Exposure to childhood abuse or neglect is associated with abnormal hippocampal function in adulthood. The molecular mechanisms by which early adversity modifies hippocampal function in adulthood are currently poorly understood in humans. However, similar findings in rodents and nonhuman primates suggest that animal models may provide important insights into this issue (1). One of the main challenges of studying hippocampal tissue in animal models has been the cellular heterogeneity of the hippocampus, where different cell types (e.g., stem cells, pyramidal cells, granule cells, interneurons, astrocytes, microglia, and oligodendrocytes) respond differently to stress, yet they are closely packed and interact with one another. The challenge has been to figure out how to harvest and study the effects of early-life stress in a homogeneous cell population in a manner that recapitulates the cellular response in the intact hippocampus. In this issue of Biological Psychiatry, Boku et al. (2) used an in vitro system to show that exposure to stress early in life restricts the capacity of adult progenitor cells to differentiate into neurons. This is an important first step in studying the effects of early adversity using a homogeneous cell population harvested from the hippocampus, and it demonstrates the advantages and the pitfalls of this reductionistic approach.
The Findings
Previous work has shown that daily maternal separation (MS) of rat pups during the first weeks of life is associated with impaired hippocampal-dependent spatial memory in adulthood (3,4). Performance in this task requires the generation of new neurons in the adult hippocampus, presumably due to the enhanced capacity of these young neurons to respond and encode novel information (5). These new neurons are formed from stem cells that are localized at the subgranular zone of the dentate gyrus. These stem cells are capable of dividing asymmetrically to regenerate one stem cell and one progenitor cell that can differentiate into either a neuron or a glia cell. Interestingly, work from several groups has shown that exposure to MS inhibits the proliferation of progenitor cells in the adult hippocampus, raising the possibility that a reduced level of neurogenesis is responsible for the impaired hippocampal-dependent function seen in these rats (3,4,6,7). To better characterize this issue, Buko et al. (2) exposed rat pups to MS or control condition. Pups were weaned and allowed to grow until postnatal day 56. At this age, considered late adolescence/young adulthood, the rats were sacrificed and progenitor cells were harvested from the hippocampus and characterized in vitro. MS did not affect the capacity of progenitor cells to proliferate or undergo apoptosis. However, when induced to differentiate, the progenitor cells harvested from MS rats produced fewer neurons. These findings suggest that exposure to MS restricts the capacity of progenitor cells to become neurons in early adulthood. Since the ability of stem cells to differentiate into different cell types (e.g., neurons vs. glia) depends on epigenetic modifications, most notably DNA methylation, the authors tested whether inhibiting DNA methylation could restore normal differentiation to progenitor cells isolated from the hippocampus of MS rats. Indeed, inhibition of DNA methylation restored normal differentiation in MS progenitor cells. DNA methylation is maintained in cells by three DNA methyltransferase enzymes (DNA [cytosine-5-]-methyltransferase [DNMT]1, DNMT3a, and DNMT3b). These enzymes add methyl groups to promoter elements of genes, a process that marks these genes for reduced expression. Exposure to MS increased expression of DNMT1, but not DNMT3a or DNMT3b, suggesting that overexpression of DNMT1 increases DNA methylation of genes that are required for normal neuronal differentiation in adult progenitor cells. Since retinoic acid receptors (RARs) have been shown to play an important role in neuronal differentiation in vitro and in vivo (2), the authors hypothesized that MS increases DNA methylation at promoter elements that regulate expression of one of the three known RARs. They found that expression of RARα was reduced in progenitor cells harvested from MS rats in a manner that was associated with increased DNA methylation at the RARα promoter. Importantly, inhibiting DNA methylation restored normal RARα levels in progenitor cells harvested from MS rats. Together, these findings suggest that exposure to MS increases expression of DNMT1 leading to higher DNA methylation at the RARα promoter. Increased DNA methylation at this regulatory element decreases RARα expression and restricts the capacity of adult progenitor cells to differentiate into neurons (Figure 1).
Figure 1.
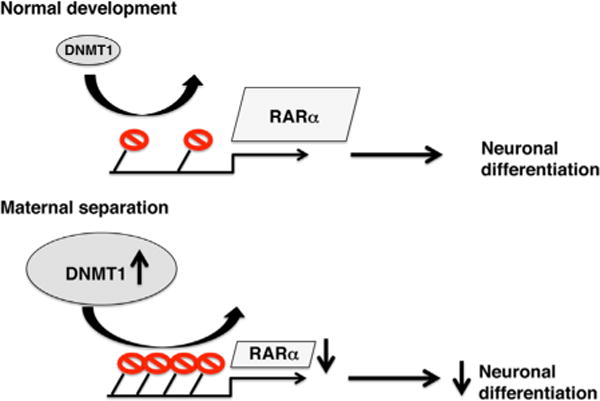
Top: under normal developmental conditions, DNMT1 levels in adult progenitor cells are low, leading to sparse DNA methylation (shown in red) at the promoter of RARα gene. Low levels of DNA methylation allow for high RARα gene expression, which is necessary for normal levels of differentiation into neurons. Bottom: exposure to maternal separation during the first 2 weeks of life increases DNMT1 levels in adult progenitor cells, leading to increased DNA methylation, reduced RARα expression, and decreased capacity to differentiate into neurons in vitro. DNMT1, DNA (cytosine-5-)-methyltransferase 1; RAR, retinoic acid receptor.
This is the first example in which the effects of MS are examined in vitro using a homogenous cell population harvested from the hippocampus. An important advantage of this reductionistic approach is that it allows the identification of cell-specific responses to MS that would otherwise be masked when looking at the heterogeneous cellular makeup that characterizes the intact hippocampus. In addition, this in vitro approach allows the use of pharmacologic agents (i.e., 5-aza-Dc) that are toxic in vivo. The finding that exposure to MS causes long-term changes in the intrinsic properties of progenitor cells demonstrates the potential of this approach to identify novel mechanisms by which stress early in life modifies hippocampal function in adulthood.
The observation that overexpression of DNMT1 restricts the capacity of progenitor cells to differentiate into neurons identifies a novel function for DNMT1. DNMT1 is the maintenance DNA methyltransferase, which is responsible for adding methyl groups to hemimethylated-DNA complex formed after DNA replication. This activity is essential for propagating and maintaining transcriptional instructions in daughter cells. DNMT1 has been shown to regulate many developmental processes and cellular functions. For example, deleting DNMT1 in stem cells leads to precocious gliogenesis during embryogenesis and reduces neuronal survival and dendritic arborization during the early postnatal period (8). Elevated levels of DNMT1 are associated with abnormal function of gamma-aminobutyric acidergic interneurons in the prefrontal cortex of individuals with schizophrenia and in mice exposed to prenatal stress (9). Together, these findings demonstrate that DNMT1 plays a critical role in maintaining the normal function of many cell types.
The Challenge and Future Questions
One of the main shortcomings of this work is the absence of confirmatory work showing that similar outcomes are present in the intact hippocampus. This is particularly important given the conflicting results reported in the literature regarding the effects of MS on adult neurogenesis in the hippocampus (3,4,6,10). For example, of the four available reports that examined the effects of MS on hippocampal neurogenesis in adult rats, two reported decreased proliferation of progenitor cells (3,6). None have shown decreased neurogenesis in young adult rats, though one report did find decreased neurogenesis in middle-aged rats (4). This discrepancy might be due to differences in experimental procedures and/or compensatory mechanisms that might occur in vivo. For example, MS may increase levels of retinoic acid or other neurotrophic factors (4) in the hippocampus in a way that compensates for the decrease in RARα in progenitor cells. Additionally, in this article (2), progenitor cells harvested from the hippocampus were incubated in vitro for 24 hours before assessing levels of DNMT1 and RARα. This incubation in vitro may be responsible for the elevated DNMT1 levels seen in progenitor cells harvested from MS rats. Future work will need to determine whether levels of DNMT1 are similarly elevated in progenitor cells that are acutely isolated from the hippocampus of MS rats. Even if some of the findings are due to specific in vitro conditions, these can still be informative for elucidating differences in intrinsic properties of these progenitor cells in the intact hippocampus.
The findings reported here raise several key follow-up questions. For example, what is the molecular mechanism by which MS modifies levels of DNMT1 in adult progenitor cells? What other cellular targets, besides RARα, are affected by increased DNMT1 levels in progenitor cells? Are high levels of DNMT1 also present in differentiated daughter cells (neurons or glia cells) in vitro? If yes, how do these high levels of DNMT1 affect the function of these differentiated cells?
The MS procedure takes place during a period in which large numbers of progenitor cells proliferate and differentiate to form the dentate gyrus. The in vivo data are confusing, with one report indicating that MS increases proliferation (4) and another report finding that MS decreases (7) proliferation of progenitor cells during the first weeks of life. Characterizing these issues in vitro using progenitor cells harvested from 14-day-old pups exposed to MS may shed important light on these confusing results. Moreover, abnormal neurogenesis during the perinatal period is likely to have far more deleterious effects on hippocampal-dependent function compared with abnormal adult neurogenesis. The reason for this is that many more progenitor cells proliferate and differentiate into granule cells during the first 2 weeks of life compared with adulthood.
In conclusion, the study by Boku et al. (2) identifies a novel mechanism by which stress early in life modifies the capacity of adult progenitor cells to differentiate into neurons in vitro. As such, it provides an important example of the advantages and the challenges of studying the effects of early adversity on a defined population of cells harvested from the hippocampus.
Acknowledgments
This work was supported by National Institute of Mental Health R21MH098181, National Institute of Mental Health R01MH100078, and DANA Foundation Program in Brain and Immuno-Imaging 2011.
I thank Dr. Evelyn Cumberbatch for helpful comments on the manuscript.
Footnotes
Disclosures
The author reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Korosi A, Naninck EF, Oomen CA, Schouten M, Krugers H, Fitzsimons C, Lucassen PJ. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res. 2012;227:400–409. doi: 10.1016/j.bbr.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 2.Boku S, Toda H, Nakagawa S, Kato A, Inoue T, Koyama T, et al. Neonatal maternal separation alters the capacity of adult neural precursor cells to differentiate into neurons via methylation of retinoic acid receptor gene promoter. Biol Psychiatry. 2015;77:335–344. doi: 10.1016/j.biopsych.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: Implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- 4.Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, et al. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry. 2013;73:658–666. doi: 10.1016/j.biopsych.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 7.Lajud N, Roque A, Cajero M, Gutierrez-Ospina G, Torner L. Periodic maternal separation decreases hippocampal neurogenesis without affecting basal corticosterone during the stress hyporesponsive period, but alters HPA axis and coping behavior in adulthood. Psychoneuroendocrinology. 2012;37:410–420. doi: 10.1016/j.psyneuen.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, et al. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, et al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–194. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wortwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res. 2005;79:772–778. doi: 10.1002/jnr.20418. [DOI] [PubMed] [Google Scholar]


