Abstract
Peripheral artery disease (PAD) is caused by atherosclerosis, hardening and narrowing arteries over time due to buildup of fatty deposit in vascular bed called plaque. Severe blockage of an artery of the lower extremity markedly reduce blood flow, resulting in critical limb ischemia (CLI) manifested by a variety of clinical syndromes including rest pain in the feet or toes, ulcer and gangrene with infection. Despite significant advances in clinical care and interventions for revascularization, patients with CLI remain at high risk for amputation and cardiovascular death. To overcome this unmet need, therapeutic angiogenesis using angiogenic growth factors has evolved in an attempt to increase blood flow in ischemic limb. Initial animal studies and phase I clinical trials with vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) demonstrated promising results, inspiring scientists to progress forward. However, more rigorous phase II and III clinical trials have failed to demonstrate beneficial effects of these angiogenic growth factors to date. Recently, two multicenter, double-blind, placebo-controlled clinical trials in Japan (phase III) and US (phase II) demonstrated that hepatocyte growth factor (HGF) gene therapy for CLI significant improved primary end points and tissue oxygenation up to two years in comparison to placebo. These clinical results implicate a distinct action of HGF on cellular processes involved in vascular remodeling under pathological condition. This review presents data from phase I-III clinical trials of therapeutic angiogenesis by gene therapy in patients with PAD. Further, we discuss the potential explanation for the success or failure of clinical trials in the context of the biological mechanisms underlying angiogenesis and vascular remodeling, including cellular senescence, inflammation, and tissue fibrosis.
Keywords: Clinical limb ischemia, fibroblast growth factor, gene therapy, hepatocyte growth factor, peripheral artery disease, therapeutic angiogenesis, vascular endothelial growth factor.
INTRODUCTION
With advances in medical treatment for acute disease, chronic non-communicable diseases are now the major cause of morbidity and mortality in both developing and developed countries [1]. One typical example of non-communicable disease is peripheral artery disease (PAD), the third leading cause of atherosclerotic morbidity, after coronary artery disease (CAD) and stroke [2]. The prevalence of PAD increases with age, affecting 6% of individuals aged 50-60 years, and 10-20% of those aged >70 years [3, 4]. Both smoking and diabetes are risk factors for CAD and PAD, but these risk factors have a greater role in PAD than in CAD [5], suggesting that the frequency of PAD is likely to increase in the western world.
As atherosclerosis is a widespread disease of arteries, PAD is frequently associated with CAD [6-8]. Therefore, therapies such as statins, angiotensin-converting-enzyme inhibitors and antiplatelet drugs have been used for systemic treatment. However, at present, none of these agents can improve perfusion to the lower extremity in patients with PAD [4, 9]. Revascularization by surgical bypass or catheter is the first option for PAD patients with stenosis or occlusion of large arteries, but many patients with critical limb ischemia (CLI) are poor candidates for these revascularization treatments because of the existence of diffuse stenosis or calcification of arteries. Moreover, re-stenosis of the treated artery or graft failure can arise as complications after successful revascularization [10, 11]. Clearly, the need exists to develop a new strategy for establishing biological grafts in PAD patients with no treatment option.
The concept of “therapeutic angiogenesis” for PAD, achieved by gene and cell therapy, has recently raised a great deal of hope for patients who cannot undergo standard revascularization treatment. Cell therapy using bone marrow stem cells is still at a primitive stage, but holds great hope [12]. On the other hand, gene therapy for PAD patients has been intensively validated by large randomized, multi-center, double-blinded, placebo-controlled trials. Although preclinical studies using vascular endothelial growth factor (VEGF) [13] and fibroblast growth factor (FGF) [14] gave very promising data, the enthusiasm generated by these early studies has been let down by the results from large randomized, multi-center, double-blinded, placebo-controlled trials [15, 16]. Recently, a multicenter, double-blind, placebo-controlled phase III clinical trial in Japan and a US phase II clinical trial of hepatocyte growth factor (HGF) gene therapy for CLI demonstrated a significant improvement in primary end points and an increase in transcutaneous partial oxygen pressure even after one year in comparison to placebo [17, 18]. These results indicate that there might be a distinct beneficial function of HGF among angiogenic growth factors in a clinical setting.
This review provides an overview of the outcomes of clinical trials of gene therapy for PAD patients. A potential explanation for the success or fail of clinical trials is discussed later in the context of the biological mechanisms underlying angiogenesis and vascular remodeling, including cellular senescence, inflammation, and tissue fibrosis in pathological condition.
PROCESS OF VESSEL FORMATION AND REMODELING
Acute arterial occlusion in the lower extremity, i.e. a blood clot, embolism, dissection or trauma, usually causes severe tissue ischemia, resulting in tissue necrosis necessitating limb amputation. Conversely, gradually developing arterial stenosis, i.e. atherosclerosis, stimulates the growth of pre-existing bypass circulation. This formation of collateral vessel, with 20-100 ìm diameter and fully developed tunica media that allow vasodilatation and regulation of blood flow, is called ‘arteriogenesis’. These vessels can grow considerably, enough to take over the role of a large artery when occluded. Arteriogenesis is independent of oxygen levels and ischemia. Instead, it is enhanced by the change in shear stress forces sensed by endothelial cells through NO production. Tissue ischemia also leads to the sprouting of new capillaries from pre-existing vessels, termed ‘angiogenesis’. These 8-12 µm diameter vessels lie within the ischemic region, connecting to the capillaries derived from normally perfused arterioles. As this process leads predominantly to the development of small capillaries, angiogenesis is unable to fully compensate for the occlusion of larger arteries [1, 19]. In contrast to arteriogenesis, angiogenesis is stimulated by ischemia and hypoxia signaling. Another type of vessel formation termed ‘vasculogenesis’ is defined as the in situ formation of blood vessels. It was thought that vasculogenesis was limited to embryonic development. However, in 1997, Asahara et al. demonstrated that circulating endothelial progenitor cells (EPC) can be incorporated into newly formed vessels following limb ischemia [20]. EPC can be involved in the processes of both arteriogenesis and angiogenesis by secreting cytokines or forming new endothelial cells. Although their actual role in physiological condition in adulthood remains largely unknown, interestingly, BM-derived EPC could be detected even in the wall of quiescent vessels without neovascularization events. This finding suggests that BM-derived EPC may relate even to the turnover of EC in quiescent vessels [21].
ANGIOGENIC GROWTH FACTORS AND CLINICAL OUTCOMES
Angiogenic growth factors have the ability to increase collateral vessels, and augment tissue perfusion and oxygenation, limiting ischemic lesions in rodents [22, 23]. Therefore, they have been expected to improve the function and symptoms of patients with CLI. Initially, interest in utilizing angiogenic cytokines for clinical intervention focused on VEGF and FGF. Later, the therapeutic potential of HGF has been identified (Table 1).
Table 1.
Human Clinical Trials of Angiogenic Growth Factors for Patients with PAD
| Trials (Reference) | Strategy | Phase | Outcome |
|---|---|---|---|
| Baumgartner et al. [13] | phVEGF165 | I | Tolerated |
| Makinen et al. [31] | phVEGF165 | I | Tolerated |
| RAVE [32] | AdenoVEGF121 | II | No improvement of exercise performance or QOL |
| Groningen [15] | phVEGF165 | II | No reduction in amputation rate |
| Comerota et al. [14] | phFGF-1 | I | Tolerated |
| TALISMAN [37] | phFGF-1 | II | Reduction in amputation rate |
| TAMARIS [16] | phFGF-1 | III | No reduction in amputation rate or death |
| No improvement of QOL or ABI | |||
| Morishita et al. [40] | phHGF | I/IIa | Tolerated |
| Makino et al. [41] | phHGF | I/IIa | Improvement of ABI |
| Reduction in rest pain and ulcer size up to 2 years | |||
| HGF-STAT [17] | phHGF | II | Improvement in TcPO2 |
| TREAT-HGF [18] | phHGF | III | Improvement in rest pain and ABI |
| Reduction in ulcer size |
ABI: ankle-brachial index
TBI: toe-brachial index
TcPO2: transcutaneous oxygen tension
VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF)
VEGF has been widely and intensively studied for its angiogenic potential in both cancer and tissue ischemia [24, 25]. The VEGF family consists of VEGF-A to E. VEGF-A and B have alternative splicing variant isoforms (e.g., VEGFA121, VEGF165). Among VEGF-A isoforms, VEGF165 is the most abundant, and the best characterized [26]. Experimentally, plasmid- and adenovirus vector-mediated VEGF over-expression significantly ameliorated tissue perfusion and oxygenation with neovascularization in a hind-limb ischemia model [27, 28]. VEGF over-expression results in proliferation of resident endothelial cells (EC) and endothelial progenitor cell (EPC) recruitment, with subsequent neovascularization [29, 30]. Clinically, VEGF gene therapy provides inconsistent findings. Phase I clinical trials demonstrated that naked-plasmid VEGF165 gene therapy is safe and feasible, and over-expression of the cytokine in skeletal muscle significantly improved tissue perfusion and wound healing [13]. Consistently, Makinen et al. showed that intra-arterial administration of Ad-VEGF165 markedly increased vascular density compared to the placebo-control group [31]. Contrary to VEGF165 administration, intramuscular injection of the Ad-VEGF121 isoform failed to show an improvement in claudication, ankle-brachial index, and quality of life in a phase II clinical trial [32]. Moreover, up to this time, no phase III clinical trial using VEGF gene transfer has shown an improvement. It has long been documented that VEGF therapy was strongly associated with dose-dependent microvascular permeability and peripheral edema; 60% of patients developed moderate or severe edema in a clinical trial. VEGF-induced vascular permeability is caused by Rac-mediated generation of ROS, which, in turn, regulates adherens junction integrity [33]. However, a recent publication by Ehrbar et al. demonstrated that controlling the level of VEGF in mouse model with unique delivery systems (α2PI1-8-VEGF121) generated non-leaky vessels and ameliorated vessel formation more potently than did native VEGF121 [34]. Slow-release, low-dose VEGF might be effective and feasible in clinical trials.
FIBROBLAST GROWTH FACTOR (FGF)
The FGF family consists of approximately 22 members that bind to various spliced isoforms of the FGF receptors. Activation of the FGF receptors, which are expressed on EC, smooth muscle cells (SMC), and EPC, stimulates the proliferation of the respective cell types [35]. In particular, FGF-1 (acidic), FGF-2 (basic), and FGF-4 are highly angiogenic, and have the ability to enhance blood-vessel formation to facilitate angiogenesis with sprouting of capillaries from vessels. Based on these experimental data, a naked DNA plasmid vector (non-viral FGF vector; NV1FGF) carrying human FGF-1 has been developed for gene therapy. Pre-clinical studies demonstrated its potential to generate a functional vascular network in the ischemic region [36]. A phase I clinical trial enrolling 51 patients demonstrated a significant improvement in ulcer size, claudication, ankle brachial index, and transcutaneous tissue oxygen in the ischemic limb [14]. The subsequent phase II clinical trial (TALISMAN) also showed promising results, with improvement of ulcer healing and decreased risk of amputation or death [37]. With great hope, a phase III randomized clinical trial (TAMARIS) was conducted in patients who were not eligible for revascularization [16], but unexpectedly, no benefit of NV1FGF was detected in either the primary efficacy endpoint (major amputation or death) or secondary endpoints (minor amputation, skin lesion status, pain index, quality of life, and ankle and toe brachial index). It has been reported that FGF gene therapy is associated with potential hypertension and membranous nephropathy; these adverse effects have to be carefully considered in determining the dose and duration of administration of FGF.
HEPATOCYTE GROWTH FACTOR (HGF)
HGF was initially discovered as a potent mitogen for hepatocytes [38]. Later, its angiogenic potential; EC proliferation and migration, via tyrosine phosphorylation of its receptor, c-Met, was discovered. The receptor c-Met is expressed on EC, SMC, and also EPC [39]. Data obtained from clinical trials using the HGF gene for the treatment of hind-limb ischemia are particularly appealing, because at least three randomized placebo-controlled clinical trials using naked human HGF plasmid DNA verified its beneficial effect in healing of ulcers, decrease in rest pain, and increase in transcutaneus oxygen tension [17, 18, 40]. Recently, our group demonstrated long-term efficacy of HGF gene therapy with an increase in ankle-brachial pressure index, and a reduction of rest pain and ulcer size two years after gene therapy [41]. It is also worth mentioning that, unlike VEGF, HGF gene therapy was not associated with edema as a side effect.
These clinical results obtained from VEGF, FGF, and HGF gene therapy for PAD patients made us think about the distinct molecular mechanism of HGF and the difference between animal experiments and clinical trials which might provide a cue to improve therapeutic angiogenesis with growth factors.
WHAT IS SUCCESSFUL THERAPEUTIC ANGIOGENESIS?
The goal of therapeutic angiogenesis using gene therapy for CLI patients is to provide relief from ischemic pain, heal ulcers, reduce amputation risk, and afford a better quality of life. The advanced disease pathology features characteristically observed in the elderly introduces several obstacles, including severe inflammation, atherosclerosis, and ischemia, which all confound the efficacy of therapeutic angiogenesis by gene therapy for revascularization. At the cellular level, these undesirable features of CLI cause cell apoptosis, senescence, and fibrosis.
Cellular Apoptosis
Capillary density in human leg skeletal muscle varies only slightly between persons [42]. However, it can be increased by exercise and decreased by age, hypertension, or reduction in muscle use. CLI and PAD are typical age-associated disease, and patients with intermittent claudication have decreased exercise tolerance due to exercise-induced muscle ischemia. Moreover, hypertension, hypercholesterolemia, current smoking, and low kidney function were positively associated with prevalent PAD. More than 95% of persons with PAD had one or more cardiovascular disease risk factors [43]. These findings are due to the fact that PAD is associated with lower capillary density, and capillary density is related to the functional impairment as defined by a reduced peak oxygen consumption and peak walking time [44].
Cellular Senescence
It is also noteworthy that capillaries in PAD patients may have a senescent phenotype due to vascular risk factors, inflammation, and aging. This hypothesis is supported by the fact that Angiotensin II-, TNF-α, and diabetes-induce premature senescence in vivo animal experiment and in vitro study [45-49]. Additionally, EPC senescence is associated with the cardiovascular risk factors.
Tissue Fibrosis
Another important factor that prevent tissue repair is fibrosis. Tissue fibrosis often presents as the final outcome of chronic disease and is a significant cause of morbidity and mortality worldwide. Fibrosis is driven by continuous expansion of fibroblasts and myofibroblasts. Epithelial-mesenchymal transition (EMT) is a form of cell plasticity in which epithelia acquire mesenchymal phenotypes, and is increasingly recognized as an integral aspect of tissue fibrogenesis. Tissue fibrosis prevents oxygen diffusion and resident stem/progenitor cell migration toward the region where tissue repair is required.
Thus, several complications in human that have not been well examined in pre-clinical studies need to be overcome for successful angiogenesis in clinical trials. The next paragraph discussed possible beneficial mechanism of HGF in comparison to VEGF and FGF.
POSSIBLE DIFFERENCE BETWEEN HGF AND OTHER GROWTH FACTORS
HGF was initially discovered as a hepatotrophic factor present in the plasma of patients with fulminant hepatic failure [38]. Subsequently, several lines of evidence have implied that the HGF/c-Met system has several roles in different aspects of cellular biology including cell survival, cell differentiation and proliferation, and cytokine production [50-52]. Therefore, HGF is now appreciated as a key growth cytokine in the prevention and attenuation of both acute and chronic disease progression in the heart [53], kidney [50], liver [54], and vascular repair [46, 47]. Among HGF’s beneficial functions, its anti-inflammatory and anti-fibrotic action differentiate HGF from VEGF and bFGF. Inflammation, characterized by infiltration of inflammatory response cells, has been thought to be essential in the initiation and progression of a wide range of chronic diseases, including heart, kidney, and lung diseases [55-57]. Importantly, inflammation has recently been shown to be associate with cellular senescence [58-60]. IL-6 and IL-8 have been shown to play a pivotal role in the initiation and maintenance of cellular senescence. Reactive oxygen species (ROS) induced by inflammation also cause cellular senescence [61]. Thus, relation between inflammation and cellular senescence is evident. Nonetheless, most of the preclinical studies, including hind-limb ischemia model and acute myocardial infarction model, have been performed in the absence of vascular risk factor, which are present in a clinical setting.
To elucidate the discrepancy between the HGF and VEGF clinical trial outcomes, we compared the effects of HGF and VEGF on EC and EPC under angiotensin II (Ang II) stimulation, which is secreted by inflammatory cells and promotes atherosclerosis. We found that HGF, but not VEGF, attenuated Ang II-induced senescence of EC and EPC through a reduction of oxidative stress by inhibition of the PIP3/rac1 pathway [46]. Potent induction of neovascularization through recruitment of EPC by HGF under Ang II was also confirmed by in vivo experiments using several models, including HGF transgenic mice. However, this action was not seen with VEGF. Surprisingly, HGF and its receptor, c-Met, down-regulate epithelial growth factor receptor (EGFR) in ligand-dependent manner through the ubiquitin proteasome system [47]. Moreover, HGF down-regulates EGFR expression following administration of lipopolysaccharide (LPS), endothelin-1 (ET-1), and transforming growth factor alpha (TGF-a), which transactivate EGFR, suggesting that ligand-dependent EGFR down-regulation of HGF might be the major anti-inflammatory and anti-oxidant mechanism of HGF [47, 62]. Also, Kaga et al. demonstrated that bFGF alone, but not HGF, significantly activated a fundamental transcription factor for inflammation, NFκB, and gene expression of its downstream inflammation-associated cytokines (IL-8 and MCP-1) in VSMC, accompanied by an increase in vascular permeability in a rat paper disc model [63]. Also, Ohtani et al. proved that expression of VEGF increases after neointimal injury, recruiting monocyte-lineage cells [64]. Importantly, HGF was shown to have a synergistic action with VEGF on EC proliferation and chemotactic response and neovascularization [65, 66]. The authors also revealed that HGF up-regulated VEGF mRNA transcript, while HGF gene therapy did not induce leaky vessels or edema. Min et al. reported that HGF markedly reduced VEGF-induced leukocyte adhesion and adhesion molecule expression. This effect was mediated by HGF suppression of VEGF-induced NFκB signaling [67]. Thus, HGF stimulates new vessel growth accompanied by inhibition of complication of inflammation, edema, and cellular senescence.
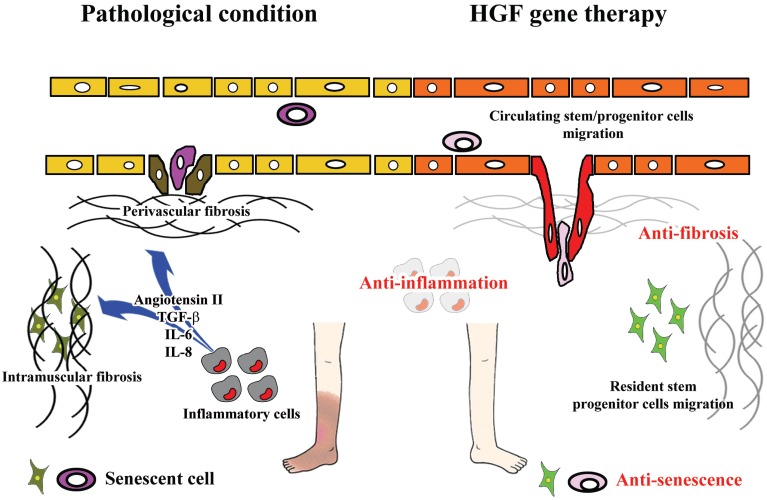
A further unique function of HGF is its anti-fibrotic action, while VEGF and FGF were shown to induce tissue fibrosis [68, 69]. We demonstrated that up-regulation of HGF resulted in a significant decrease in fibrotic tissue following acute myocardial infarction [52]. Moreover, our recent work demonstrated that HGF significantly attenuates endothelial-mesenchymal transition, which is considered to be involved in perivascular fibrosis in the heart [70] and kidney [50]. These actions would serve to minimize obstacles to tissue regeneration. Intramuscular fibrosis might prevent resident stem/progenitor cell migration. In the same way, perivascular fibrosis would limit engraftment of circulating stem/progenitor cells and oxygen diffusion, leading to im-pairment of tissue regeneration and oxygenation. Since local HGF expression was decreased in the muscle of an ischemic hind-limb model, where vascular and intramuscular fibrosis cause disease progression [71], administration of HGF by gene therapy would be expected to have a significant influence on neovascularization and regeneration of the organ. In fact, HGF stimulates resident cardiac stem cell improving cardiac function in the infarct mice heart where fibrosis complicates pathological condition [72]. Thus, these different properties of HGF, VEGF, and bFGF might affect the efficiency of therapeutic angiogenesis (Fig. 1).
Fig. (1).
Possible mechanisms underlying effectiveness of HGF gene therapy for patients with CLI.
Although, differences in the dose and duration of gene therapy, vector selection, and endpoint selection should be considered in the interpretation of the results of clinical trials, we have to take into account limitations of preclinical studies and the complexities of clinical settings.
CONCLUSION
Despite significant advances in medical, interventional, and surgical therapy, peripheral arterial disease patients are increasing with the aging world population [73]. To address this unmet need, scientists have accumulated data of therapeutic angiogenesis by gene therapy for more than ten years from clinical and preclinical studies. Unfortunately, we now recognize that the dramatic efficacy of single-angiogenic growth factor gene therapy that was shown in several animal studies was not fully translated into clinical practice. Comprehensive understanding of the basic biology of neovascularization in pathological conditions would provide critical information for future successful therapeutic angiogenesis. Although it is challenging to translate basic research to the clinical situations, HGF has unique potential and might be more ideal for the treatment of complex conditions in advanced peripheral artery disease patients.
LIMITATION OF CLINICAL DATA AND FUTURE PERSPECTIVES
The optimal dose, duration and timing of gene therapy have yet to be determined. In preclinical non-ischemic animal model, it is suggested that angiogenesis might need to be induced for weeks or months before the newly formed vasculatures mature [74, 75]. However, it is unclear whether persistent angiogenic stimuli is required for the ischemia in clinical setting. Naked plasmid DNA is expressed for only few days, and likewise adenovirus gene expression is expected to sustain for few weeks. Thus, a more robust exploration of dose and duration strategies may need to be tested after safety data accumulates regarding these approaches. Delivery route and vector selection should also be optimized as well. Intramuscular gene administration is a simple procedure. New methodology such as ultrasound –mediated administration of plasmid DNA [76] and intravenous retrograde gene delivery [77] can be applied for CLI patient with main artery occlusion. The ideal vector would combine low immunogenicity and safety profile with high transfection efficiency and gene expression for certain period of time. These optimization will provide benefit for patients with CLI.
The number of patients with cardiovascular disease will increase dramatically, as the population of developed nation ages. Thus, angiogeninc gene therapy and other strategies including stem cell therapy are needed to lessen the burden of ischemic disease and to improve quality of life. The challenges in clinical and the lesson from preclinical studies might offer therapeutic development. We are currently establishing strategy to treat patient with heart failure by administration of HGF plasmid.
ACKNOWLEDGEMENTS
We thank the members of the Department of Clinical Gene Therapy, Osaka University Graduate School of Medicine, for much helpful discussion and technical support.
CONFLICT OF INTEREST
R. Morishita received honoraria, consulting fees, and funds from Novartis, Takeda, Shionogi, Astellas, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer.
SOURCES OF FUNDING
This work was partially supported by a grant-in-aid from the Organization for Pharmaceutical Safety and Research, a grant-in-aid from the Ministry of Public Health and Welfare, a grant-in-aid from Japan Promotion of Science, and funds from the Ministry of Education, Culture, Sports, Science and Technology, of the Japanese Government.
ABBREVIATIONS
- Ang II
= Angiotensin II
- CAD
= Coronary Artery Disease
- CLI
= Critical Limb Ischemia
- EC
= Endothelial Cells
- EGFR
= Epithelial Growth Factor Receptor
- EMT
= Epithelial-mesenchymal Transition
- ROS
= Reactive Oxygen Species
- EPC
= Endothelial Progenitor Cells
- ET-1
= Endothelin-1
- FGF
= Fibroblast Growth Factor
- HGF
= Hepatocyte Growth Factor
- LPS
= Lipopolysaccharide
- PAD
= Peripheral Artery Disease
- SMC
= Smooth Muscle Cells
- TGF-a
= Transforming Growth Factor Alpha
- VEGF
= Vascular Endothelial Growth Factor
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010 A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000, and 2010,: A systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA. Critical issues in peripheral arterial disease detection and management A call to action. Arch. Intern. Med. 2003;163(8):884–892. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes F G. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007;45(Suppl. S ):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis Epidemiology pathophysiology and management. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 6.Lau JF, Weinberg MD, Olin JW. Peripheral artery disease.Part 1 Clinical evaluation and noninvasive diagnosis. Nat. Rev. Cardiol. 2011;8(7):405–418. doi: 10.1038/nrcardio.2011.66. [DOI] [PubMed] [Google Scholar]
- 7.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 2001;344(21):1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 8.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013;10(7):387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 9.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295(5):547–553. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2005, guidelines for the management of patients with peripheral arterial disease (lower extremity renal mesenteric and abdominal aortic) Executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery Society for Cardiovascular Angiography and Interventions Society for Vascular Medicine and Biology Society of Interventional Radiology and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation National Heart Lung and Blood Institute; Society for Vascular Nursing Transatlantic Inter-society Consensus and Vascular Disease Foundation. J. Am. Coll. Cardiol. 2006;47(6):1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, Sixt S, Schwarz T, Brechtel K, Bohme C, Neumann FJ, Zeller T. Sirolimus-eluting stents vs.bare-metal stents for treatment of focal lesions in infrapopliteal arteries A double-blind multi-centre randomized clinical trial. Eur. Heart. J. 2011;32(18):2274–2281. doi: 10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 12.Blum A, Balkan W, Hare JM. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis. 2012;223(2):269–277. doi: 10.1016/j.atherosclerosis.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97(12):1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 14.Comerota AJ, Throm. RC, Miller KA, Henry T, Chronos N, Laird J, Sequeira R, Kent CK, Bacchetta M, Goldman C, Salenius JP, Schmieder FA, Pilsudski R. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia Preliminary results of a phase I trial. J. Vasc. Surg. 2002;35(5):930–936. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- 15.Kusumanto YH, van Weel V, Mulder NH, Smit AJ, van den Dungen JJ, Hooymans JM, Sluiter WJ, Tio RA, Quax PH, Gans RO, Dullaart RP, Hospers GA. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia A double-blind randomized trial. Hum. Gene Ther. 2006;17(6):683–691. doi: 10.1089/hum.2006.17.683. [DOI] [PubMed] [Google Scholar]
- 16.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E. Effect of fibroblast growth factor NV1 FGF on amputation and death A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377(9781):1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 17.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a double-blind placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118(1):58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 18.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, Yamaguchi T, Ogihara T, Morishita R. Randomized double-blind placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010;17(9):1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 19.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J. Mol. Cell. Cardiol. 2002;34(7):775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 20.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 21.Gunsilius E, Duba HC, Petzer AL, Kahler CM, Grunewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J, Gastl G. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355(9216):1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 22.Muhlhauser J, Merrill MJ, Pili R, Maeda H, Bacic M, Bewig B, Passaniti A, Edwards NA, Crystal RG, Capogrossi MC. VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ. Res. 1995;77(6):1077–1086. doi: 10.1161/01.res.77.6.1077. [DOI] [PubMed] [Google Scholar]
- 23.Gowdak LH, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A, Palumbo R, Straino S, Emanueli C, Marrocco-Trischitta M, Lakatta EG, Anversa P, Spencer RG, Talan M, Capogrossi MC. Adenovirus-mediated VEGF (121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation. 2000;102(5):565–571-0. doi: 10.1161/01.cir.102.5.565. [DOI] [PubMed] [Google Scholar]
- 24.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isner JM. Therapeutic angiogenesis A new frontier for vascular therapy. Vasc. Med. 1996;1(1):79–87. doi: 10.1177/1358863X9600100114. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. (Lond). 2005;109(3):227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 27.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis.A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 1994;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walder CE, Errett CJ, Bunting S, Lindquist P, Ogez JR, Heinsohn HG, Ferrara N, Thomas GR. Vascular endothelial growth factor augments muscle blood flow and function in a rabbit model of chronic hindlimb ischemia. J. Cardiovasc. Pharmacol. 1996;27(1):91–98. doi: 10.1097/00005344-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann. Thorac. Surg. 2000;70(3):829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 30.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO. J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Yla-Herttuala S. Increased vascularity detected by digital subtraction angiography after vegf gene transfer to human lower limb artery A randomized placebo-controlled double-blinded phase II study. Mol. Ther. 2002;6(1):127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Mohler E, 3rd, Lederman RJ, Saucedo J, Mendelsohn FO, Olin J, Blebea J, Goldman C, Trachtenberg JD, Pressler M, Rasmussen H, Annex BH, Hirsch AT. Regional angiogenesis with vascular endothelial growth factor (VEGF) in peripheral arterial disease Design of the rave trial. Am. Heart. J. 2003;145(6):1114–1118. doi: 10.1016/S0002-8703(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 33.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J. Biol. Chem. 2009;284(38):25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 35.Burger PE, Coetzee S, McKeehan WL, Kan M, Cook P, Fan Y, Suda T, Hebbel RP, Novitzky N, Muller WA, Wilson EL. Fibroblast growth factor receptor-1 is expressed by endothelial progenitor cells. Blood. 2002;100(10):3527–3535. doi: 10.1182/blood.V100.10.3527. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves LM. Fibroblast growth factor-mediated angiogenesis for the treatment of ischemia.Lessons learned from experimental models and early human experience. Rev. Port. Cardiol. 1998;17(Suppl. 2 ):II11–20. [PubMed] [Google Scholar]
- 37.Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC, Ferreira-Maldent N, Gallino A, Wyatt MG, Wijesinghe LD, Fusari M, Stephan D, Emmerich J, Pompilio G, Vermassen F, Pham E, Grek V, Coleman M, Meyer F. Therapeutic angiogenesis with intramuscular NV1 FGF improves amputation-free survival in patients with critical limb ischemia. Mol. Ther. 2008;16(5):972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc. Natl. Acad. Sci. USA. 1986;83(17):6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+ CD34/CD117+ c-met+ stem cells and mononuclear cells expressing early cardiac muscle and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110(20):3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 40.Morishita R, Aoki M, Hashiya N, Makino H, Yamasaki K, Azuma J, Sawa Y, Matsuda H, Kaneda Y, Ogihara T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44(2):203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 41.Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Sawa Y, Kaneda Y, Ogihara T, Morishita R. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2012;32(10):2503–2509. doi: 10.1161/ATVBAHA.111.244632. [DOI] [PubMed] [Google Scholar]
- 42.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J. Appl. Physiol. . 1985;72(5):1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 43.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States Results from the national health and nutrition examination survey 1999,-2000,. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 44.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J. Appl. Physiol. 2011;111(1):81–86. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minamino T, Komuro I. Vascular cell senescence Contribution to atherosclerosis. Circ. Res. 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 46.Sanada F, Taniyama Y, Azuma J, Iekushi K, Dosaka N, Yokoi T, Koibuchi N, Kusunoki H, Aizawa Y, Morishita R. Hepatocyte growth factor but not vascular endothelial growth factor attenuates angiotensin II-induced endothelial progenitor cell senescence. Hypertension. 2009;53(1):77–82. doi: 10.1161/HYPERTENSIONAHA.108.120725. [DOI] [PubMed] [Google Scholar]
- 47.Sanada F, Taniyama Y, Iekushi K, Azuma J, Okayama K, Kusunoki H, Koibuchi N, Doi T, Aizawa Y, Morishita R. Negative action of hepatocyte growth factor/c-met system on angiotensin ii signaling via ligand-dependent epithelial growth factor receptor degradation mechanism in vascular smooth muscle cells. Circ. Res. 2009;105(7):667–675. doi: 10.1161/CIRCRESAHA.109.202713. [DOI] [PubMed] [Google Scholar]
- 48.Yokoi T, Fukuo K, Yasuda O, Hotta M, Miyazaki J, Takemura Y, Kawamoto H, Ichijo H, Ogihara T. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55(6):1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki M, Ikeda H, Sato Y, Nakanuma Y. Proinflammatory cytokine-induced cellular senescence of biliary epithelial cells is mediated via oxidative stress and activation of atm pathway A culture study. Free. Radic. Res. 2008;42(7):625–632. doi: 10.1080/10715760802244768. [DOI] [PubMed] [Google Scholar]
- 50.Iekushi K, Taniyama Y, Kusunoki H, Azuma J, Sanada F, Okayama K, Koibuchi N, Iwabayashi M, Rakugi H, Morishita R. Hepatocyte growth factor attenuates transforming growth factor-beta-angiotensin ii crosstalk through inhibition of the pten/akt pathway. Hypertension. 2011;58(2):190–196. doi: 10.1161/HYPERTENSIONAHA.111.173013. [DOI] [PubMed] [Google Scholar]
- 51.Koibuchi N, Kaneda Y, Taniyama Y, Matsumoto K, Nakamura T, Ogihara T, Morishita R. Essential role of HGF (hepatocyte growth factor) in blood formation in xenopus. Blood. 2004;103(9):3320–3325. doi: 10.1182/blood-2003-02-0352. [DOI] [PubMed] [Google Scholar]
- 52.Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei K, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential contribution of a novel antifibrotic factor hepatocyte growth factor to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102(2):246–252. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 53.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40(1):47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 54.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 1999;5(2):226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 55.Vlassara H, Cai W, Chen X, Serrano EJ, Shobha MS, Uribarri J, Woodward M, Striker GE. Managing chronic inflammation in the aging diabetic patient with ckd by diet or sevelamer carbonate A modern paradigm shift. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67(12):1410–1416. doi: 10.1093/gerona/gls195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansson GK. Inflammation atherosclerosis and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 57.Mantzouranis EC, Rosen FS, Colten HR. Reticuloendothelial clearance in cystic fibrosis and other inflammatory lung diseases. N. Engl. J. Med. 1988;319(6):338–343. doi: 10.1056/NEJM198808113190604. [DOI] [PubMed] [Google Scholar]
- 58.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 59.Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv. Anat. Pathol. 2010;17(1):42–48. doi: 10.1097/PAP.0b013e3181c66f4e. [DOI] [PubMed] [Google Scholar]
- 60.Mooi WJ. Oncogene-induced cellular senescence Causal factor in the growth arrest of pituitary microadenomas? Horm. Res. 2009;71(Suppl. 2 ):78–81. doi: 10.1159/000192442. [DOI] [PubMed] [Google Scholar]
- 61.Imanishi T, Tsujioka H, Akasaka T. Endothelial progenitor cell senescence--is there a role for estrogen? Ther. Adv. Cardiovasc. Dis. 2010;4(1):55–69. doi: 10.1177/1753944709353173. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu K, Taniyama Y, Sanada F, Azuma J, Iwabayashi M, Iekushi K, Rakugi H, Morishita R. Hepatocyte growth factor inhibits lipopolysaccharide-induced oxidative stress via epithelial growth factor receptor degradation. Arterioscler. Thromb. Vasc. Biol. 2012;32(11):2687–2693. doi: 10.1161/ATVBAHA.112.300041. [DOI] [PubMed] [Google Scholar]
- 63.Kaga T, Kawano H, Sakaguchi M, Nakazawa T, Taniyama Y, Morishita R. Hepatocyte growth factor stimulated angiogenesis without inflammation Differential actions between hepatocyte growth factor vascular endothelial growth factor and basic fibroblast growth factor. Vasc. Pharmacol. 2012;57(1):3–9. doi: 10.1016/j.vph.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inoue S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110(16):2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 65.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor The case for paracrine amplification of angiogenesis. Circulation. 1998;97(4):381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 66.Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001;158(3):1111–1120. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial icam-1 and vcam-1 by inhibiting the nuclear factor-kappa B pathway. Circ. Res. 2005;96(3):300–307. doi: 10.1161/01.RES.0000155330.07887.EE. [DOI] [PubMed] [Google Scholar]
- 68.Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, Nakashima N, Maeyama T, Yoshimi M, Nakanishi Y. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J. Immunol. 2005;175(2):1224–1231. doi: 10.4049/jimmunol.175.2.1224. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhary NI, Roth GJ, Hilberg F, Muller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 2007;29(5):976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 70.Okayama K, Azuma J, Dosaka N, Iekushi K, Sanada F, Kusunoki H, Iwabayashi M, Rakugi H, Taniyama Y, Morishita R. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial-mesenchymal transition. Hypertension. 2012;59(5):958–965. doi: 10.1161/HYPERTENSIONAHA.111.183905. [DOI] [PubMed] [Google Scholar]
- 71.Nakano N, Morishita R, Moriguchi A, Nakamura Y, Hayashi SI, Aoki M, Kida I, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Negative regulation of local hepatocyte growth factor expression by angiotensin ii and transforming growth factor-beta in blood vessels Potential role of hgf in cardiovascular disease. Hypertension. 1998;32(3):444–451. doi: 10.1161/01.hyp.32.3.444. [DOI] [PubMed] [Google Scholar]
- 72.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium improving ventricular function and long-term survival. Circ. Res. 2005;97(7):663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 73.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease Morbidity and mortality implications. Circulation. 2006;114(7):688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 74.Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, Goelman G, Keshet E. Conditional switching of VEGF provides new insights into adult neovascularization and pro angiogenic therapy. EMBO. J. 2002;21(8):1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gounis MJ, Spiga MG, Graham RM, Wilson A, Haliko S, Lieber BB, Wakhloo AK, Webster KA. Angiogenesis is confined to the transient period of VEGF expression that follows adenoviral gene delivery to ischemic muscle. Gene. Ther. 2005;12(9):762–771. doi: 10.1038/sj.gt.3302481. [DOI] [PubMed] [Google Scholar]
- 76.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105(10):1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 77.Fumoto S, Nishi J, Nakamura J, Nishida K. Gene therapy for gastric diseases. Curr. Gene Ther. 2008;8(3):187–200. doi: 10.2174/156652308784746431. [DOI] [PubMed] [Google Scholar]