Abstract
Background:
Decreased nitric oxide (NO) bioavailability and increased lipid oxidation are associated with progressive endothelial dysfunction. L-Citrulline, the effective precursor of L-arginine which is essential as a substrate for endothelial NO synthase (eNOS), is effective in enhancing NO-dependent signaling. However, little is known about the efficacy of L-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction.
Methods:
Twenty-two patients (aged 41 - 64 years old) diagnosed with vasospastic angina with flow-mediated dilation (FMD) of the brachial artery (< 5.5 %) received 800 mg/day of L-citrulline for 8 weeks. FMD (%), blood NOx, asymmetric dimethylarginine (ADMA), small dense LDL, oxidized lipids, amino acids concentrations were measured before and after supplementation.
Results:
Compared with baseline values, FMD (%) was significantly improved at 4 and 8 weeks as well as at 4 weeks after the end of intake. L-Citrulline supplementation caused a significant lowering of plasma ADMA levels. Plasma L-arginine/ADMA ratio and NOx levels rose markedly throughout the study period. Moreover, significant reductions of serum oxidized LDL and lectin-like oxidized LDL receptor 1 (LOX-1) ligand containing ApoB (LAB), an indicator of the biological activity of oxidized lipoprotein binding to LOX-1, were observed after L-citrulline intake.
Conclusions:
L-Citrulline supplementation improves endothelial dysfunction, probably due to potentiating NO-dependent reactions and decreasing the state of lipoprotein oxidation in humans.
Keywords: Asymmetric dimethylarginine, endothelial dysfunction, flow-mediated dilation, L-Citrulline, nitric oxide, oxidized LDL.
INTRODUCTION
Endothelial dysfunction is one of the initial factors in cardiovascular events, and is involved in other pathological conditions such as renal disorders, diabetes mellitus, and systemic hypertension [1, 2]. Growing evidence suggests increased oxidative stress to be associated with high levels of oxidized low density lipoprotein (LDL), which induces a wide variety of cellular responses, including induction of adhesion molecules, and accounts for significant advancement of endothelial impairment [3].
Coronary vascular endothelium modulates vascular stiffness through the release of vasodilating and vasoconstricting molecules. Nitric oxide (NO), which is a major endothelium-derived relaxing factor (EDRF) that is generated from L-arginine by endothelial NO synthase (eNOS), plays an important role in the regulation of endothelium-dependent vasodilatation [4-6]. Reduced NO bioavailability caused by endothelial impairment is associated with atherosclerotic coronary artery disease. The results of several clinical studies in which L-arginine was orally administered suggest the potential for therapeutic or preventive strategies to combat cardiovascular diseases by restoring physiological levels of NO and augmenting NO-dependent signalling [7-9], however, relatively large doses of 5 - 15 g/day would be required to improve endothelial function in humans.
L-Citrulline is a colorless, water-soluble α-amino acid that is a potent endogenous precursor of L-arginine. L-Citrulline from dietary supplementation enters the kidney, vascular endothelium, and other tissues [10]. It is then converted to L-argininosuccinate by argininosuccinate synthase and subsequently to L-arginine by argininosuccinate lyase, thus contributing to the elevation of plasma and tissue levels of L-arginine [10, 11]. A recent study reported that oral intake of L-citrulline dose-dependently increased plasma L-arginine levels in healthy human volunteers more effectively than equivalent doses of L-arginine [12]. L-Citrulline can therefore be considered an effective precursor of L-arginine.
A recent study demonstrated that oral supplementation with L-citrulline upregulates endothelial eNOS expression, causes dramatic regression in atheromatous lesions and plays an inhibitory role in the progression of atherosclerosis in animal models [13, 14]. Moreover, we and others have revealed in clinical trials that L-citrulline supplementation functionally improves arterial stiffness [15], causes a reduction in the heart-rate corrected QT interval as a marker of sudden cardiac death [16], and decreases ankle blood pressure and carotid wave reflection [17]. L-Citrulline may thus be considered a useful ingredient for protecting against atherosclerotic coronary artery disease.
However, there are few reports describing the benefit of L-citrulline on the impairment of endothelial function and oxidative stress, a fundamental part of the progression of vascular disorders. The present study, therefore, was undertaken to investigate the effects of L-citrulline on endothelial dysfunction and lipid oxidation in patients with vasospastic angina, a clinical syndrome caused by coronary disorders. The influence on asymmetric dimethylarginine (ADMA), which is an endogenous competitive inhibitor of eNOS and a strong predictor of adverse cardiovascular outcomes [18], was also assessed.
METHODS
Subjects
This study included 25 subjects with vasospastic angina, screened from 34 patients after an initial physical examination, blood tests, and urinary analysis. Three were excluded for reasons of physical conditions not related to the present study; hense, we studied 22 subjects (aged 41 - 64 years old). They had impaired flow-mediated dilation (FMD) of the brachial artery (< 5.5 %) at the cutoff value for cardiovascular risk prediction [19]. The patients were advised not to change any medical therapy that had continued for several years or longer. The exclusion criteria included diabetes, liver or kidney disease, defects in amino acid metabolism, and serious coronary stenosis as assessed by a catheterization and CT check. None of the subjects were taking any supplements, amino acids, or vitamins. Written informed consent was obtained from each subject after the experimental procedures and information on L-citrulline had been fully explained. Subject recruitment, informed consent procedures, and all the experimental methods were approved by the Clinical Research Ethical Committee at Tsukiji Futaba Clinic and were conducted in compliance with the principles of the Declaration of Helsinki.
Study Design
After baseline measurements, including FMD, blood tests, and urinary analysis, the subjects received 800 mg/day of L-citrulline (KYOWA HAKKO BIO CO., LTD., Tokyo, Japan) orally in capsule form, for 8 weeks in an open-label trial. The daily dose was taken at night before going to bed. Blood samples were drawn before supplementation, then after 4 and 8 weeks, and at 4 weeks after the end of the supplementation (follow-up period, 12 weeks in total) to determine plasma NOx (nitrite + nitrate), ADMA, amino acids, hematological and biochemical markers, and serum oxidized lipids. Endothelial function was assessed by using FMD measurement on the same day as the blood collection. Subjects were asked to refrain from drinking alcohol for 48 h before the measurement day. All sampling processes were carried out in the morning after overnight fasting.
Evaluation of Endothelial Function
In most clinical studies, endothelial function is evaluated by FMD measurement [8, 9, 12, 19], an index that represents endothelium-mediated dilation in response to the local bioavailability of NO. FMD measurements were performed in the morning in a quiet temperature-controlled room (22 - 26 °C) after overnight fasting, as shown previously [8, 9], using an ultrasonography system with a linear-array 10-MHz transducer (UNEXEF18G; UNEX Corporation, Nagoya, Japan). Briefly, following 10 minutes’ rest, arterial diameters were scanned at the baseline and during reactive hyperemia. To induce reactive hyperemia, a pneumatic cuff, set to a pressure calculated as 50 mmHg plus the patient’s systolic blood pressure was placed on the right forearm for 5 min. After releasing the pneumatic cuff, the brachial diameter was measured continuously for 2 min, and FMD was determined as the percentage change in diameter after the release of the ischemia cuff compared with the baseline. The same experienced investigator performed all the measurements.
Clinical and Biological Analysis of Blood
Blood samples were collected into heparinized tubes as plasma or collection tubes containing serum separator gel as serum and immediately centrifuged to obtain the supernatant. Plasma NOx (nitrite + nitrate) was measured by NO-detector high performance liquid chromatography (HPLC) (ENO10; Eicom, Kyoto, Japan) [13]. Concentrations of amino acids and ADMA in plasma were determined using an amino acid analyzer (JLC-500/V; JEOL, Tokyo, Japan) and HPLC system after deproteinization, as described previously [18, 20]. Serum small dense LDL was assayed by quantitative technique for LDL subfractionation using the lipoprint LDL system [21]. Serum oxidized LDL, lectin-like oxidized LDL receptor 1 (LOX-1) ligand containing ApoB (LAB), an indicator of the biological activity of oxidized lipoprotein binding to LOX-1, and soluble LOX-1 (sLOX-1) which reflects its cellular expression levels, were quantified using a high-sensitivity ELISA system based on immobilized recombinant human LOX-1 and antihuman LOX-1 antibody respectively, as previously published [22]. We also calculated the LOX index, a novel predictive marker for coronary heart disease, by multiplying serum concentrations of sLOX-1 by those of LAB [22]. For the measurement of plasma concentrations of glucose, lipids (total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol), parameters for liver and renal function (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, total protein, albumin, γ-glutamyl-transpeptidase, creatinine, alkaline phosphatase, urea nitrogen) and hematological markers (leukocytes, erythrocytes, Hb, platelets), blood samples were transported to Mitsubishi Chemical Medience Corporation (Tokyo, Japan) and determined using their standard laboratory methods.
Statistical Analysis
Data are presented as means ± SEM. The paired t-test (two-sided) and Wilcoxon signed-ranks test were used to test for statistical significance between the study and baseline results. Statistical comparisons between the baseline and sequential values were analyzed using Bonferroni’s test following one-way ANOVA. A p-value of less than 0.05 was considered to indicate significance. Statistical analysis was performed with Statcel software for Windows (Version 2, OMS Publishing, Inc., Saitama, Japan) and the ystat 2000 Statistical Program File (Igaku Tosho Shuppan, Tokyo, Japan).
RESULTS
The baseline characteristics and changes in physical parameters in the subjects throughout the study period are given in Table 1. There were no significant differences between baseline and supplementation period. None of the subjects experienced clinically relevant or any side effects.
Table 1.
Baseline characteristics and changes in physical parameters during the study period.
| Study Period (Week) | Intake | Follow-up | ||
|---|---|---|---|---|
| Baseline | 4 | 8 | 12 | |
| Age (years) | 57.0 ± 1.3 | — | — | — |
| Sex (Male/Female) | 7 M, 15 F | |||
| Height (cm) | 159.7 ± 1.7 | — | — | — |
| Weight (kg) | 57.2 ± 2.1 | — | — | — |
| Body mass index (kg /m2) | 22.4 ± 0.7 | — | — | — |
| Systolic blood pressure (mmHg) | 117.0 ± 2.9 | 114.9 ± 2.6 | 117.9 ± 2.7 | 117.1 ± 2.8 |
| Diastolic blood pressure (mmHg) | 69.0 ± 1.8 | 68.2 ± 1.7 | 67.9 ± 1.6 | 66.1 ± 1.8 |
| Heart rate | 68.7 ± 2.7 | 65.6 ± 2.8 | 65.5 ± 2.7 | 65.9 ± 2.7 |
Values are expressed as mean with their standard errors. n = 22.
Table 2 shows the changes in blood biochemical and hematological markers, amino acids, NOx levels before and after L-citrulline supplementation. L-Citrulline supplementation significantly increased plasma L-arginine concentrations at 8 weeks compared with the baseline. A marked but not statistically significant increase in plasma NOx levels were observed after supplementation. The other markers assessed did not differ between before and after the study period, except for a slight but significant change in HDL cholesterol levels within the normal range.
Table 2.
Changes in blood biochemical and hematological markers, amino acids, NOx levels before and after L-citrulline supplementation.
| Study Period (Week) | Intake | Follow-up | ||
|---|---|---|---|---|
| Baseline | 4 | 8 | 12 | |
| Glucose (mg/dl) | 88.9 ± 2.7 | 88.2 ± 1.7 | 88.7 ± 2.4 | 88.3 ± 2.1 |
| Total cholesterol (mg/dl) | 199.6 ± 6.7 | 198.0 ± 6.0 | 195.5 ± 5.6 | 195.4 ± 6.1 |
| Triglycerides (mg/dl) | 109.8 ± 13.2 | 115.2 ± 12.9 | 115.0 ± 18.1 | 110.2 ± 11.4 |
| LDL cholesterol (mg/dl) | 109.5 ± 5.7 | 110.4 ± 5.4 | 108.7 ± 4.6 | 108.4 ± 5.1 |
| HDL cholesterol (mg/dl) | 63.4 ± 3.8 | 62.0 ± 3.5 | 59.8 ± 3.5 * | 59.7 ± 3.4 |
| AST (IU/l) | 21.5 ± 1.1 | 21.5 ± 0.9 | 20.3 ± 0.9 | 21.6 ± 1.3 |
| ALT (IU/l) | 20.4 ± 2.5 | 20.6 ± 2.2 | 18.7 ± 2.0 | 20.5 ± 2.8 |
| LDH (IU/l) | 203.7 ± 7.5 | 201.4 ± 7.3 | 200.6 ± 7.0 | 208.4 ± 7.4 |
| Total protein (g/dl) | 7.6 ± 0.13 | 7.6 ± 0.12 | 7.6 ± 0.09 | 7.6 ± 0.08 |
| Albumin (g/dl) | 4.6 ± 0.1 | 4.6 ± 0.1 | 4.5 ± 0.1 | 4.5 ± 0.1 |
| γ-GTP (IU/l) | 29.0 ± 3.3 | 30.5 ± 4.7 | 27.9 ± 2.7 | 26.9 ± 2.6 |
| Creatinine (mg/dl) | 0.7 ± 0.03 | 0.7 ± 0.03 | 0.7 ± 0.04 | 0.7 ± 0.04 |
| ALP (IU/l) | 233.2 ± 7.9 | 230.1 ± 6.9 | 228.2 ± 6.5 | 228.2 ± 8.3 |
| Urea nitrogen (mg/dl) | 13.1 ± 0.6 | 13.1 ± 0.6 | 13.2 ± 0.7 | 13.8 ± 0.7 |
| Leukocytes (µl-1) | 5618.2 ± 346.9 | 5822.7 ± 415.0 | 5377.3 ± 343.1 | 5623.8 ± 319.4 |
| Erythrocytes (×104/µl) | 450.6 ± 8.1 | 447.0 ± 7.4 | 445.1 ± 7.8 | 445.7 ± 7.6 |
| Hb (g/dl) | 13.7 ± 0.3 | 13.6 ± 0.3 | 13.6 ± 0.3 | 13.5 ± 0.3 |
| Platelet (×104/µl) | 26.2 ± 1.1 | 25.6 ± 1.1 | 26.3 ± 1.2 | 26.2 ± 1.4 |
| L-Citrulline (µmol/l) | 40.2 ± 1.3 | 40.4 ± 1.6 | 42.1 ± 2.0 | 42.1 ± 1.4 |
| L-Arginine (µmol/l) | 49.1 ± 2.6 | 52.8 ± 4.1 | 56.3 ± 3.7 * | 48.4 ± 3.9 |
| NOx (µmol/l) | 33.0 ± 3.4 | 54.3 ± 14.1 | 46.5 ± 10.9 | 41.7 ± 6.8 |
Values are expressed as mean with their standard errors. LDL, low density lipoprotein; HDL, high density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; γ-GTP, γ-glutamyltranspeptidase; ALP, alkaline phosphatase; Hb, hemoglobin. Significant difference from baseline score, *p < 0.05. n = 22.
The changes in serum concentrations of small dense LDL and biochemical markers related to oxidized lipoprotein are summarized in Table 3. L-Citrulline supplementation resulted in a significant reduction in serum levels of oxidized LDL and LAB as compared with the baseline, while small dense LDL and sLOX-1 were unchanged. Small changes in the LOX index were observed after L-citrulline supplementation, but were not statistically significant.
Table 3.
Changes in blood concentrations of small dense LDL and biochemical markers related to oxidized lipoprotein at baseline and at 8 weeks.
| Study Period | ||
|---|---|---|
| Baseline | 8 Weeks | |
| Small dense LDL (mg/dl) | 5.0 ± 1.8 | 4.8 ± 1.9 |
| Oxidized LDL (U/l) | 128.2 ± 9.0 | 113.7 ± 7.6 * |
| LAB (ng/ml) | 3661.4 ± 311.3 | 3007.3 ± 206.7* |
| Soluble LOX-1 (pg/ml) | 1039.0 ± 180.5 | 989.9 ± 162.1 |
| LOX-1 index | 3544.1 ± 553.0 | 2890.6 ± 462.9 |
Values are expressed as mean with their standard errors. LDL, low density lipoprotein; LOX-1, lectin-like oxidized LDL receptor 1; LAB, LOX-1 ligand containing apolipoprotein B. Significant difference from baseline score, *p < 0.05. n = 22.
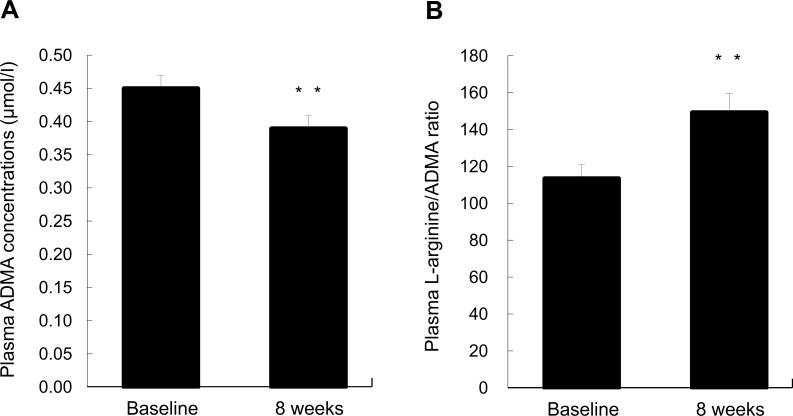
Fig. 1 shows the effects of L-citrulline on plasma ADMA concentrations and L-arginine/ADMA ratio. Compared with the baseline, plasma ADMA was significantly reduced by L-citrulline supplementation. A significantly improved L-arginine/ADMA ratio was also observed after oral administration of L-citrulline.
Fig. (1).
Plasma ADMA concentrations (A) and L-arginine/ADMA ratio (B) at baseline and at 8 weeks. The subjects received 800 mg/day of L-citrulline orally for 8 weeks. Following overnight fasting, blood samples were drawn to analyze plasma ADMA and L-arginine concentrations before and after L-citrulline supplementation. Values are expressed as mean with their standard errors. ADMA, asymmetric dimethylarginine. Significant difference from baseline score, **p < 0.01. n = 22.
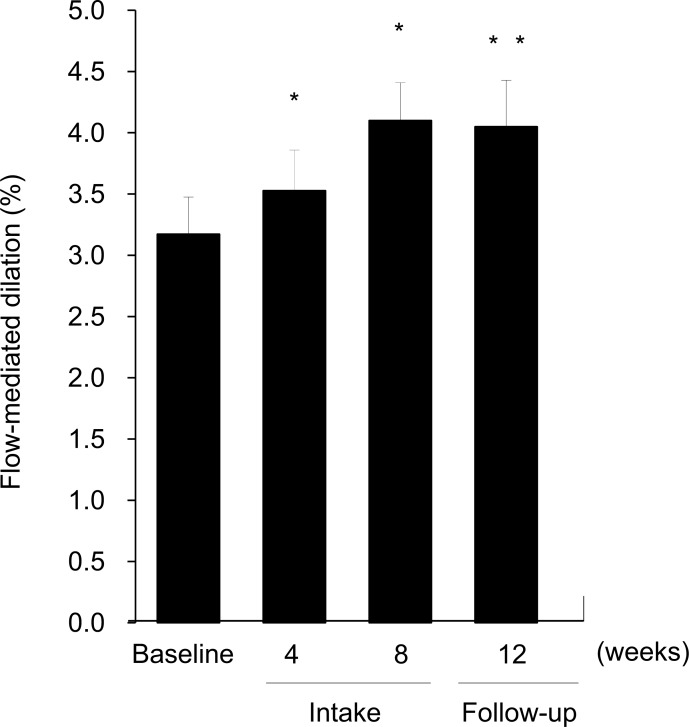
The changes in FMD (%) before and after L-citrulline supplementation are given in (Fig. 2). Our subjects showed a markedly impaired endothelial function at baseline, that was much lower than the normal value (approximately 8.0 % or higher) of FMD (%) in healthy people as reported previously [9, 23]. L-Citrulline supplementation then exerted a significant improvement in FMD (%) at 4 and 8 weeks, and maintained its effects at 4 weeks after the end of intake.
Fig. (2).
Percent changes in flow-mediated dilation (FMD) of the brachial artery before and after L-citrulline supplementation. The subjects received 800 mg/day of L-citrulline orally for 8 weeks. Endothelial function was assessed using FMD measurement before supplementation, after 4 and 8 weeks, and at 4 weeks after the end of supplementation (follow-up period, 12 weeks in total). Values are expressed as mean with their standard errors. Significant difference from baseline score,**p < 0.05, **p < 0.01. n = 22.
DISCUSSION
The objective of the present study was to evaluate the influence of oral L-citrulline intake on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. The major findings of our trial were that oral administration of L-citrulline has important beneficial effects in that it improves endothelial dysfunction and reduces oxidative stress.
FMD measurement is a well-established method for studying endothelium-dependent vasodilation in humans. In our study patients, FMD (%) was considerably impaired, and was close to or below values seen in coronary artery disease [19, 23]. In this trial, L-citrulline supplementation significantly improved FMD (%).
The endothelium is an important modulator of vascular tone through many signal molecules [3]. The endothelium-derived relaxing factor NO is produced from L-arginine by eNOS. It has been reported that intervention with L-arginine can improve endothelial dysfunction in humans and animal models [7-9, 24]. However, the recent literature has reported no effects of chronic administration of L-arginine [25, 26], since orally administered L-arginine passes through the gastrointestinal tract and is trapped in the hepatic tissue, where it is extensively catabolized by arginase that also can be induced by dietary L-arginine [10, 27], suggesting its limited oral bioavailability as a substrate for eNOS. On the other hand, L-citrulline is an effective precursor of L-arginine and also suppresses arginase activity as an allosteric inhibitor [28]. Moreover, extracellular L-arginine, not its intracellular level, is the major determinant of NO production via the cationic amino acid transporter 1 (CAT-1) which is localized in the caveolae of endothelial cells, contributing to the uptake of extracellular L-arginine directly to eNOS [29], although intracellular L-arginine concentration (~ 800 μmol/l or higher) is the saturated level for the km of eNOS. The present study showed L-citrulline supplementation to significantly increase plasma L-arginine and markedly enhance NOx levels, as mentioned above. These findings reveal the role played by L-citrulline in increasing L-arginine levels and upregulating NO bioavailability.
Our results indicate a significant reduction in plasma ADMA levels, and a significant improvement in L-arginine/ADMA ratio, a marker of endothelial dysfunction [15, 30], after L-citrulline supplementation. In this study, baseline ADMA levels in patients with vasospastic angina were 20 % higher than those found in healthy middle-aged men [15], and similar to those seen in patients with coronary artery disease [31]. Endothelial dysfunction has been linked to elevated concentration of ADMA [18, 30]. A previous clinical study has reported a high correlation between increased plasma ADMA levels and decreased NO-mediated vasodilation [32]. One study has shown that treatment with watermelon juice, which is rich in L-citrulline, significantly decreased serum concentration of ADMA in an animal model of diabetes [14]. In the present study, we speculate that L-citrulline supplementation elevates L-arginine availability, ameliorates the interference of ADMA with eNOS, and thus effectively improves NO signalling.
Oxidative stress has been linked to impaired endothelial function in atherosclerosis [3, 28]. The activation of adhesion molecules elicited by oxidized LDL and the uptake of oxidized LDL by macrophages are key steps in the progression of atherothrombotic cardiovascular diseases [33]. In this present study, we demonstrated that L-citrulline supplementation caused significant reduction in serum oxidized LDL and LAB levels. Small changes in LOX index were also observed as a result of L-citrulline supplementation. Here, LOX-1 is the receptor for oxidized LDL in endothelial cells, and upregulated LOX-1 expression is involved in inflammation as well as atherogenesis [34]. It is strongly suggested that LAB, which indicates the biological activity of oxidized lipoprotein binding to LOX-1, is more relevant to atherogenic risk than oxidized lipids, including oxidized LDL [22]. On the other hand, NO has potent antioxidant properties that exert anti-inflammatory effects and can inhibit LDL oxidation [35]. It has also been reported that oral administration of L-citrulline decreased superoxide production from the aorta in animal models of atherosclerosis [13, 28]. In addition, L-citrulline itself is an efficient radical scavenger and is a strong antioxidant [36]. Therefore, the efficacy of L-citrulline in suppressing lipoprotein oxidation is likely to be partially mediated by the antioxidant effect of NO. This lowering effect of oxidized lipoprotein may play an important role in improving endothelial dysfunction.
There is growing evidence that small dense LDL is a risk factor for cardiovascular diseases [37]. In one clinical trial, the blood levels of small dense LDL, assayed using the lipoprint LDL system, the same method that we used here, in an Asian population without coronary artery disease, were generally below 7 mg/dl [38]. Accordingly, we performed a differential analysis in subjects with serum small dense LDL of > 8 mg/dl (n = 5) for blood concentrations in patients with diabetes and coronary artery disease [38]. A statistically significant decrease in the serum level of small dense LDL was observed as a result of L-citrulline intake (p < 0.01, data not shown). It may contribute in a minor way to suppressing the penetration of this lipoprotein species to the arterial wall.
Interestingly, L-citrulline supplementation improved FMD (%) at the first time point, 4 weeks, and still maintained a marked improvement at 4 weeks after the end of intake, although plasma L-arginine either did not significantly increase or returned to the baseline levels. L-Citrulline itself is transported into the endothelial cells by neural amino acid system N transporter 1 (SN1) [39], and is used as a substrate to form L-arginine. A laboratory study has indicated that L-citrulline added to cultured endothelial cells sustained NOS activity and NO production under L-arginine-deficient conditions [10]. This could provide an explanation in that direct uptake of L-citrulline by endothelial cells might contribute to improvement of FMD (%) at the basal concentration of plasma L-arginine. It has also been reported that oral L-citrulline supplementation caused an up-regulation of eNOS protein expression in the rabbit aorta [13]. eNOS up-regulation is an important process for supplying bioavailable NO to vascular tissue. In our study we speculate that a functionally active form of eNOS might be increased at 4 weeks by taking L-citrulline and maintained until 12 weeks. In fact, plasma NOx levels tended to be higher at 4 and 12 weeks, showing a 65 % and 26 %-increases over baseline respectively. Moreover, the effects of L-citrulline in lowering blood ADMA and oxidized lipoprotein, both strongly associated with the maintenance of endothelial function, found at 8 weeks may be sustained until 12 weeks, which could lead to preserved endothelial function. We believe a further study needs to be done to investigate continuing effects after ending L-citrulline intake in the future.
Coronary spasm is the leading cause of myocardial ischemia [40]. The precise mechanisms underlying vasospastic angina remain unknown. However, a previous study has demonstrated that a deficiency in endothelial NO activity in spastic arteries is associated with the pathogenesis of coronary spasm [41]. It is also known that endothelial dysfunction related to the impaired endothelium-derived NO bioavailability is an independent risk factor responsible for vasospastic angina [42]. Therefore, our results showing that oral L-citrulline intake significantly improved endothelial dysfunction suggest a therapeutic strategy for decreasing coronary spasms in patients with vasospastic angina.
In conclusion, our results demonstrate that oral L-citrulline supplementation for 8 weeks at a dose of 800 mg/day improves endothelial dysfunction. This effect is probably due to improved L-arginine bioavailability and L-arginine/ADMA ratio in association with potentiating NO-dependent reactions, combined with reduced oxidative stress. This study proposes therapeutic functions for L-citrulline supplementation in heart failure patients with vasospastic angina.
Heart diseases including vasomotor angina have become one of the most important health problems worldwide, especially among Asian population [43], and the number of young patients is also increasing recently. L-Citrulline is a potentially novel amino acid, and its no obvious side effects have been observed in studies to date. Thus, dietary L-citrulline may be beneficial for the supplemental treatment of heart diseases.
ACKNOWLEDGEMENTS
The authors thank Biomarker Science Co. Ltd., for their technical assistance with LOX-1 and LAB analysis.
CONFLICT OF INTEREST
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that there is no conflict of interest.
ABBREVIATIONS
- ADMA
= Asymmetric dimethylarginine
- eNOS
= Endothelial NO synthase
- FMD
= Flow-mediated dilation
- LAB
= LOX-1 ligand containing apolipoprotein B
- LDL
= Low density lipoprotein
- LOX-1
= Lectin-like oxidized LDL receptor 1
- NO
= Nitric oxide
REFERENCES
- 1.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60(1):119–126. [PubMed] [Google Scholar]
- 2.Vogel RA. Coronary risk factors endothelial function, and atherosclerosis a review . Clin Cardiol. 1997;20(5):426–432. doi: 10.1002/clc.4960200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 4.Elahi MM, Naseem KM, Matata BM. Nitric oxide in blood.The nitrosative-oxidative disequilibrium hypothesis on the pathogenesis of cardiovascular disease. . FEBS J. 2007;274(4):906–923. doi: 10.1111/j.1742-4658.2007.05660.x. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Bolanos JP. Nitric oxide cell bioenergetics and neurodegeneration. J Neurochem. 2006;97(6):1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 7.Lerman A, Burnett JC, Jr., Higano ST, McKinley LJ, Holmes DRJr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 8.Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, Andreou I, Zisimos K, Papavassiliou AG, Stefanadis C. Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol. 2008;0126(3):394–399. doi: 10.1016/j.ijcard.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Tsai WC, Chen JY, Li YH, Lin LJ, Chen JH. Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int J Cardiol. 2008;127(3):337–341. doi: 10.1016/j.ijcard.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006;24(3-4):275–290. doi: 10.1111/j.1527-3466.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 11.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids. 2005;29(3):177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 12.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Boger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin. Pharmacol. 2008;65(1):51–59. doi: 10.1111/j.1365-2125.2007.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. L-Citrulline and L-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl. Acad Sci USA. 2005;102(38):13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137(12):2680–2685. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanabe F, Morishita K. Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol. 2012;155(2):257–261. doi: 10.1016/j.ijcard.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kameda N, Okigawa T, Kimura T, Fujibayashi M, Asada T, Kinoshita R, Baba S, Morita M, Morishita K, Moritani T. The effect of L-citrulline ingestion on ECG QT interval and autonomic nervous system activity. J Physiol. Anthropol. 2011;30(2):41–45. doi: 10.2114/jpa2.30.41. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa A, Sanchez-Gonzalez MA, Wong A, Arjmandi BH. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am J. Hypertens. 2012;25(6):640–643. doi: 10.1038/ajh.2012.20. [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Boger R. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 2002;62(1):339–345. doi: 10.1046/j.1523-1755.2002.00437.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll. Cardiol. 2004;44(8):1636–1640. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Moinard C, Nicolis I, Neveux N, Darquy S, Benazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr. 2008;99(4):855–862. doi: 10.1017/S0007114507841110. [DOI] [PubMed] [Google Scholar]
- 21.Hoefner DM, Hodel SD, O'Brien JF, Branum EL, Sun D, Meissner I, McConnell JP. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001;47(2):266–274. [PubMed] [Google Scholar]
- 22.Inoue N, Okamura T, Kokubo Y, Fujita Y, Sato Y, Nakanishi M, Yanagida K, Kakino A, Iwamoto S, Watanabe M, Ogura S, Otsui K, Matsuda H, Uchida K, Yoshimoto R, Sawamura T. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56(4):550–558. doi: 10.1373/clinchem.2009.140707. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell AJ, Zapien MP, Pearce GL, MacCallum G, Stone PH. Randomized trial of a medical food for the dietary management of chronic stable angina. J Am Coll Cardiol. 2002;39(1):37–45. doi: 10.1016/s0735-1097(01)01708-9. [DOI] [PubMed] [Google Scholar]
- 24.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J.utr. . 2005; 135(4):714–721. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 25.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Susic D, Francischetti A, Frohlich ED. Prolonged L-arginine on cardiovascular mass and myocardial hemodynamics and collagen in aged spontaneously hypertensive rats and normal rats. Hypertension. 1999;33(1 Pt. 2):451–455. doi: 10.1161/01.hyp.33.1.451. [DOI] [PubMed] [Google Scholar]
- 27.Morris SM Jr. Regulation of enzymes of urea and arginine synthesis. Annu Rev Nutr. 1992;12:81–101. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- 28.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ. Res. 2008;102(1):95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin S, Mohan S, Fung HL. Intracellular L-arginine concentration does not determine NO production in endothelial cells: implications on the "L-arginine paradox". Biochem Biophys. Res. Commun. 2011;414(4):660–663. doi: 10.1016/j.bbrc.2011.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119(12):1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enseleit F, Sudano I, Periat D, Winnik S, Wolfrum M, Flammer AJ, Frohlich GM, Kaiser P, Hirt A, Haile SR, Krasniqi N, Matter CM, Uhlenhut K, Hogger P, Neidhart M, Luscher TF, Ruschitzka F, Noll G. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease a double-blind randomized placebo-controlled cross-over study. Eur Heart J. 2012;33(13):1589–1597. doi: 10.1093/eurheartj/ehr482. [DOI] [PubMed] [Google Scholar]
- 32.Bode-Boger SM, Muke J, Surdacki A, Brabant G, Boger RH, Frolich JC. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8(2):77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 33.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature . 1997;386(6620 ):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 34.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl. Acad Sci USA. 2003;100(3):1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli C, Ackah E, De Nigris F, Del Soldato P, D'Armiento FP, Crimi E, Condorelli M, Sessa WC. Chronic treatment with nitric oxide-releasing aspirin reduces plasma low-density lipoprotein oxidation and oxidative stress arterial oxidation-specific epitopes, and atherogenesis in hypercholesterolemic mice. Proc Natl Acad. Sci USA. 2002;99(19):12467–12470. doi: 10.1073/pnas.192244499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akashi K, Miyake C, Yokota A. Citrulline a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001;508(3):438–442. doi: 10.1016/s0014-5793(01)03123-4. [DOI] [PubMed] [Google Scholar]
- 37.Packard C, Caslake M, Shepherd J. The role of small dense low density lipoprotein (LDL): a new look. Int. J. Cardiol . 2000;74(Suppl. 1 ):S17–22. doi: 10.1016/s0167-5273(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 38.Mohan V, Deepa R, Velmurugan K, Gokulakrishnan K. Association of small dense LDL with coronary artery disease and diabetes in urban Asian Indians- the Chennai Urban Rural Epidemiology Study (CURES-8). J Assoc Physicians India. 2005;53:95–100. [PubMed] [Google Scholar]
- 39.Simon A, Plies L, Habermeier A, Martine U, Reining M, Closs EI. Role of neutral amino acid transport and protein breakdown for substrate supply of nitric oxide synthase in human endothelial cells. Circ Res. 2003;93(9):813–820. doi: 10.1161/01.RES.0000097761.19223.0D. [DOI] [PubMed] [Google Scholar]
- 40.Maseri A, Chierchia S. Coronary vasospasm in ischemic heart disease. Chest. 1980;78( 1 Suppl.):210–215. doi: 10.1378/chest.78.1_supplement.210. [DOI] [PubMed] [Google Scholar]
- 41.Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, Yoshimura M, Motoyama T, Inobe Y, Kawano H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94(3):266–271. doi: 10.1161/01.cir.94.3.266. [DOI] [PubMed] [Google Scholar]
- 42.Teragawa H, Kato M, Kurokawa J, Yamagata T, Matsuura H, Chayama K. Endothelial dysfunction is an independent factor responsible for vasospastic angina. Clin Sci. 2001;101(6):707–713. [PubMed] [Google Scholar]
- 43.Sasayama S. Heart disease in Asia. Circulation. 2008;118(25):2669–2671. doi: 10.1161/CIRCULATIONAHA.108.837054. [DOI] [PubMed] [Google Scholar]