoto T, Chiba K, Tani M, Shimada N, Ishizaki T, Kuroiwa Y. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos. 1998;26:818–822. [PubMed] [Google Scholar]
- 11.Nekhayeva IA, Nanovskaya TN, Hankins GDV, Ahmed MS. Role of human placental efflux transporter P-glycoprotein in the transfer of buprenorphine, Levo-α-acetylmethadol, and paclitaxel. Am J Perinatol. 2006;23:423–430. doi: 10.1055/s-2006-951301. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Ishii S, Arizono H, Nishimura S, Tsuruda K, Saito N, nemoto H, Jin Y, Esumi Y. Pharmacokinetics of buprenorphine hydrochloride. Absorption, Distribution, Metabolism, Excretion after percutaneous or subcutaneous administration of buprenorphine hydrochloride in rats. Xenobiotic Metabolism and Disposition. 2001;16:569–583. [Google Scholar]
- 13.Hytten FE, Leitch I. The physiology of human pregnancy. Oxford: Blackwell Scientific Publications; 1971. [Google Scholar]
- 14.Pritchard JA, Macdonald PC. Williams obstetrics. 15. New York: Appleton-Century-Crofts; 1976. [Google Scholar]
- 15.Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha 1-acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. Br J Obstet Gynecol. 1984;91:875–881. doi: 10.1111/j.1471-0528.1984.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 16.Hill MD, Abramson FP. The significance of plasma protein binding on the fetal/maternal distribution of drugs at steady-state. Clin Pharmacokinet. 1988;14:156–170. doi: 10.2165/00003088-198814030-00004. [DOI] [PubMed] [Google Scholar]
- 17.McNamara PJ, Alcorn J. Protein binding predictions in infants. [Accessed 2008 May 8];AAPS Pharm Sci [serial online] 2002 4(1) doi: 10.1208/ps040104. Available: http://www.aapspharmsci.org/view.asp?art=ps040104 via the INTERNET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RK, Wier PJ, Maulik D, di Sant’Agnese PA. Human placenta in vitro: characterization during 12 h of dual perfusion. Contrib Gynec Obstet. 1985;13:77–84. [PubMed] [Google Scholar]
- 19.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 20.Jagsch R, Gombas W, Schindler SH, Eder H, Moody DE, Fischer G. Opioid plasma concentrations in methadone-and buprenorphine-maintained patients. Addict Biol. 2005;10:365–371. doi: 10.1080/13556210500358441. [DOI] [PubMed] [Google Scholar]
- 21.Schneider H. Techniques: in vitro perfusion of human placenta. In: Sastry BVR, editor. Placental toxicology. Boca Raton: CRC Press; 1995. pp. 1–26. [Google Scholar]
- 22.Sastry BVR. Technique to study human placental transport. Adv Drug Deliv Rev. 1999;38:17–39. doi: 10.1016/s0169-409x(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 23.Nanovskaya TN, Patrikeeva S, Hemauer S, Fokina V, Mattison DR, Hankins GDV, Ahmed MS. Effect of albumin on transplacental transfer and distribution of rosiglitazone and glyburide. J Matern Fetal Neonatal Med. 2008;21:197–207. doi: 10.1080/14767050801929901. [DOI] [PubMed] [Google Scholar]