Abstract
Background
A high prevalence of obesity has been increasingly recognized in survivors of pediatric ALL. However, longitudinal patterns of weight change during and after treatment, and associated factors, are less well elucidated.
Procedure
In a retrospective cohort of 83 pediatric patients with ALL diagnosed between 1985 and 2010, we examined body mass index (BMI) status at several key time points: diagnosis; end of induction; end of consolidation; every 6 months during maintenance; and yearly for up to 5 years post-treatment.
Results
At diagnosis, 21% were overweight (BMI = 85–94.9th percentile) or obese (BMI ≥95th percentile). At the end of treatment and 5 years post-treatment, approximately 40% were overweight or obese. The mean BMI z-score was 0.2 (58th percentile) at diagnosis and increased significantly during induction (Δ = 0.5, P <0.0001). It increased again during the first 6 months of maintenance (Δ = 0.2, P <0.01) and did not significantly change over the remainder of maintenance (BMI z-score at the end of treatment = 0.8, 79th percentile) and 5 years post-treatment (BMI z-score = 0.7, 76th percentile). High BMI z-score at diagnosis was associated with an increased risk of being overweight/obese at treatment completion (OR = 2.9, 95% CI: 1.6–5.1). Weight gain during treatment was associated with being overweight/obese 5 years post-treatment (OR = 3.8, 95% CI: 1.1–12.5).
Conclusion
Children with ALL are at risk of becoming overweight/obese early in treatment. Increases in weight are maintained throughout treatment and beyond. Lifestyle interventions are needed targeting weight control early during treatment, particularly for patients overweight/obese at diagnosis and those who experience substantial weight gain during treatment. Pediatr Blood Cancer 2014;61:1263–1269.
Keywords: childhood cancer, obesity, survivors
INTRODUCTION
Advances in cancer treatment have resulted in significant improvements in survival rates of childhood cancer. The 2010 5-year survival rate of those diagnosed with childhood cancer was estimated to be over 80% [1]. This success translates into a growing population of long-term survivors [2]. At the same time, cancer treatment at a young age also produces late-effects, including obesity, that substantially contribute to morbidity and mortality of childhood cancer survivors [3,4]. Evidence from the Childhood Cancer Survivor Study (CCSS) suggests that survivors of acute lymphoblastic leukemia (ALL), the most common cancer diagnosed in children, experience a higher rate of obesity than their same-sex siblings, especially for female survivors (ALL: 31.7% vs. siblings: 22.2%) [5]. Obesity may further compound the risk of other late-effects, such as increased rate of cardiovascular diseases observed in childhood cancer survivors [6].
Patterns and predictors of weight gain in childhood cancer survivors need to be identified so that targeted intervention can be developed. Previous studies have attributed obesity to the cranial irradiation (CRT) patients received to prevent central nervous system (CNS) relapse [7]. However, since the 1990s, CNS prophylaxis with CRT protocols has gradually been replaced with intrathecal and systemic chemotherapy by several consortia. A study of the Children’s Oncology Group (COG) found excessive weight gain also occurred in children receiving chemotherapy alone [8]. The relative magnitude of excess weight associated with chemotherapy, compared to those who also received CRT, remains unclear. The associations of obesity in survivors with various patient characteristics, such as gender and age at diagnosis, have also been inconsistent. Equally important, the sensitive window for weight gain has not been clearly specified. Because childhood cancer survivors may experience weight gain early in treatment, interventions will need to be implemented early to prevent late-effect obesity. Longitudinal studies that provide detailed assessment on weight patterns during and after cancer treatment may better elucidate the pattern of weight gain and predictors for obesity in childhood cancer survivors.
We report herein results of a retrospective analysis that evaluated longitudinal changes in weight in children with ALL and identified factors possibly contributing to overweight or obesity during and after cancer treatment.
METHODS
Study Population
We assembled a retrospective cohort of patients with pediatric ALL diagnosed and treated in the Division of Pediatric Hematology/Oncology at the Floating Hospital for Children at Tufts Medical Center between 1985 and 2010. Eligible patients were patients diagnosed <21 years with standard or high risk precursor B-cell or T-cell ALL. Precursor B-cell patients were classified as standard or high risk based on their age and white blood cell count at diagnosis [9]. All T-cell patients were classified as high risk. Four patients with Down syndrome were excluded due to well-described difference in growth patterns [10]. Initially, 105 patients were identified from medical records, 22 of whom were excluded from the analyses due to death or disease recurrence before the completion of initial treatment (n = 16), or loss to follow-up resulting from transfer of care to another facility (n = 6). This resulted in our study cohort of 83 patients. The study was approved by the Institutional Review Board (IRB) at Tufts Medical Center/ Tufts University.
Data Collection
Medical records were reviewed for each eligible patient to extract cancer-related information and growth data. Cancer-related information including cancer diagnosis, medical history, and treatment information were extracted using the Summary of Cancer Treatment Form, developed by COG [11]. Growth data on weight and height were extracted for several key time points including diagnosis, end of induction, end of consolidation, every 6 months during maintenance, and yearly up to 5 years post-treatment.
Anthropometrics
Body mass index (BMI) was calculated using the standard approach of dividing weight in kilograms by height in meters squared (kg/m2). For patients aged 2–20 years old, BMI z-score and BMI percentile were calculated using the 2000 Center for Disease Control and Prevention (CDC) growth charts for children [12]. For patients <2 years old, weight-for-length z-score and percentile were calculated using the 2000 CDC growth charts for children [12]. For simplicity, weight-for-length z-score is referred to as BMI z-score hereafter. The z-score indicates the number of standard deviations the measurement is away from the mean for the age- and sex-specific US general population mean, and a z-score >0 indicates a higher-than-average BMI or weight-for-length. The percentile indicates the relative position of a child’s BMI or weight-for-length among children of same sex and age. A percentile >50th indicates a higher-than-average BMI or weight-for-length. For patients >20 years, BMI z-score and percentile were calculated based on the reference data for age 20 in the 2000 CDC growth charts [13].
Weight Status
Obesity was defined as BMI z-score ≥1.645 (≥95th percentile), overweight as BMI z-score = 1.036–1.644 (85th–94.9th percentile), healthy weight as BMI z-score = −1.645 to 1.035 (5th–84.9th percentile), and underweight as BMI z-score <−1.645 (<5th percentile). These definitions are based on the current recommendations of the US CDC [14] and are in accordance with previous studies [15].
Statistical Analysis
We first summarized demographic and cancer-related variables of the study cohort using descriptive statistics. We then evaluated the longitudinal pattern of changes in percentage of overweight/ obesity and in BMI z-score during and after cancer treatment. Changes were evaluated for each treatment phase, every 6 months during maintenance until the end of treatment, and yearly after completion of treatment until 5 years post-treatment. One-sample t-tests were performed to evaluate whether BMI z-score at each time point or a change in BMI z-score was significantly different from 0. Data were censored at time of disease recurrence after the completion of initial treatment, or at the time of last follow-up, whichever occurred earlier. For patients who had incomplete data for weight or height at a particular time point (n = 30, 2.5% of observations), data were imputed by taking the average of the weight/height data for the previous and subsequent time points, or using the last observed weight/height to estimate the age- and sex-specific weight/height according to the 2000 CDC growth charts.
Logistic regression models were constructed to examine potential predictors for being overweight or obese at two time points: (i) the end of treatment, and (ii) 5 years post-treatment. Outcome at each time point was specified as a binary variable: overweight/obese versus underweight/healthy weight. Overweight and obesity were combined as one group to increase statistical power. Potential predictors for being overweight/obese at the end of treatment were determined a priori and included age at diagnosis (<10 vs. ≥10 years old), gender, treatment risk category (standard vs. high risk), cranial radiation therapy (yes vs. no), weight status at diagnosis (overweight/obese vs. healthy weight/underweight), BMI z-score at diagnosis (continuous), increase in BMI z-score during induction, increase in BMI z-score during consolidation, and increase in BMI z-score during maintenance. For being overweight/ obese 5 years post-treatment, an overall increase in BMI z-score from diagnosis to end of treatment was also examined as a potential predictor. We also estimated repeated measures models to examine the predictors for the trajectory of BMI z-score (i) from diagnosis to end of treatment and (ii) from the end of treatment to 5 years post-treatment. Predictors included in the models were gender, age at diagnosis (continuous), treatment risk category (standard vs. high risk), months since diagnosis (continuous), BMI z-score at diagnosis (continuous), and weight status at diagnosis (overweight/ obese vs. healthy weight/underweight). However, separate models were built to evaluate the effect of BMI z-score at diagnosis and weight status at diagnosis to avoid collinearity. In addition, the model from the end to treatment to 5 years post-treatment also included change in BMI z-score during treatment (continuous). All statistical analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
The median age at diagnosis of the cohort was 4.0 years (range: 0–19). Of the 83 patients, 40 (48%) were males. The majority had precursor B-cell ALL (95%). Fifty-two (63%) were classified as standard risk and 31 (37%) were classified as high risk. Patients were treated primarily on Pediatric Oncology Group (POG) protocols (66%), followed by Dana–Farber Cancer Institute (DFCI) ALL Consortium protocols (25%), COG protocols (4%), and others (5%). When stratified by treatment protocol era, 58 (73%) patients were treated on 1990–1999 protocols, 13 (17%) were treated on 2000–2010 protocols, and 8 (10%) were treated on pre-1990 protocols (Table I). Among 83 patients with ALL, 13 (16%) received cranial radiation therapy (CRT).
TABLE I.
Characteristics of Pediatric ALL Patients (N = 83) at Tufts Medical Center, 1985–2010
| Characteristics | N (%) |
|---|---|
| Age at diagnosis, years, median (Q1, Q3) | 4.0 (2.0, 6.0) |
| Years since diagnosis, years, mean (SD) | 13.4 (6.9) |
| Gender | |
| Female | 43 (51.8) |
| Male | 40 (48.2) |
| Immunophenotype | |
| Pre B ALL | 79 (95.2) |
| T cell ALL | 4 (4.8) |
| Risk treatment category | |
| Standard risk | 52 (62.7) |
| High risk | 31 (37.4) |
| Cranial radiation therapy | |
| Yes | 13 (15.7) |
| No | 70 (84.3) |
| Treatment protocol era | |
| Pre-1990 | 8 (10.1) |
| 1990–1999 | 58 (73.4) |
| 2000–2010 | 13 (16.5) |
| Weight status at diagnosis | |
| Underweight (BMI percentile <5th) | 5 (6.0) |
| Healthy weight (BMI percentile = 5th–84.9th) | 61 (73.5) |
| Overweight (BMI percentile = 85th–94.9th) | 8 (9.6) |
| Obese (BMI percentile ≥95th) | 9 (10.8) |
Longitudinal Changes in Weight Status
At diagnosis, 20.5% were overweight (9.6%, n = 8) or obese (10.8%, n = 9). The mean BMI z-score was 0.2 (SD = 1.2), corresponding to a BMI of 58th percentile. However, BMI z-score at diagnosis was not significantly different from 0 (P = 0.20).
At the end of induction, the percentage of overweight/obese doubled (41.0%). Although it dropped during consolidation (25.3% at the end of consolidation), the percentage of overweight/obese increased again during the first 6 months of maintenance therapy (39.7%) and remained at approximately 40% thereafter: 38.2% were overweight/obese at the end of cancer treatment and 40.5% were overweight/obese 5 years post-treatment (Table II, Fig. 1). Among those who were not overweight/obese at diagnosis, 26.7% were overweight/obese at the end of treatment and 36.1% were overweight/obese 5 years post-treatment; among those who were overweight/obese at diagnosis, 81.3% remained overweight/ obese at the end of treatment and 66.7% remained overweight/ obese 5 years post-treatment.
TABLE II.
Changes in Percentage of Overweight or Obese and BMI z-Score of Pediatric ALL Patients During and After Treatment
| Diagnosis (N = 83) | End of induction (N = 78) | End of consolidation (N = 79) | 6 months into maintenance (N = 78) | 12 months into maintenance (N = 74) | 18 months into maintenance (N = 50) | End of treatment (N = 76) | 1 year post-treatment (N = 61) | 3 years post-treatment (N = 51) | 5 years post-treatment (N = 42) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of overweight or obese, N (%) | ||||||||||
| All | 17 (20.5) | 32 (41.0) | 20 (25.3) | 31 (39.7) | 31 (41.9) | 18 (36.0) | 29 (38.2) | 25 (41.0) | 22 (43.1) | 17 (40.5) |
| Females | 5 (11.6) | 12 (29.3) | 7 (17.5) | 14 (35.9) | 17 (44.7) | 8 (29.6) | 13 (33.3) | 14 (40.0) | 13 (43.3) | 9 (36.0) |
| Males | 12 (30.0) | 20 (54.1) | 13 (33.3) | 17 (43.6) | 14 (38.9) | 10 (43.5) | 16 (43.2) | 11 (42.3) | 9 (42.9) | 8 (47.1) |
| BMI z-score, mean (SD) | ||||||||||
| All | 0.2 (1.2) | 0.7 (1.2) | 0.5 (1.0) | 0.7 (0.9) | 0.8 (0.9) | 0.6 (1.0) | 0.8 (0.8) | 0.7 (1.0) | 0.8 (1.0) | 0.7 (0.9) |
| Females | −0.1 (1.1) | 0.4 (1.2) | 0.2 (1.1) | 0.6 (0.9) | 0.7 (0.9) | 0.5 (0.9) | 0.6 (0.8) | 0.5 (1.0) | 0.6 (0.9) | 0.7 (0.9) |
| Males | 0.4 (1.2) | 1.1 (1.1) | 0.8 (0.9) | 0.9 (1.0) | 0.9 (0.9) | 0.6 (1.1) | 0.9 (0.9) | 0.9 (0.9) | 1.2 (1.1) | 0.8 (1.0) |
Fig. 1.
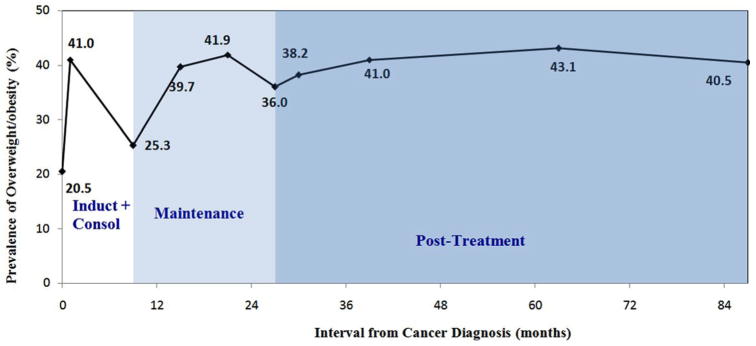
Prevalence of overweight/obesity during and after treatment in 83 survivors of pediatric ALL.
The longitudinal change in BMI z-score reflected a similar pattern (Table II). The mean BMI z-score increased significantly during induction (Δ = 0.5, P <0.0001). At the end of induction, the mean BMI z-score was 0.7 (SD = 1.2), and was significantly greater than 0 (P <0.0001). After a reduction during consolidation, BMI z-score increased again during the first 6 months of maintenance therapy (Δ = 0.2, P <0.01). At 6 months post-maintenance, the mean BMI z-score was 0.7 (SD = 0.9) and was also significantly greater from 0 (P <0.0001). The BMI z-score did not significantly change over the remainder of maintenance or at 5 years post-treatment (BMI z-score = 0.8 at the end of treatment, corresponding to the 79th BMI percentile; BMI z-score = 0.7 5-year post-treatment, corresponding to the 76th BMI percentile).
Predictors for Overweight/Obesity at the End of Treatment and 5 Years Post-Treatment
Weight status at diagnosis and BMI z-score at diagnosis were both significant predictors for being overweight/obese at the end of treatment. Patients who were overweight/obese at diagnosis were 11.9 times more likely to be overweight/obese at the end of treatment than those who were underweight or had healthy weight at diagnosis (OR = 11.9, 95% CI: 3.0–47.4). A 1-unit increase in BMI z-score at diagnosis was associated with nearly threefold increased risk of being overweight/obese at the end of treatment (OR = 2.9, 95% CI: 1.6–5.1) (Table III).
TABLE III.
Predictors of Being Overweight or Obese in Pediatric ALL Patients at the End of Treatment and 5 Years Post-Treatment
| Overweight/obese at the end of treatment
|
Overweight/obese 5 years post-treatment
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Yes (N = 29) | No (N = 47) | OR (95%CI)a | P | Yes (N = 17) | No (N = 25) | OR (95%CI)a | P | |
| N (%) | N (%) | |||||||
| Sex | ||||||||
| Female | 13 (44.8%) | 26 (55.3%) | 1.0 | 9 (52.9%) | 16 (64.0%) | 1.0 | ||
| Male | 16 (55.2%) | 21 (44.7%) | 1.5 (0.6–3.9) | 0.38 | 8 (47.1%) | 9 (36.0%) | 1.6 (0.5–5.4) | 0.48 |
| Cranial radiation therapy | ||||||||
| No | 23 (79.3%) | 41 (87.2%) | 1.0 | 17 (100.0%) | 22 (88.0%) | 1.0 | ||
| Yes | 6 (20.7%) | 6 (12.8%) | 1.8 (0.5–6.2) | 0.36 | 0 (0.0%) | 3 (12.0%) | N/A | |
| Age at diagnosis | ||||||||
| <10 years | 24 (82.8%) | 38 (80.9%) | 1.0 | 15 (88.2%) | 21 (84.0%) | 1.0 | ||
| ≥10 years | 5 (17.2%) | 9 (19.2%) | 0.9 (0.3–2.9) | 0.84 | 2 (11.8%) | 4 (16.0%) | 0.7 (0.1–4.3) | 0.70 |
| Treatment risk category | ||||||||
| Standard risk | 21 (72.4%) | 28 (59.6%) | 1.0 | 12 (70.6%) | 16 (64.0%) | 1.0 | ||
| High risk | 8 (27.6%) | 19 (40.4%) | 0.6 (0.2–1.5) | 0.26 | 5 (29.4%) | 9 (36.0%) | 0.7 (0.2–2.8) | 0.66 |
| Weight status at diagnosis | ||||||||
| Healthy/underweight | 16 (55.2%) | 44 (93.6%) | 1.0 | 13 (76.5%) | 23 (92.0%) | 1.0 | ||
| Overweight/obese | 13 (44.8%) | 3 (6.4%) | 11.9 (3.0–47.4) | 0.0004 | 4 (23.5%) | 2 (8.0%) | 3.5 (0.6–22.0) | 0.18 |
| Mean (SD) | Mean (SD) | |||||||
| BMI z-score at diagnosis | 0.9 (1.2) | −0.2 (1.0) | 2.9 (1.6–5.2) | 0.0004 | 0.3 (0.9) | −0.1 (0.8) | 1.9 (0.8–4.2) | 0.13 |
| Change in BMI z-score during inductionb | 0.6 (0.8) | 0.4 (1.1) | 3.1 (1.4–6.8) | 0.006 | 0.5 (0.8) | 0.5 (1.0) | 1.3 (0.6–2.7) | 0.57 |
| Change in BMI z-score during consolidationb | −0.2 (0.7) | −0.1 (1.0) | 1.3 (0.7–2.4) | 0.46 | 0.1 (0.8) | −0.2 (0.8) | 2.6 (0.9–7.5) | 0.07 |
| Change in BMI z-score during maintenanceb | 0.4 (0.6) | 0.1 (0.7) | 2.7 (1.1–6.9) | 0.03 | 0.3 (0.7) | 0.3 (0.7) | 1.1 (0.4–2.9) | 0.79 |
| Change in BMI z-score during treatmentb | — | — | — | — | 0.8 (0.9) | 0.6 (1.0) | 3.8 (1.1–12.5) | 0.03 |
Univariate analyses;
Adjusted for BMI z-score at diagnosis.
After controlling for BMI z-score at diagnosis, an increase in BMI z-score during induction (OR = 3.1, 95% CI: 1.4–6.8) and an increase in BMI z-score during maintenance (OR = 2.7, 95% CI: 1.1–6.9) were each associated with about a threefold increased risk of being overweight/obese at the end of treatment (Table III).
At 5 years post-treatment, BMI z-score at diagnosis and an increase in BMI z-score during each phase of cancer treatment (induction, consolidation, and maintenance) were not significantly associated with overweight/obesity (Table III). However, an overall increase in BMI z-score from diagnosis to the end of treatment was associated with a more than threefold increased risk of being overweight/obese 5 years post-treatment (OR = 3.8, 95% CI: 1.1–12.5).
Predictors for Changes in BMI z-Score During and After Cancer Treatment
After adjusting for age at diagnosis, gender, receipt of CRT, risk status and months since diagnosis, being overweight/obese at diagnosis (β = 1.02, 95% CI: 0.64, 1.41) and a high BMI z-score at diagnosis (β = 0.46, 95% CI: 0.34, 0.59) were both significantly associated with an increase in BMI z-score from diagnosis to the end of treatment (Table IV). Being overweight/obese at diagnosis (β = 0.80, 95% CI: 0.10, 1.50) and a high BMI z-score at diagnosis (β = 0.91, 95% CI: 0.69, 1.12) were also associated with an increase in BMI z-score from the end of treatment to 5 years post-treatment. In addition, an increase in BMI z-score from diagnosis to the end of treatment was significantly associated with BMI changes post-treatment (β = 0.82, 95% CI: 0.59, 1.04). High risk ALL was negatively associated with change in BMI z-score during treatment (β = −0.37, 95% CI: −0.69, −0.06) but not post-treatment (β = −0.002, 95% CI: −0.36, 0.35). Male gender, receiving CRT and age at diagnosis were not significant predictors of BMI z-score in survivors of pediatric ALL during or after treatment.
TABLE IV.
Repeated Measures Models for Predictors of BMI z-Score During and After Treatment in Pediatric ALL Survivors
| From diagnosis to end of treatment
|
From end of treatment to 5 years post-treatment
|
|||||
|---|---|---|---|---|---|---|
| β | 95% CIa | P | β | 95% CIb | P | |
| Male gender | 0.17 | −0.11, 0.46 | 0.22 | 0.21 | −0.09, 0.52 | 0.17 |
| Receiving CRT | 0.16 | −0.24, 0.56 | 0.43 | −0.42 | −0.94, 0.10 | 0.11 |
| Age at diagnosis | −0.01 | −0.04, 0.03 | 0.67 | −0.004 | −0.04, 0.03 | 0.82 |
| High risk ALL | −0.37 | −0.69, −0.06 | 0.02 | −0.002 | −0.36, 0.35 | 0.99 |
| Months since diagnosis | −0.004 | −0.01, 0.005 | 0.38 | 0.001 | −0.003, 0.005 | 0.56 |
| BMI z-score at diagnosis | 0.46 | 0.34, 0.59 | <0.0001 | 0.91 | 0.69, 1.12 | <0.001 |
| Overweight/obese at diagnosis | 1.02 | 0.64, 1.41 | <0.0001 | 0.80 | 0.10, 1.50 | 0.03 |
| Change in BMI z-score during treatment | — | 0.82 | 0.59, 1.04 | <0.001 | ||
Adjusted for gender, receiving CRT, age at diagnosis (continuous), treatment risk category (high vs. standard risk ALL), months since diagnosis (continuous), and BMI z-score at diagnosis (continuous). Weight status at diagnosis (overweight/obese vs. healthy weight/underweight) was evaluated in separate models with all predictors except for BMI z-score to avoid collinearity;
β and 95%CI were additionally adjusted for change in BMI z-score during treatment (continuous).
DISCUSSION
We evaluated longitudinal changes in obesity rate and BMI z-scores in survivors of pediatric ALL during and after cancer treatment. Our results revealed that patients with pediatric ALL were at risk becoming overweight or obese early in treatment, and increases in weight were maintained throughout treatment and beyond. Being overweight or obese at the time of diagnosis and excessive weight gain during treatment were significant predictors of being overweight or obese after cancer treatment.
Several studies have demonstrated that childhood cancer survivors are more overweight or obese than their peers [8,16–22]. However, it remains unclear at which time point childhood cancer survivors gained excessive weight and whether obesity persists after treatment. Our study is among the few studies that assessed change in BMI and obesity in several key points from diagnosis until several years after completion of treatment. We found a rapid increase in obesity rate from 20% at diagnosis to 40% at the end of treatment. In particular, a rapid weight gain was observed during induction and the first 6 months of maintenance therapy. This is consistent with findings from two previous studies evaluating the trajectory of changes in BMI during various phases of treatment: the study by Esbenshade et al. [13] in 183 patients with pediatric ALL diagnosed during 2000–2008 reported a rapid increase in BMI z-score during induction and the first 22 months of maintenance therapy; the study by Withycombe et al. [8] in 1,638 patients with high risk ALL from the COG diagnosed between 1996 and 2002 also found a substantial increase in weight gain during early maintenance cycles. These findings suggest that the early cancer treatment period represents a sensitive window for excessive weight gain in childhood cancer survivors.
Importantly, we found survivors did not return to their pre-treatment weight classification and were consistently more overweight or obese than their peers several years beyond treatment. The obesity rate ranged between 38% and 43% from the end of treatment to 5 years post-treatment. This overweight/ obesity prevalence was similar to the level reported in several other studies, ranging between 40% and 50% [7,17,18,23–26], and is higher than the national average (33–34%) in children age 6–19 years in the National Health and Nutrition Examination Survey (NHANES) 2003–2006 [27]. These findings suggest that excessive weight gain that occurred early in treatment is unlikely to be reversed after completion of cancer treatment. Studies are warranted to identify underlying mechanisms for excessive weight gain during treatment, and lifestyle interventions targeting weight control early during treatment are needed to prevent obesity in this at-risk population.
Research has demonstrated that cancer treatments, both radiotherapy and chemotherapy, can cause damage to the hypothalamus [28]. The subsequent hypothalamic-pituitary dysfunction may change energy regulation and thereby promote obesity. Possible mechanisms may include growth hormone insufficiency that leads to altered substrate oxidation and fat deposition, abnormal appetite control that leads to food craving or increased energy intake, and altered resting metabolic rate that leads to reduced energy expenditure. This impact, although occurring primarily during active cancer treatment, may last beyond cancer treatment and become permanent. There are some suggestions that this impact may be more profound in survivors who received CRT or were diagnosed at a young age. Female survivors of pediatric ALL have also been found to have a higher prevalence of hyperleptinemia that male survivors [29,30], possibly due to the continuous increase in leptin and body fatness that occurs during puberty in girls but not in boys. Genetic variations in leptin receptors have also been associated with obesity in female survivors [31]. Nevertheless, evidence remains inconsistent for the potential effect of CRT, gender and age at diagnosis on obesity in survivors of pediatric ALL. Some earlier studies reported a higher rate of obesity in association with CRT [7,21,32–34] while more recent studies that typically include survivors treated with no or less intense CRT [8,24] do not support such findings. Some studies have suggested female survivors are at a higher risk of becoming overweight/obese than male survivors [7,8,17,30,34,35], whereas others did not support a gender difference [21,22,36] or reported the opposite disparity [18,37]. A young age at diagnosis has been associated with a high rate of obesity in some [7,8,24] but not all studies [21]. In the current study, we did not find that the trajectory of changes in BMI z-score differed significantly by receipt of CRT, gender and age at diagnosis where about 15% of the patients were treated with CRT, We did find that while patients with high risk ALL were subject to less weight gain than patients with standard risk ALL during treatment, their patterns of weight after treatment did not differ from the standard risk group. More studies, preferably with a large sample size, are needed to further elucidate how ALL treatment, in particular induction and early maintenance therapy, affect hypothalamic-pituitary function and subsequent energy balance. This not only helps us understand the role of energy balance in obesity development in childhood cancer survivors, but also may provide clues for other groups of children who suffer from obesity.
Our study highlights that being overweight/obese at diagnosis or having a high BMI z-score at diagnosis was strongly associated with being overweight/obese at the end of treatment. This is consistent with findings from two previous studies that examined weight status at diagnosis as a potential predictor for post-treatment obesity. The study by Esbenshade et al. [13] reported that a high BMI z-score was strongly associated with elevated BMI z-score throughout maintenance. In 456 children with pediatric ALL and lymphoma followed beyond treatment completion, Razzouk et al. [24] found being overweight/obese at diagnosis was associated with a ninefold increased risk of being overweight/obese when children reached adult height. Beyond confirming that weight status at diagnosis affects obesity after treatment, we also found weight gain during ALL treatment significantly predicted overweight/obesity after treatment completion. Rapid weight gain during treatment might be due to the effect of glucocorticoids, which are known to increase energy intake and accumulate body fat. Cancer treatment may also cause fatigue and lead to a sedentary lifestyle in survivors. Another unexplored factor is the adoption of parenting style that promotes a sedentary lifestyle and more permissive diet after a child is diagnosed with cancer. These psychosocial factors, along with the secular trends of rising childhood obesity, may have a substantial impact on survivors’ behaviors related to energy balance, and emphasize the importance of promoting good nutrition and physical activity, not only in survivors but also in their families and communities at large.
We acknowledge the limitations of our study. Our ALL survivors were diagnosed over a wide range of calendar years during which treatment for pediatric ALL went through substantial changes. However, we analyzed whether treatment era affected patterns of weight gain and did not find a significant impact. Second, we had to rely on information documented in administrative databases on race/ethnicity, rather than self-identification. Nevertheless, the majority of our survivors are likely to be non-Hispanic whites; and our findings may not be generalized to survivors of other race/ethnicity. Third, our study did not prospectively collect data on diet, physical activity and parenting style, which prevents a direct evaluation of their effects on survivors’ weight gain during and after treatment. Despite these limitations, our study is among the few studies that assessed change in BMI and obesity in several keys points from diagnosis until several years after completion of treatment. Our findings convey important messages in the clinical care for childhood cancer survivors in obesity prevention.
In conclusion, we found that patients with pediatric ALL are at risk becoming overweight or obese early in treatment, and that increase in weight is likely to be maintained throughout treatment and beyond. Interventions are needed to address weight control early during treatment, particularly for patients who are overweight or obese at diagnosis and for those who experience substantial weight gain during treatment.
Acknowledgments
This research is supported by a Catalyst Grant, awarded by the Tufts Clinical and Translational Science Award, funded by the National Center for Research Resources Grant Number UL1 RR025752, National Center for Advancing Translational Sciences, the National Institutes of Health Grant Number UL1 TR000073, and the Boston Nutrition Obesity Research Center Grant Number P30DK46200. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: Nothing to report
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 3.Huang TT, Hudson MM, Stokes DC, et al. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest. 2011;140:881–901. doi: 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased risk of cardiovascular disease? Pediatr Blood Cancer. 2008;50:462–467. doi: 10.1002/pbc.21410. discussion 468. [DOI] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 8.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53:1249–1254. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Cronk C, Crocker AC, Pueschel SM, et al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81:102–110. [PubMed] [Google Scholar]
- 11.Children’s Oncology Group. [Accessed April 25, 2011];Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. 2008 www.childrensoncologygroup.org.
- 12.Centers for Disease Control and Prevention. [Accessed January 27, 2012];CDC growth charts. 2000 http://www.cdc.gov/growthcharts/zscore.htm.
- 13.Esbenshade AJ, Simmons JH, Koyama T, et al. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:372–378. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 15.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 16.Collins L, Zarzabal LA, Nayiager T, et al. Growth in children with acute lymphoblastic leukemia during treatment. J Pediatr Hematol Oncol. 2010;32:e304–e307. doi: 10.1097/MPH.0b013e3181ece2bb. [DOI] [PubMed] [Google Scholar]
- 17.Love E, Schneiderman JE, Stephens D, et al. A cross-sectional study of overweight in pediatric survivors of acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2011;57:1204–1209. doi: 10.1002/pbc.23010. [DOI] [PubMed] [Google Scholar]
- 18.Nathan PC, Jovcevska V, Ness KK, et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J Pediatr. 2006;149:518–525. doi: 10.1016/j.jpeds.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Odame I, Reilly JJ, Gibson BE, et al. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71:147–149. doi: 10.1136/adc.71.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly JJ, Ventham JC, Newell J, et al. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24:1537–1541. doi: 10.1038/sj.ijo.0801403. [DOI] [PubMed] [Google Scholar]
- 21.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: Role of cranial irradiation. Med Pediatr Oncol. 2000;35:91–95. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, et al. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38:86–90. doi: 10.1203/00006450-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Kourti M, Tragiannidis A, Makedou A, et al. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol. 2005;27:499–501. doi: 10.1097/01.mph.0000181428.63552.e9. [DOI] [PubMed] [Google Scholar]
- 24.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 25.Shaw MP, Bath LE, Duff J, et al. Obesity in leukemia survivors: The familial contribution. Pediatr Hematol Oncol. 2000;17:231–237. doi: 10.1080/088800100276406. [DOI] [PubMed] [Google Scholar]
- 26.Tylavsky FA, Smith K, Surprise H, et al. Nutritional intake of long-term survivors of childhood acute lymphoblastic leukemia: Evidence for bone health interventional opportunities. Pediatr Blood Cancer. 2010;55:1362–1369. doi: 10.1002/pbc.22737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer CA, Gietema JA, Kamps WA, et al. Changes in body composition after childhood cancer treatment: Impact on future health status–a review. Crit Rev Oncol Hematol. 2007;63:32–46. doi: 10.1016/j.critrevonc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Karaman S, Ercan O, Yildiz I, et al. Late effects of childhood ALL treatment on body mass index and serum leptin levels. J Pediatr Endocrinol Metab. 2010;23:669–674. doi: 10.1515/jpem.2010.23.7.669. [DOI] [PubMed] [Google Scholar]
- 30.Kohler JA, Moon RJ, Wright S, et al. Increased adiposity and altered adipocyte function in female survivors of childhood acute lymphoblastic leukaemia treated without cranial radiation. Horm Res Paediatr. 2011;75:433–440. doi: 10.1159/000324412. [DOI] [PubMed] [Google Scholar]
- 31.Skoczen S, Tomasik PJ, Bik-Multanowski M, et al. Plasma levels of leptin, soluble leptin receptor, polymorphisms of leptin gene -18G> leptin receptor genes K109R, Q223R, in survivors of childhood acute lymphoblastic leukemia. J Exp Clin Cancer Res. 2011;30:64. doi: 10.1186/1756-9966-30-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer EI, Reuter M, Dopfer RE, et al. Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Horm Res. 2000;53:193–199. doi: 10.1159/000023566. [DOI] [PubMed] [Google Scholar]
- 33.Papadia C, Naves LA, Costa SS, et al. Incidence of obesity does not appear to be increased after treatment of acute lymphoblastic leukemia in Brazilian children: Role of leptin, insulin, and IGF-1. Horm Res. 2007;68:164–170. doi: 10.1159/000100781. [DOI] [PubMed] [Google Scholar]
- 34.Veringa SJ, van Dulmen-den Broeder E, Kaspers GJ, et al. Blood pressure and body composition in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58:278–282. doi: 10.1002/pbc.23251. [DOI] [PubMed] [Google Scholar]
- 35.Warner JT, Evans WD, Webb DK, et al. Body composition of long-term survivors of acute lymphoblastic leukaemia. Med Pediatr Oncol. 2002;38:165–172. doi: 10.1002/mpo.1304. [DOI] [PubMed] [Google Scholar]
- 36.van der Sluis IM, van den Heuvel-Eibrink MM, Hahlen K, et al. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35:415–420. doi: 10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]


