Abstract
The Rapid Opioid Dependence Screen (RODS) is an 8-item measure of opioid dependence designed for quick, targeted screening in clinical and research settings. Based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, criteria, the RODS has an average administration of less than 2 minutes and can easily be administered as a stand-alone instrument or as part of a comprehensive interview. This study reports on the initial validation of the RODS among a sample of 97 newly incarcerated, HIV-positive individuals. Using the Mini International Neuropsychiatric Interview as the primary measure of opioid dependence, the RODS showed good-to-strong sensitivity (.97), specificity (.76), positive predictive value (.69), and negative predictive value (.98), while concordance analysis revealed moderate diagnostic agreement (κ = .67). Psychometric properties revealed strong internal consistency (α = .92) and inter-item correlations (.66 to .87).
Keywords: opioids, opiates, substance dependence, screen, measurement, instrument
Introduction
Illicit opioid abuse is increasing and remains a global health threat, with misuse reported in 148 countries (Mathers et al., 2008). Global estimates suggest there are more than 15 million active users of illicit opioids, with a global prevalence of 0.3% to 0.5% among persons 15 to 64 years of age (United Nations Office on Drugs and Crime [UNODC], 2010). Heroin and morphine account for more than half of all illicit opioid use, a proportion that has remained largely unchanged over the past 3 years (UNODC, 2010). Despite this stability, there have been alarming increases in the nonmedical use of synthetic prescription opioids, such as hydrocodone and oxycodone, particularly in North America and Western Europe (Maxwell, 2011; Substance Abuse and Mental Health Services Administration [SAMHSA], 2009a; UNODC, 2010). In the United States, while illicit opioid abuse continues to rise, it is increasingly fueled by illicit use of prescription synthetic opioids (Maxwell, 2011). Between 1997 and 2007, substance abuse treatment admissions for prescription opioids in the United States increased by more than 450%, representing only 6% of all opioid admissions in 1997 but more than 27% of those in 2007 (UNODC, 2010). Likewise, a recent survey found that 2.1% (5.2 million people) of individuals 12 years of age or older in the United States reported having used nonmedical prescription painkillers in the past month (SAMHSA, 2009b).
Syndemic with the crisis of opioid dependence is a high rate of HIV and viral hepatitis from the associated injection practices, particularly among heroin users. The overall prevalence of HIV among people who inject drugs is estimated at 18% but varies greatly across countries (Mathers et al., 2008). Not surprisingly, illicit opioid use is associated with a 6- to 20-fold increase in mortality due to increased risks of overdose, suicide, and comorbid HIV infection (SAMHSA, 2008; UNODC, 2010; World Health Organization [WHO], 2009). In most countries, opioids are responsible for the majority of drug-related deaths (UNODC, 2010).
Pharmacological Treatments for Opioid Dependence
Fortunately, a number of effective and evidence-based medication-assisted treatments (MATs) are available for the treatment of opioid dependence (Altice, Kamarulzaman, Soriano, Schechter, & Friedland, 2010). Methadone maintenance treatment (MMT), an effective treatment available in the United States since 1965, prevents relapse to opioids, reduces criminal activity and criminal justice involvement, lowers HIV transmission risk, and improves retention to health care (Basu, Smith-Rohrberg, Bruce, & Altice, 2006; Centers for Disease Control and Prevention, 2002; Mattick, Breen, Kimber, & Davoli, 2009). Buprenorphine, a partial opioid agonist and partial antagonist, is another effective treatment for opioid dependence. Like MMT, buprenorphine maintenance treatment reduces relapse to opioid use and the risk of HIV transmission but has a safer profile and is less likely to be abused (Altice et al., 2006). Lastly, the complete opioid antagonist, naltrexone, is another Food and Drug Administration (FDA)-approved treatment for prevention of relapse to opioid use. The extended-release injectable form (Vivitrol®) administered once monthly was developed to overcome adherence issues noted with oral naltrexone (ReVia®) and was found to be effective in preventing relapse to opioid use. It was FDA approved in 2010 for treatment of opioid dependence (Altice et al., 2010; Krupitsky et al., 2011) and was FDA approved in 2006 for treatment of alcohol dependence (Altice et al., 2010).
Although in 2005 the WHO added methadone and buprenorphine to its Model List of Essential Medicines, access to these medications remains severely limited due to enforced bans in multiple countries around the world (Wolfe, Carrieri, & Shepard, 2010; Wolfe & Cohen, 2010). These restrictions persist despite numerous studies that show that for every dollar spent on effective drug treatment programs, between three and seven dollars are saved in other health care costs (Bradley, French, & Rachal, 1994; Dunlap & French, 1998; French, Bradley, Calingaert, Dennis, & Karuntzos, 1994; French, Galinis, Dunlap, Zarkin, & Rachal, 1994; Gerstein et al., 1994; Rydell & Everingham, 1994; UNODC & WHO, 2008).
Validated Screening Instruments for Diagnosing Opioid Dependence
To effectively treat and manage an individual’s dependence on opioids, one must first have a valid and reliable diagnostic instrument for identifying the affected population. Although a number of such tools are available (see Table 1), many are cumbersome or time consuming, require extensive training and certification to administer, do not have rapid scoring that is immediately available, or are clinician-rated only. Moreover, most screening instruments are subcomponents of larger assessments. Some are also deficient in that they do not recognize that opioid dependence is a chronic, relapsing disease and most screening instruments exclude those who have not used opioids in the past 12 months (e.g., those in detention or in recovery). Among the most widely used tools are the Structured Clinical Interview for DSM Disorders (SCID; Peters, Greenbaum, Edens, Carter, & Ortiz, 1998), Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), Composite International Diagnostic Interview (Robins et al., 1988), Composite International Diagnostic Interview Substance Abuse Module (Cottler, Robins, & Helzer, 1989), Schedule for Clinical Assessment in Neuropsychiatry (Wing et al., 1990), Severity of Opiate Dependence Questionnaire (Gossop et al., 1995), and the Alcohol Use Disorder and Associated Disabilities Interview Schedule–Alcohol/Drug Revised (Chatterji et al., 1997; Grant et al., 2003; Grant, Harford, Dawson, Chou, & Pickering, 1995).
Table 1.
Comparison of Screening and Diagnostic Tools for Opioid Dependence and Abuse.
| Instrument (Author, Year) | Type of Measure | Content | Validity | Reliability | Administration Time |
|---|---|---|---|---|---|
| MINI (Sheehan et al., 1998) |
|
Based on DSM-IV criteria; validated against the SCID-P | MINI-CR against SCID
MINI-PR against SCID
|
MINI·CR inter-rater reliability for drug abuse and dependence: κ ≥ .88 MINI-CR test– retest reliability for drug abuse and dependence: κ ≥ .89 |
15 to 21 Minutes on average |
| SCID (Spitzer et al., 1992) |
|
Based on DSM-IV criteria | Gold standard instrument | Cronbach’s α = .98 (for opiates) Inter-rater reliability for drug dependence: κ = .95 |
30 Minutes on average |
| CIDI-2 (also known as Diagnostic Interview Schedule) (Robins et al., 1988) |
|
Based on DSM-III/IV and ICD-10 criteria | Good concordance with DSM-III diagnosis of substance use disorders: κ = .83 | Test–retest reliability for drug abuse and dependence: κ = .73 | ~2 Hours (drug section takes ~20 minutes) |
| LDS (Raistrick et al., 1994) |
|
Based on a psychological view of dependence | Opiate LDQ sample scores correlated with scores on: SODQ (r = .30) GHQ (r = .33) SFQ (r = .27) |
Cronbach’s α = .94 Test–retest reliability: κ = .95 |
10 Minutes |
| SODQ (Sutherland et al., 1986) |
|
Based on the psychobiological nature of dependence | Correlations between SODQ total score and: Need for more of the drug (r = .50) Drug use being out of control (r = .49) Missing a fix (r = .48) Worrying about drug use (r = .43) Wish to stop using (r = .29) Average fixes per day (r = .50) |
Section 1 Cronbach’s α = .81 Section 2 Cronbach’s α = .88 Section 3 Cronbach’s α = .86 Section 4 Cronbach’s α = .70 |
Not reported–Modeled on the SADQ, which takes 5 minutes to complete |
| SDS (Gossop et al., 1995) |
|
Based on a psychological view of dependence | SDS against CIDI
|
Cronbach’s α = .81 to .92 | ≤ 1 Minute |
| CAGE-AID (adapted to include drugs) (Brown et al., 1995) |
|
Based on a psychological view of dependence | CAGE-AID against CIDI
|
X | ~ 1 Minute |
| SMAST-AID (Brown et al., 1995) |
|
Based on a psychological, social, and legal view of dependence | SMAST-AID against DIS-R
|
X | ~2 Minutes |
| ASI (McLellan et al., 1980) |
|
Based on quantitative, psychological, social, and legal views of dependence | Correlations between ASI drug severity and: CAGE-AID (r = .64) DAST (r = .73) Clinical Use, Abuse and Dependence Scale (r = .70) |
Cronbach’s α = .58 to .79 Test–retest reliability for drug dependence: κ ≥ .92 Inter-rater reliability for drug dependence: κ = .89 |
45 to 60 Minutes |
| DAST (Skinner, 1982; Yudko et al., 2007) |
|
Based on a psychological, social, and legal view of dependence | DAST against MAST:
|
Cronbach’s α = .92 | ≤ 5 Minutes |
| RODS (Wickersham et al., 2015) |
|
Based on DSM-IV criteria; validated against MINI | RODS against MINI
|
Cronbach’s α = .92 Item-total correlations (range: .66 to .87) |
≤ 2 Minutes |
Note. MINI = Mini International Neuropsychiatric Interview; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth edition; SCID = Structured Clinical Interview for DSM Disorders; SCID-P = patient-rated SCID; PPV = positive predictive value; NPV = negative predictive value; CIDI = Composite International Diagnostic Interview; ICD-10 = International Classification of Diseases; LDS = Leeds Dependence Questionnaire; SODQ = Severity of Opiate Dependence Questionnaire; GHQ = General Health Questionnaire; SFQ = Social Functioning Questionnaire; SADQ = Severity of Alcohol Dependence Questionnaire; SDS = Severity of Dependence Scale; SMAST-AID = Short Michigan Alcoholism Screening Test–Adapted to Include Drugs; DIS-R = Diagnostic Interview Schedule–Revised; ASI = Addiction Severity Index; DAST = Drug Abuse Screening Test; MAST = Michigan Alcoholism Screen Test; RODS = Rapid Opioid Dependence Screen; LDS = Leeds Dependence Questionnaire.
Among these major diagnostic assessments of opioid dependence, the SCID is well established as the “gold standard” (Peters et al., 1998) but requires considerable time for the subject and requires specialized training in both the administration and the scoring of the instrument. Other less time-consuming instruments have also been used, including the MINI, based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) and International Classification of Diseases (ICD-10) criteria and validated against the SCID, which screens for 17 Axis I diagnoses and has demonstrated high reliability and validity across diverse samples (Black, Arndt, Hale, & Rogerson, 2004; Lecrubier, Sheehan, & Weiller, 1997; Pinninti, Madison, Musser, & Rissmiller, 2003; Sheehan et al., 1998). Concordance analysis of the clinician-rated MINI (MINI-CR) and the patient-rated MINI (MINI-PR) against the patient-rated SCID (SCID-P) reveals κ scores ranging from acceptable (.45 to .59) to good (.60 to .74) among the various diagnostic sections (Sheehan et al., 1998). κ scores on current drug dependence, however, showed poor agreement on both the MINI-PR (.30) and MINI-CR (.43), while lifetime drug dependence scores were more robust (MINI-PR = .62, MINI-CR = .64). With an average administration time of 15 to 21 minutes (Jones, 2005; Samet, Waxman, Hatzenbuehler, & Hasin, 2007), the MINI is considerably shorter than its predecessors. Despite its reduced administration time, the MINI remains ill suited for rapid, targeted diagnoses in clinical settings due to its considerable length and complexity. For example, the MINI’s subsection on drug dependence and abuse screens for 63 drug types among 9 drug classes. Each drug class with an affirmative response is then fully explored for both dependence (2 to 7 questions) and abuse (1 to 4 questions), creating a thorough, but also highly complex, screen.
Such an approach is suboptimal in the context of rapid and targeted screening of opioid dependence, especially when only opioid dependence is usually needed to determine eligibility for MAT for treatment of opioid dependence. Moreover, the shift in recent years to a primary care model for delivering physical and mental health care has necessitated the development of succinct screening tools that can be efficiently integrated into routine care, especially when integrating MAT into clinical care settings (Altice et al., 2011; Basu et al., 2006). In response, the Modified MINI Screen (MMS) was developed (New York State Office of Alcoholism and Substance Abuse Services, 2005). It is an abbreviated 22-item measure of anxiety and mood disorders, trauma exposure, post-traumatic stress disorder, and nonaffective psychoses; absent from the MMS, however, is a screen for opioid dependence.
The present study addresses these limitations by presenting a new, brief measure of opioid dependence, the Rapid Opioid Dependence Screen (RODS). The RODS was developed to provide a brief, targeted measure of opioid dependence that could be deployed in a variety of contexts, including both clinical and research.
Method
Ninety-seven newly incarcerated HIV-infected individuals from New Haven County, Connecticut, were recruited to participate in this study from 2009 to 2011 as part of a novel jail-release program that aims to create a comprehensive transitional program for people living with HIV/AIDS, including the provision of buprenorphine/naloxone upon release (ClinicalTrials.gov ID NCT00841711). Eligibility criteria for the study were (1) 18 years of age or older, (2) documented HIV-seropositive status, (3) incarcerated in jail but not sentenced, (4) within 30 days of their scheduled release from jail, and (5) returning to the greater New Haven or Waterbury areas. The details of this study site and the multisite evaluation have been previously described (Draine et al., 2011).
Procedures
On incarceration to jail (typically within 1 week), study participants were administered a series of baseline questionnaires by a trained research assistant. As such, the screened population was not comprised of a “drug treatment–seeking” population. The measures included the MINI-CR (Sheehan et al., 1998) and the RODS. Both the MINI and RODS were administered in a retrospective manner, asking participants to recall their use of opioids during previous 12 months.
Instruments
MINI
Participants were administered all 16 modules of the MINI (Version 5) during the baseline interview. The module on substance dependence and abuse (Module K) was used as the gold standard for diagnosing opioid dependence in this study. Response options to items on the MINI are structured in a binary “yes/no” format. The MINI has also been validated to the SCID among prison inmates, which is the target population of the current investigation (Black et al., 2004; Gunter et al., 2008).
RODS
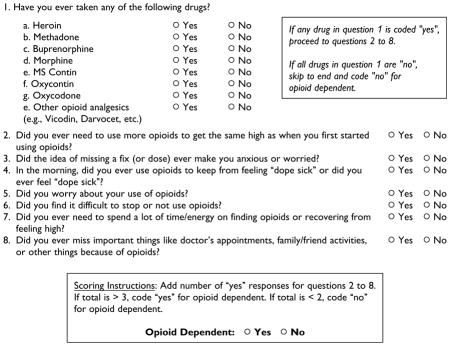
The RODS (see the Appendix) is a brief, 8-item measure designed to assess dependence for opioid drugs. Like the MINI, all response options are coded in a dichotomous yes/no format. The first item assesses lifetime use of the following eight types of opioids: heroin, methadone, buprenorphine, morphine, MS Contin, Oxycontin, oxycodone, and other opioid analgesics. Next, Items 2 to 8 measure physiological, behavioral, and cognitive factors associated with opioid use. A “no” response to all eight drug types in Item 1 results in an immediate outcome of nondependence, skipping Items 2 through 8. Participants with an affirmative response to at least one drug type in Item 1, however, proceed to answer Items 2 through 8. A diagnosis of opioid dependence is made if three or more affirmative responses are given to Items 2 through 8, reflecting the diagnostic criteria outlined in DSM-IV.
Development of the RODS
The RODS was originally developed as a tool to assist in the rapid screening for opioid dependence in a pilot research study evaluating the use of buprenorphine for relapse prevention among a group of HIV-infected, opioid-dependent released prisoners (Saber-Tehrani et al., 2012). It was designed so that research assistants, who administered this screen to consented participants while incarcerated, could inform the subjects of how likely they would be to meet criteria for the sub-study of buprenorphine upon release. In turn, this allowed the clinician (S.A.S.) to be prepared quickly on day of release for buprenorphine induction if necessary. Upon release, the subjects who screened positive for opioid dependence by the RODS were then confirmed for DSM-IV criteria for opioid dependence based on the results of the MINI subcategory of opioid dependence that had been administered to subjects prior to release. If confirmed opioid dependent, the subjects were offered buprenorphine as relapse prevention on the same day as release. When creating the RODS assessment tool, author S.A.S. used the DSM-IV criteria for opioid dependence and turned them into questions that could be understood by the anticipated subjects easily and, importantly, could be asked quickly (less than 5 minutes) and scored easily by nonclinical research assistants (i.e., not persons with MD/PhD). Table 2 lists the DSM-IV criteria for opioid dependence and the associated RODS questions.
Table 2.
DSM-IV Criteria for Substance Dependence and Comparison to Items in the RODS.
| DSM-IV Criteria for Substance Dependence | Corresponding RODS Question |
|---|---|
| Tolerance (marked increase in amount; marked decrease in effect) | Did you ever need to use more opioids to get the same high as when you first started using drugs?a |
| Characteristic withdrawal symptoms; substance taken to relieve withdrawal | In the morning, did you ever use opioids to keep from feeling dope sick or did you ever feel dope sick? |
| Substance taken in larger amount and for longer period than intended | Did you ever need to use more opioids to get the same high as when you first started using drugs?a |
| Persistent desire or repeated unsuccessful attempt to quit | Did you find it difficult to stop or not use opioids? |
| Much time/activity to obtain, use, recover | Did you ever need to spend a lot of time/energy on finding opioids or recovering from feeling high? |
| Important social, occupational, or recreational activities given up or reduced | Did you ever miss important things like doctors’ appointments, family/friend activities, or other things because of opioids? |
| Use continues despite knowledge of adverse consequences (e.g., failure to fulfill role obligation, use when physically hazardous) | Did the idea of missing a fix (or dose) ever make you anxious or worried? |
| Clinical experience question (not associated with DSM-IV criteria for opioid dependence or opioid abuse) | Did you worry about your use of opioids? |
Note. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth edition; RODS = Rapid Opioid Dependence Screen.
Denotes question in RODS that measured both items of DSM-IV criteria for opioid dependence.
Originally, the RODS comprised eight questions, with the first assessing whether the subject had ever used opioids (as listed in the current RODS). If the answer was “yes” to any one of them, the subject was then asked six questions that were based on DSM-IV criteria for OD (Questions 2 to 4, 6 to 8 in the RODS) and one question that was not based on DSM-IV criteria for OD or opioid abuse (Question 5 in the RODS). The reasoning by S.A.S. for including Question 5 (“Did you worry about your use of opioids?”) was due to her clinical history of treating patients with co-occurring opioid dependence and HIV infection where this question was found to be valuable in assessment of opioid problems. When developing the RODS, the initial intention was to remove this question; however, upon validating this tool to the MINI, this question was found to be very important in assessing for opioid use problems and was therefore kept in the final RODS version for this study.
Results
The baseline characteristics of the sample are summarized in Table 3. The majority of participants were male (70%) and identified as African American (54.6%). The mean age for the sample was 47.2 years (SD = 8.3) and mean lifetime opioid use was 10.4 years (SD = 11.3).
Table 3.
Sample Demographics.
| Variable | % (n) |
|---|---|
| Gender | |
| Female | 29.9 (29) |
| Male | 70.1 (68) |
| Race | |
| White | 19.6 (19) |
| Black | 54.6 (53) |
| Hispanic | 25.8 (25) |
| Age (mean years, SD) | 47.2 (8.3) |
| Lifetime opioid use (mean years, SD) | 10.4 (11.3) |
Concordance of the RODS and MINI
Results of the concordance analysis between the MINI and the RODS are reported in Table 4. For the diagnosis of opioid dependence, a substantial level of agreement was found between the two measures (Cohen’s κ = .67). Sensitivity (.97) and specificity (.76) were also strong, while positive predictive value (PPV = .69) was considered good and negative predictive value (NPV = .98) was excellent. These values represent a substantial improvement over the MINI’s validation against the SCID, which exhibited only a moderate level of agreement (Cohen’s κ = .43) and weaker operating characteristics (sensitivity = .45, specificity = .96, PPV = .50, NPV = .95).
Table 4.
RODS-MINI Contingency Table.
| MINI
|
Total | |||
|---|---|---|---|---|
| Dependent | Not Dependent | |||
| RODS | Dependent | 34 (TP) | 15 (FP) | 49 |
| Not dependent | 1 (FN) | 47 (TN) | 48 | |
| Total | 35 | 62 | 97 | |
Note. MINI = Mini International Neuropsychiatric Interview; RODS = Rapid Opioid Dependence Screen; TP = true positive; TN = true negative; FP = false positive; FN = false negative.
Reliability
Items 2 through 8 showed strong internal consistency (α = .92). Item-total correlations (ϕ) were also good, ranging from .66 to .87. Item 1 was excluded from this analysis since it is an indicator of opioid use rather than dependence.
Discussion
Controlling the global epidemic of opioid dependence and abuse is among the greatest public health challenges facing individuals, clinicians, policy makers, and law enforcement agencies today. While interruptions in controlling opioid supply have largely failed, the first and requisite step toward treatment for those already dependent on opioids is accurate identification of those who would be eligible for evidence-based treatment, including MAT. Existing diagnostic tests that appear to be the most accurate, the SCID and the MINI, are long and assess myriad psychiatric and substance use disorders that do not necessarily direct an opioid-dependent person to evidence-based treatment. Therefore, a brief, valid, and reliable screening instrument to identify opioid dependence will contribute greatly to increased access to available treatment. The ideal instrument would be brief, reliable, accurate, and able to be administered by professionals and nonprofessionals alike. Since 1990, the SCID has served as the gold standard for evaluating neuropsychiatric well-being, including drug dependence and abuse (Spitzer, Williams, Gibbon, & First, 1990). For the past decade, the briefer, self-scoring MINI, however, has largely replaced the SCID as a comprehensive diagnostic tool. The MINI is still a time-consuming yet comprehensive assessment of DSM-IV mental disorders, making it ill suited for the rapid and targeted screening of opioid dependence when the primary purpose for the tool is to accurately determine eligibility for opioid substitution or medication-assisted therapy.
The RODS addresses this unmet need by providing a rapid, self-scoring, stand-alone, and opioid-specific measure for use in dynamic clinical and research environments targeting adults at risk for opioid dependence. In our experience, the average time for administration of the RODS by a non-clinician was less than 2 minutes. While substantially shorter than the MINI, results from this initial validation study suggest that the RODS is an equally rigorous tool for the screening of opioid dependence. Moreover, the RODS’ high sensitivity (97%) makes it a preferred screening mechanism since few individuals with opioid dependence would be missed. Likewise, the relatively low false negative rate (specificity = 76%) accurately diagnoses most individuals who “screen in,” making it an ideal first-line assessment tool.
Though this study now provides clinicians and researchers with a convenient screening tool for opioid dependence, it does have several limitations. First, data used for validation of the RODS relied on newly incarcerated, HIV-infected individuals—populations that have both been demonstrated to have a higher prevalence of opioid dependence compared to the general population (Smith-Rohrberg, Bruce, & Altice, 2004). Moreover, the sample population is slightly older than most drug treatment–seeking individuals, and the prior years with drug use and experience with the drug treatment may have increased the accuracy of self-reports. Therefore, these results may be limited in their generalizability to other populations. Second, scale validation studies generally require larger samples, with a recommended minimum of 10 to 20 participants per scale item (Gorsuch, 1997; Guadagnoli & Velicer, 1988); therefore, our relatively small sample size limits full-scale validation. The high reliability of items, nevertheless, suggests that the findings will be reproducible among larger and more diverse samples, especially since there were just three major questions with one having multiple items. Third, this study is also limited by its use of the MINI, instead of the SCID, as the measurement standard for evaluating the RODS. Further work on the RODS should apply the SCID as the gold standard measure to confirm the present findings.
Future studies of the RODS should also examine its validity across populations and cultures, incorporate inter-rater reliability testing, and assess its use among a larger sample of HIV-negative individuals. Such studies should also be confirmed in Eastern Europe and Asia, where opioid abuse is growing exponentially (Mathers et al., 2008) and MAT is urgently needed and beginning to expand (Wolfe et al., 2010).
Rapidly and correctly identifying individuals with opioid dependence, by having access to a reliable and brief screening assessment tool, will not only facilitate access and entry into evidence-based treatment but also likely reduce the generalization of HIV transmission among people who inject drugs (Alistar, Owens, & Brandeau, 2011). Rapid screening will result in quick diagnosis and treatment initiation that will also contribute to reductions in morbidity and mortality associated with opioid dependence (e.g., overdose, skin and soft tissue infections, pneumonia, trauma, criminal justice problems, unemployment) and conditions that are syndemic with opioid use, such as viral hepatitis and other sexually transmitted infections.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data were collected from research supported by a Special Project of National Significance funded by the U.S. Department of Health and Human Services (Grant H97HA08541; Altice, P.I.) and from career development awards from the National Institute on Drug Abuse (K23 DA019381 and K02 DA032322 for SAS; K24 DA017072 for FLA; and K01 DA038529 for JAW). These funding agencies had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Appendix
Rapid Opioid Dependence Screen (RODS)
Instructions: [Interviewer reads] The following questions are about your prior use of drugs. For each question, please indicate “yes” or “no” as it applies to your drug use during the last 12 months.
Footnotes
Declaration of Conflicting Interests
The authors disclosed the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Frederick L. Altice: speaker honorarium from Bristol-Myers Squibb, Gilead Sciences, Merck, and RUSH Simply Speaking; research support from Gilead Sciences. For information about JCHC’s disclosure policy, please see the Self-Study Exam.
References
- Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: A modeling analysis for Ukraine. PLoS Medicine. 2011;8:e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP BHIVES Collaborative. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: Results from a multisite study. Journal of Acquired Immune Deficiency Syndromes. 2011;56:S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:59–79. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clinical Infectious Diseases. 2006;43:S178–S183. doi: 10.1086/508181. CID40075 [pii] [DOI] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clinical Infectious Diseases. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Black DW, Arndt S, Hale N, Rogerson R. Use of the Mini International Neuropsychiatric Interview (MINI) as a screening tool in prisons: Results of a preliminary study. Journal of the American Academy of Psychiatry and the Law. 2004;32:158–162. [PubMed] [Google Scholar]
- Bradley CJ, French MT, Rachal JV. Financing and cost of standard and enhanced methadone treatment. Journal of Substance Abuse Treatment. 1994;28:155–173. doi: 10.1016/0740-5472(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Methadone maintenance treatment. Fact sheet. 2002 Retrieved from http://www.cdc.gov/idu/facts/MethadoneFin.pdf.
- Chatterji S, Saunders JB, Vrasti R, Grant BF, Hasin D, Mager D. Reliability of the alcohol and drug modules of the Alcohol Use Disorder and Associated Disabilities Interview Schedule—Alcohol/Drug Revised (AUDADIS-ADR): An international comparison. Drug and Alcohol Dependence. 1997;47:171–185. doi: 10.1016/s0376-8716(97)00088-4. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. British Journal of Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Draine J, Ahuja D, Altice FL, Arriola KJ, Avery AK, Beckwith CG, Tinsley MJ. Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS Care. 2011;23:366–377. doi: 10.1080/09540121.2010.507738. [DOI] [PubMed] [Google Scholar]
- Dunlap LJ, French MT. A comparison of two methods for estimating the costs of substance abuse treatment. Journal of Maintenance in the Addictions. 1998;1:29–44. [Google Scholar]
- French MT, Bradley CJ, Calingaert B, Dennis ML, Karuntzos GT. Cost analysis of training and employment services in methadone treatment. Evaluation and Program Planning. 1994;17:107–120. [Google Scholar]
- French MT, Galinis DN, Dunlap LJ, Zarkin GA, Rachal JV. The impact of health care reform and managed care on the availability, financing, and costs of substance abuse treatment. Research Triangle Park, NC: National Institute on Drug Abuse; 1994. [Google Scholar]
- Gerstein DR, Johnson RA, Harwood HJ, Fountain D, Suter N, Malloy K. Evaluating recovery services: The California Drug and Alcohol Treatment Assessment (CALDATA) Sacramento, CA: California Department of Alcohol and Drug Programs; 1994. [Google Scholar]
- Gorsuch RL. Exploratory factor analysis: Its role in item analysis. Journal of Personality Assessment. 1997;68:532–560. doi: 10.1207/s15327752jpa6803_5. [DOI] [PubMed] [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. The Severity of Dependence Scale (SDS): Psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 1995;90:607–614. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and Alcohol Dependence. 2003;71:7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): Reliability of alcohol and drug modules in a general population sample. Drug and Alcohol Dependence. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- Guadagnoli E, Velicer WF. Relation of sample size to the stability of component patterns. Psychological Bulletin. 1988;103:265–275. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- Gunter TD, Arndt S, Wenman G, Allen J, Loveless P, Sieleni B, Black DW. Frequency of mental and addictive disorders among 320 men and women entering the Iowa prison system: Use of the MINI-Plus. Journal of the American Academy of Psychiatry and the Law. 2008;36:27–34. [PubMed] [Google Scholar]
- Jones JE. Clinical assessment of Axis I psychiatric morbidity in chronic epilepsy: A multicenter investigation. Journal of Neuropsychiatry and Clinical Neuroscience. 2005;17:172–179. doi: 10.1176/jnp.17.2.172. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E. The Mini International Neuropsychiatric Interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. doi: 10.1016/S0924-9338(97)86748-X. [DOI] [PubMed] [Google Scholar]
- Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee S, Mattick R. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. 2009:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. The prescription drug epidemic in the United States: A perfect storm. Drug and Alcohol Review. 2011;30:264–270. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- New York State Office of Alcoholism and Substance Abuse Services. Screening for co-occurring disorders using the Modified MINI Screen (MMS): Provider Implementation Plan. Albany, NY: Author; 2005. [Google Scholar]
- Peters RH, Greenbaum PE, Edens JF, Carter CR, Ortiz MM. Prevalence of DSM-IV substance abuse and dependence disorders among prison inmates. American Journal of Drug and Alcohol Abuse. 1998;24:573–587. doi: 10.3109/00952999809019608. [DOI] [PubMed] [Google Scholar]
- Pinninti NR, Madison H, Musser E, Rissmiller D. MINI International Neuropsychiatric Schedule: Clinical utility and patient acceptance. European Psychiatry. 2003;18:361–364. doi: 10.1016/j.eurpsy.2003.03.004. [DOI] [PubMed] [Google Scholar]
- Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C. Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction. 1994;89(5):563–572. doi: 10.1111/j.1360-0443.1994.tb03332.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Towle L. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rydell CP, Everingham SS. Controlling cocaine: Supply versus demand programs. Washington, DC: Office of National Drug control Policy, United States Army; 1994. [Google Scholar]
- Saber-Tehrani AS, Springer SA, Qiu J, Herme M, Wickersham J, Altice FL. Rationale, study design and sample characteristics of a randomized controlled trial of directly administered antiretroviral therapy for HIV-infected prisoners transitioning to the community: A potential conduit to improved HIV treatment outcomes. Contemporary Clinical Trials. 2012;33:436–444. doi: 10.1016/j.cct.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet S, Waxman R, Hatzenbuehler M, Hasin DS. Assessing addiction: Concepts and instruments. Addiction Science and Clinical Practice. 2007;4:19–31. doi: 10.1151/ascp074119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Bruce RD, Altice FL. Review of corrections-based therapy for opiate-dependent patients: Implications for buprenorphine treatment among correctional populations [Review] Journal of Drug Issues. 2004;34:451–480. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R. Washington, DC: American Psychiatric Association; 1990. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National survey on drug use and health (NSDUH): 2008 highlights. Rockville, MD: Author; 2008. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National survey on drug use and health: Trends in nonmedical use of prescription pain relievers: 2002 to 2007. Rockville, MD: Author; 2009a. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 national survey on drug use and health: National findings. Rockville, MD: Author; 2009b. [Google Scholar]
- Sutherland G, Edwards G, Taylor C, Phillips G, Gossop M, Brady R. The measurement of opiate dependence. British Journal of Addiction. 1986;81(4):485–494. doi: 10.1111/j.1360-0443.1986.tb00360.x. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World drug report 2010. New York, NY: United Nations Publication; 2010. [Google Scholar]
- United Nations Office on Drugs and Crime & World Health Organization. Principles of drug dependence treatment: Discussion paper. Vienna, Austria: World Health Organization; 2008. [Google Scholar]
- Wickersham JA, Azar MM, Cannon CM, Altice LF, Springer SA. Validation of a Brief Measure of Opioid Dependence: The Rapid Opioid Dependence Screen (RODS) Journal of Correctional Health Care. 2015 doi: 10.1177/1078345814557513. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, Sartorius N. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wolfe D, Cohen J. Human rights and HIV prevention, treatment, and care for people who inject drugs: Key principles and research needs. Journal of Acquired Immune Deficiency Syndromes. 2010;55:S56–S62. doi: 10.1097/QAI.0b013e3181f9c0de. 00126334-201012011-00011 [pii] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Model list of essential medicines. 14. Geneva, Switzerland: Author; 2005. [Google Scholar]
- World Health Organization. Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva, Switzerland: Author; 2009. [Google Scholar]
- Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32(2):189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]


