Abstract
Emerging studies suggested that murine podoplanin-positive monocytes (PPMs) are involved in lymphangiogenesis. The goal of this study was to demonstrate the therapeutic lymphangiogenesis of human PPMs by the interaction with platelets. Aggregation culture of human peripheral blood mononuclear cells (PBMCs) resulted in cellular aggregates termed hematospheres. During 5-day culture, PPMs expanded exponentially and expressed several lymphatic endothelial cell–specific markers including vascular endothelial growth factor receptor (VEGFR)-3 and well-established lymphangiogenic transcription factors. Next, we investigated the potential interaction of PPMs with platelets that had C-type lectin–like receptor-2 (CLEC-2), a receptor of podoplanin. In vitro coculture of PPMs and platelets stimulated PPMs to strongly express lymphatic endothelial markers and upregulate lymphangiogenic cytokines. Recombinant human CLEC-2 also stimulated PPMs through Akt and Erk signaling. Likewise, platelets in coculture with PPMs augmented secretion of a lymphangiogenic cytokine, interleukin (IL)-1-β, via podoplanin/CLEC-2 axis. The supernatant obtained from coculture was able to enhance the migration, viability, and proliferation of lymphatic endothelial cell. Local injection of hematospheres with platelets significantly increased lymphatic neovascularization and facilitated wound healing in the full-thickness skin wounds of nude mice. Cotreatment with PPMs and platelets augments lymphangiogenesis through podoplanin/CLEC-2 axis, which thus would be a promising novel strategy of cell therapy to treat human lymphatic vessel disease.
Introduction
Disruption of the lymphatic system can cause chronic problems such as remodeling of the skin and the subcutaneous extracellular matrix, leading to the accumulation of lipids and macrophages in the affected tissue.1 Treatment of lymphatic vascular disease relies heavily on an empirically developed lymphatic-specific massage technique termed manual lymphatic drainage,2 and care of this chronic condition requires lifelong attention and good compliance as for other vascular diseases and diabetes. Furthermore, it is well known that diabetic patients frequently have severe problems with impaired wound healing whose mechanisms are generally poorly understood. Therefore, new therapies to treat chronic lymphedema and impaired wound healing are in great demand.
Adult bone marrow or peripheral blood is most convenient therapeutic cell source and has been widely used in the treatment of various hematologic or ischemic diseases.3,4 Previous report shows that murine monocyte/macrophages contribute to the formation of lymphatic vessels and promote diabetic wound healing,5,6 and tumor-associated macrophages play a pivotal role in peritumoral lymphangiogenesis.7 Additional reports demonstrated that mouse bone marrow or circulating blood-derived cells that express lymphendothelial cell markers can function as lymphatic endothelial progenitor cells and participate in postnatal lymphatic neovascularization.8,9,10 Reviewing these reports, it could be inferred that peripheral blood–derived lymphangiogenic cells, which are relatively easily accessible, might be of therapeutic value in patients with chronic or acute lymphatic edema and impaired wound healing. However, the limitation of the previous studies is lack of evidences in human cells because the results were primarily based on murine cell sources. Therefore, identification of human lymphangiogenic cells and evaluation of their lymphangiogenic potential are required. In addition, breakthrough to overcome the barriers to obtain sufficient number of cells for lymphatic cell therapy is needed since the number of lymphangiogenic cells, which conventionally obtained from peripheral blood or bone marrow, is insufficient.
We previously reported a three-dimensional (3D) culture method with human peripheral blood mononuclear cells (PBMCs), which led to generation of cellular hematospheres termed blood-born hematospheres.11 The components of hematosphere were hematopoietic stem cells and myeloid niche supporting cells. In addition, we proved that myeloid cells derived from hematosphere actively participate in angiogenesis as well as support the angiogenic niche of the hematospheres.12 In this study, we developed a new method to generate large amount of podoplanin-positive cells out of the human circulating monocytes, dissected the mechanism of such a transdifferentiation with interaction of platelet, and the therapeutic potential to facilitate wound healing.
Results
Aggregation culture of human PBMCs induces expansion of podoplanin-positive monocytes
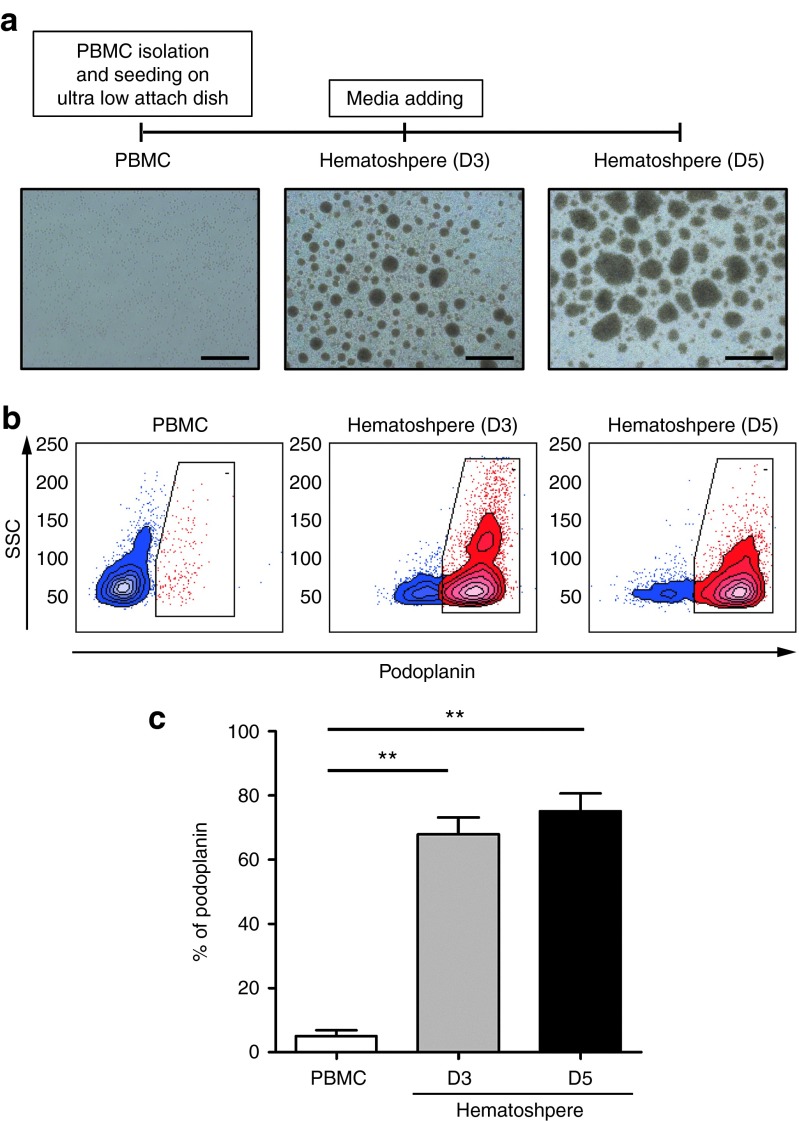
To investigate whether human monocyte aggregates have lymphangiogenic potential, we first examined lymphatic endothelial cell marker expression in the hematospheres during aggregation culture by fluorescence-associated cell sorter analysis. First, freshly isolated PBMCs were cultured on the ultralow attach dish as previously described.11 As a result, numerous cellular spheroids, which we call as hematospheres, were observed via aggregation culture and their sizes continued to increase gradually during 5-day culture (Figure 1a). During 3 days of culture, the expression of podoplanin increased dramatically in hematospheres compared to freshly isolated PBMCs, and about 75% of monocytes expressed podoplanin at day 5 (Figure 1b,c).
Figure 1.
Aggregation culture of human mononuclear cells expands podoplanin-positive monocytes (PPMs). (a) Timeline of aggregation culture. PBMCs were cultured under three-dimensional (3D) suspension condition and harvested at day 3 or 5. The number and size of hematosphere increased during 3D culture. Scale bar = 0.5 mm. (b) Flow cytometry analysis of fresh PBMCs and cultured hematospheres at days 3 and 5 was performed with podoplanin antibody. (c) Bar graph representing the percentage of PPMs during hematosphere culture. Most of the cells in hematospheres expressed podoplanin. Each value is the average of three independent experiments (**P < 0.01; n = 4 per experiment). PBMCs, peripheral blood mononuclear cells; SSC, side scatter.
Expanded podoplanin-positive monocytes express lymphangiogenesis-related genes and lymphatic markers
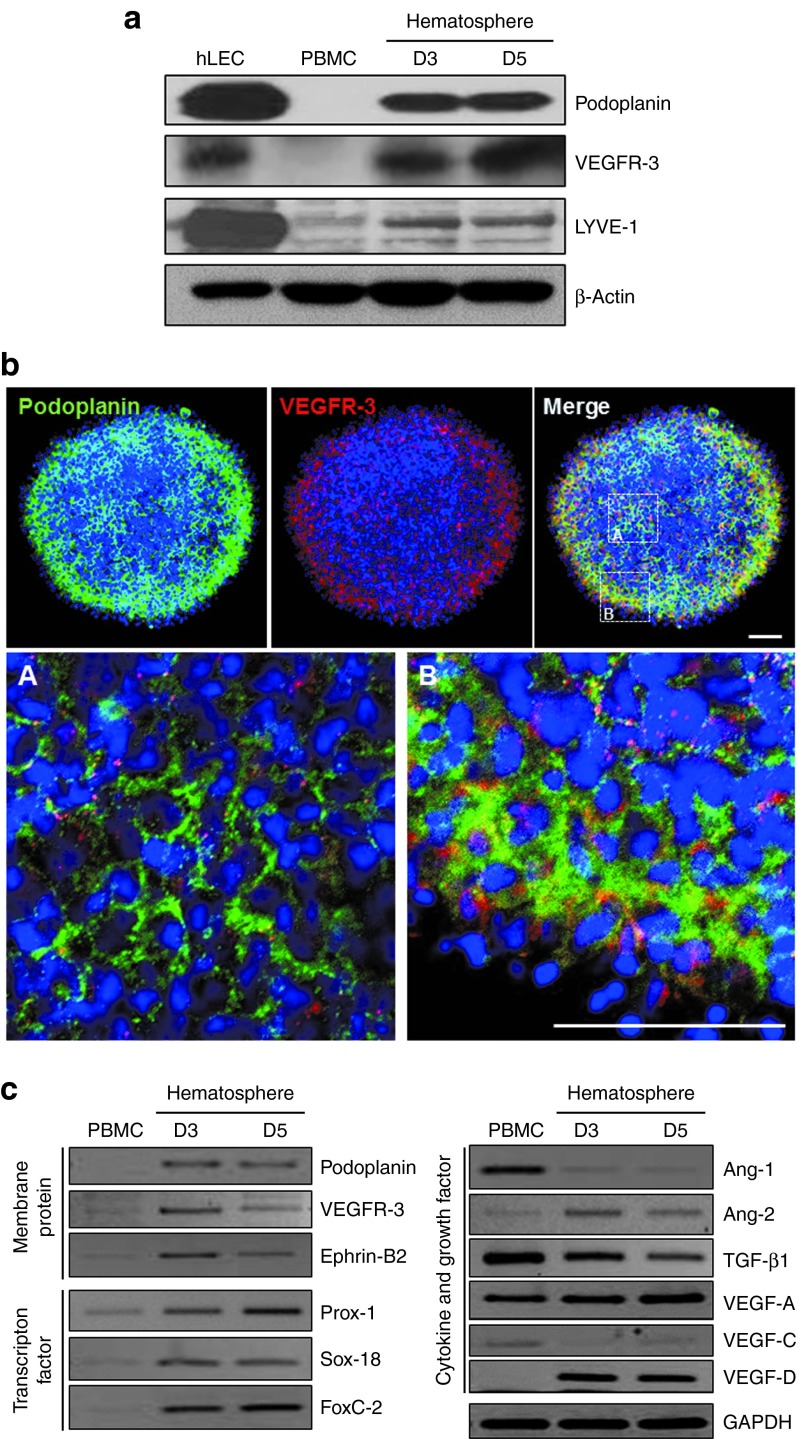
After optimization of experimental conditions regarding media and antibodies (Supplementary Figures S1 and S2), we compared the protein expression of vascular endothelial growth factor receptor (VEGFR)-3 and lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), another makers of lymphatic endothelial cell, between freshly isolated PBMCs and hematospheres as well as in human lymphatic endothelial cells (hLECs). Podoplanin, VEGFR-3, and LYVE-1 were significantly increased by aggregation culture (Figure 2a). In addition, we found that most of the podoplanin single-positive cells were located in the center of hematosphere, and podoplanin and VEGFR-3 double-positive cells were located in the periphery of hematosphere by the immunofluorescence (Figure 2b). Furthermore, we proved that VEGFR-3 and LYVE-1 were upregulated in podoplanin-positive cells by fluorescence-associated cell sorter analysis (Supplementary Figure S3a,b). To explore whether hematospheres express lymphangiogenesis-related genes, we conducted semiquantitative reverse transcription polymerase chain reaction (RT-PCR). Hematospheres showed a significant induction of lymphatic endothelial cell–specific genes, such as membrane protein (podoplanin, VEGFR-3, and Ephrin-B2), transcription factors (Prox-1, Sox-18, and FoxC-2), and cytokines or growth factors (angiopoietin-2, vascular endothelial growth factor (VEGF)-A, and VEGF-D) (Figure 2c). On the other hand, expression of transforming growth factor-β1, antilymphangiogenic cytokine,13,14,15 decreased gradually and VEGF-C maintained low level until day 5 (Figure 2c). Interestingly, the expression of angiopoietin-1 decreased during culture while expression of angiopoietin-2 increased. These results indicated that hematosphere provided a good niche for myeloid cells to transdifferentiate and obtain the characteristics of lymphatic endothelial cell.
Figure 2.
Expanded podoplanin-positive monocytes (PPMs) express lymphangiogenesis-related genes. (a) Protein expression of podoplanin, VEGFR-3, and LYVE-1 in hLEC, fresh PBMCs, and cultured hematospheres at days 3 and 5 via western blot analysis. PPMs also expressed VEGFR-3 and LYVE-1. β Actin was utilized as an internal control. (b) Whole-mount immunofluorescence staining of the cultured hematospheres on day 5 with podoplanin and VEGFR-3. Scale bar = 50 µm. (c) Gene expression of lymphangiogenesis-related genes in fresh PBMCs and cultured hematospheres at days 3 and 5 via semiquantitative RT-PCR. PPMs showed a significant induction of lymphatic endothelial cell–specific genes. GAPDH was utilized as an internal control. Ang, angiopoietin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hLEC, human lymphatic endothelial cell; PBMCs, peripheral blood mononuclear cells; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Podoplanin-positive/VEGFR-3high monocytes in hematospheres possess lymphangiogenic characteristics
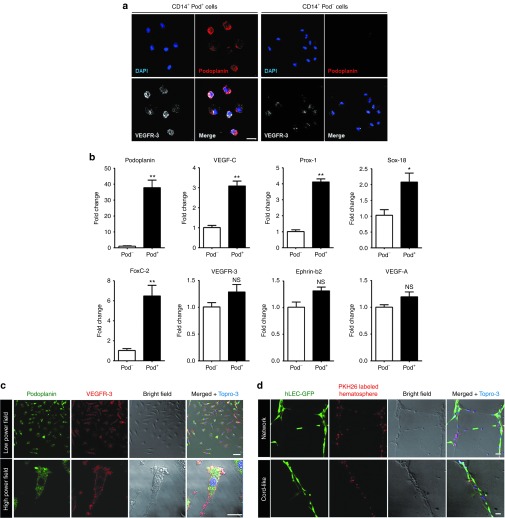
To characterize the podoplanin-positive monocytes (PPMs) in hematospheres, we isolated the podoplanin-positive or podoplanin-negative cells (Supplementary Figure S4a,b) from the 5-day-cultured hematospheres that were mainly composed of CD14+ monocytes. PPMs showed strong expression of VEGFR-3 whereas podoplanin-negative monocytes had low expression levels (Figure 3a). In addition, LYVE-1 also had a similar expression pattern to VEGFR-3, which was dependent on the presence of podoplanin among CD14+ monocytes (Supplementary Figure S5). Quantitative RT-PCR of sorted cells demonstrated that the PPMs had a significantly higher expression of podoplanin (40-fold), VEGF-C (3-fold), Prox-1 (4-fold), Sox-18 (2-fold), and FoxC-2 (6-fold) than the podoplanin-negative cells did, whereas expression of VEGFR-3 (1.2-fold), Ephrin-b2 (1.3-fold), and VEGF-A (1.2-fold) showed insignificant differences (Figure 3b).
Figure 3.
Podoplanin-positive/VEGFR-3high myeloid cells within hematospheres express lymphangiogenic characteristics. (a) Flow cytometry–sorted CD14+ Pod+ cells and CD14+ Pod− cells from day 5 hematospheres were subjected to immunofluorescence staining for podoplanin and VEGFR-3. Scale bar = 20 µm. (b) Quantitative RT-PCR of flow cytometry–sorted CD14+ Pod+ cells and CD14+ Pod− cells. Bar graphs represented the relative quantity of the lymphangiogenesis-related gene expression in the pod+ cells compared to pod− cells (*P < 0.05, **P < 0.01; n = 3 per experiment). (c) Dissociated single cells from hematospheres were seeded on 1.5% gelatin-coated dish and cultured for 24 hours. Attached cells at 24 hours were subjected to immunofluorescence staining for podoplanin and VEGFR-3. Scale bar = 50 µm. (d) Coculture of red PKH26-labeled dissociated single cells from hematospheres with lenti-GFP-transduced hLEC on Matrigel. Scale bar = 50 µm. hLEC, human lymphatic endothelial cell; NS, nonsignificant; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Furthermore, to examine the distinct characteristics of hematospheres and nonhematosphere single-cell fractions (not incorporated into hematospheres), we separated them into two populations and cultured them in endothelial cell basal medium (EBM)-2 with 5% fetal bovine serum (FBS) on 1.5% gelatin-coated dish. While dissociated single cells from hematosphere fraction attached to the dish surface and displayed spindle-shaped morphology, those from nonhematosphere fraction remained suspended in culture (Supplementary Figure S6a). The dissociated single cells from hematosphere were attached to the dish surface and cultured. They were positive for podoplanin, VEGFR-3, and LYVE-1 in immunofluorescence analysis (Figure 3c; Supplementary Figure S6b) and were able to participate in the growth of lymphendothelial tube–like structure with lenti-GFP-transduced hLECs on thin Matrigel tube formation assay (Figure 3d).
The interaction with platelets potentiates lymphangiogenic characteristics of PPMs
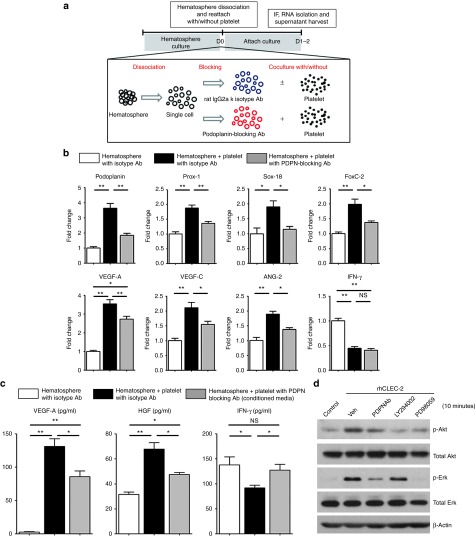
To examine the interaction between PPMs and platelets, we cocultured them with platelets. PPMs were dissociated into single-cell state to enhance a direct contact with platelets (Figure 4a). To identify the role of podoplanin in the interaction between PPMs and platelets, we used blocking antibody against podoplanin. PPMs were pretreated with podoplanin-blocking antibody or with their corresponding isotype antibody before coculturing with platelets. When exposed to platelets, PPMs were remarkably committed to lymphatic cells with significant induction of LEC-specific markers, such as podoplanin, Prox-1, Sox-18, FoxC-2, VEGF-A, VEGF-C, and angiopoietin-2 in quantitative RT-PCR. Their upregulated gene expressions were reversed when they were pretreated with podoplanin-blocking antibody (Figure 4b). In the enzyme-linked immunosorbent assay data, the representative lymphangiogenic cytokines such as VEGF-A16 and hepatocyte growth factor (HGF)17 were significantly increased in the supernatant of PPMs cocultured with platelets, which were attenuated in the presence of the podoplanin-blocking antibody (Figure 4c). Intriguingly, interferon-γ, an antilymphangiogenic cytokine,18 was decreased in the supernatant of PPMs exposed to platelets, which was also reversed by podoplanin blocking (Figure 4c).
Figure 4.
Human podoplanin-positive monocytes (PPMs) and platelets reciprocally activate each other via podoplanin/C-type lectin–like receptor-2 (CLEC-2) axis. (a) Experimental scheme for coculture of PPMs and platelets. Cultured PPMs were dissociated to single cells and pretreated with podoplanin blocking or isotype antibody (100 µg/ml) for 30 minutes. Cells were then divided into two groups, one receiving freshly isolated platelets while the other was treated with vehicle. Both groups were cultured for 24–48 hours. Cultured supernatant and RNA were harvested. (b) Quantitative RT-PCR of PPMs under single culture versus cocultured with platelets in the presence of isotype or podoplanin-blocking antibody. Bar graphs represent the relative expression quantity of the lymphangiogenesis-related genes. Coculture with platelets significantly induced the expression of lymphangiogenic gene expression in monocytes, which was remarkably prevented by blocking podoplanin–CLEC-2 interaction (*P < 0.05, **P < 0.01; n = 3 per experiment). (c) Enzyme-linked immunosorbent assay for lymphangiogenesis-related cytokines (VEGF-A and HGF) and antilymphangiogenesis-related cytokine (IFN-γ) in each conditioned medium of monocytes (*P < 0.05; **P < 0.01; n = 3 per experiment). (d) Analysis of cell signaling in PPMs after stimulation with CLEC-2. Stimulation of monocytes with rhCLEC-2 led to activation of Akt and Erk in western blot analysis, which was significantly prevented by PDPN Ab; starvation media (control), dimethyl sulfoxide (Veh), PDPN Ab, phosphoinositide 3-kinases inhibitor (LY294002), or MEK inhibitor (PD98059) were used in the treatment for 1 hour prior to stimulation with rhCLEC-2 (5 µg/ml, 5 minutes). HGF, hepatocyte growth factor; IFN, interferon; NS, nonsignificant; PDPN Ab, podoplanin-blocking antibody; rhCLEC-2, recombinant human C-type lectin–like receptor-2; VEGF, vascular endothelial growth factor.
To investigate the signaling mechanism of lymphatic commitment of monocytes by exposure to platelets, which was depedent on podoplanin, we stimulated PPMs from hematosphere with recombinant human C-type lectin–like receptor-2 (CLEC-2), a physiological counterpart for podoplanin.19 To examine the involvement of Akt and Erk activation in regulation of lymphangiogenesis, the PPMs were pretreated with podoplanin-blocking antibody, LY294002 (a specific inhibitor of phosphoinositide 3-kinases) or PD98059 (a specific inhibitor of MEK-1) followed by stimulation with recombinant human CLEC-2. Treating PPMs with recombinant human CLEC-2 increased the activation of Akt and Erk while treatment of podoplanin-blocking antibody attenuated this effect (Figure 4d). Inhibition of phosphoinositide 3-kinases by LY294002 blocked the phosphorylation of Akt, and the inhibition of MEK by PD98059 attenuated the phosphorylation of Erk significantly, both of them showing similar results to podoplanin-blocking antibody pretreatment. These results indicated that interaction between PPMs and platelets facilitated lymphatic commitment of monocytes through podoplanin/CLEC-2 axis followed by activation of phosphoinositide 3-kinases/Akt and Erk pathways.
Podoplanin activates platelets, leading to induction of interleukin-1-β
Next, the phenomenal change in platelets was evaluated during interaction with PPMs. The most dramatic change in platelets was the enlargement of platelets in coculture condition, compared with platelets in single culture condition. This enlargement of platelets was remarkably prevented by podoplanin-blocking antibody (Figure 5a,b).
Figure 5.
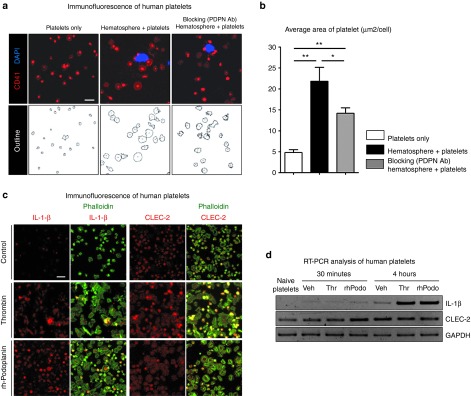
Human podoplanin-positive monocytes (PPMs) or recombinant human rh-podoplanin activates platelets and upregulates the accumulation of IL-1-β in platelets. (a,b) Platelets were left in a quiescent state or cocultured with PPMs in the presence of isotype or podoplanin-blocking antibody (100 µg/ml) on immobilized fibrinogen (200 µg/ml). After 2 hours, cells were fixed and stained with nuclei for monocytes (blue) and CD41 for platelets (red). Coculture with PPMs significantly enlarged the size of platelets, which was remarkably prevented by podoplanin-blocking antibody. Scale bar = 10 µm. (b) Bar graph representing the total area of platelets normalized by the number (*P < 0.05, **P < 0.01; n = 3). (c) Platelets were activated by rh-podoplanin (20 µg/ml), with thrombin (0.01 U/ml) and vehicle being used as positive and negative control. Platelets in each group were stained for IL-1-β or CLEC-2 (red). The green fluorescence represents polymerized actin, phalloidin. Podoplanin significantly activated platelets resulting in the induction of IL-1-β expression and morphologic change, as thrombin did. Scale bar = 10 µm. (d) After platelets were allowed to adhere to the immobilized fibrinogen for 30 minutes and 4 hours, expression of IL-1-β and CLEC-2 in each group was determined by semiquantitative RT-PCR. rh-Podoplanin significantly induced the lymphangiogenic cytokine IL-1-β in platelets as thrombin did. CLEC-2, C-type lectin–like receptor-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; PDPN Ab, podoplanin-blocking antibody; rh-podoplanin, recombinant human-podoplanin; RT-PCR, reverse transcription polymerase chain reaction.
To dissect the underlying mechanism for the activation of platelets by PPMs, first, we directly applied recombinant human (rh)-podoplanin on platelets. We cultured platelets that were allowed to adhere to immobilized human fibrinogen in the presence of rh-podoplanin or thrombin as a control stimulant. Cellular spreading of platelets on immobilized fibrinogen in the presence of rh-podoplanin demonstrated that platelets were efficiently stimulated (Supplementary Figure S7a), and the average area of platelets significantly more increased in rh-podoplanin or thrombin group, compared with that in control group (Supplementary Figure S7b). Second, we examined the level of the interleukin (IL)-1-β in the activated platelets, which was known as a potent lymphangiogenic cytokine.20 Immunofluorescence staining showed that IL-1-β protein was upregulated in the activated platelets by rh-podoplanin or thrombin while CLEC-2 protein was constitutively expressed in platelets (Figure 5c). The increased gene expression of IL-1-β in the activated platelets by rh-podoplanin was also confirmed by semiquantitative RT-PCR analysis (Figure 5d). It suggested that the rh-podoplanin could activate platelets as strongly as thrombin and increase IL-1-β at mRNA and protein level in anucleate platelets.
Taken together, the coculture of PPMs and platelets may not only induce lymphatic commitment of monocytes but also activate platelets. These reciprocal effects were mediated through the interaction between podoplanin and CLEC-2 axis.
Cocultured supernatant of PPMs with platelets augments the migration, viability, and proliferation of lymphatic endothelial cells
To investigate the in vitro paracrine effect of PPMs with platelets on hLECs, we performed functional assays of hLECs using the cocultured supernatant. We found that supernatant of PPMs that were cocultured with platelet increased migration, viability, and proliferation of hLECs compared to supernatant of either platelet only or PPMs (Figure 6a–c). All these increases by cocultured supernatant were attenuated by pretreatment of podoplanin-blocking antibody. To find active agent in the supernatants, we performed Matrigel tube formation assay of lymphatic endothelial cells using the supernatant from coculture of PPMs and platelets. The supernatant of PPMs and platelets increased tube formation, which was attenuated by VEGF- or HGF-neutralizing antibody (Supplementary Figure S8). These data provided ex vivo evidence that coculture of PPMs and platelets exerts a stimulatory paracrine action on hLECs, presumably mediated by VEGF and HGF.
Figure 6.
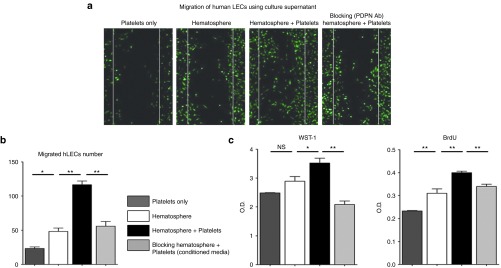
Cocultured supernatant from podoplanin-positive monocytes (PPMs) and platelets enhances the migration, viability, and proliferation of hLECs. (a) Representative figure of hLECs' migration in various conditioned media. hLECs migrated most rapidly in the presence of the conditioned medium from coculture of PPMs and platelets, while migration of hLECs was significantly attenuated when treated with blocking antibody against podoplanin. (b) Bar graph representing the number of the migrating hLECs (*P < 0.05, **P < 0.01; n = 3 per experiment). (c) Quantitative data showing the viability and proliferation of hLECs under various conditions. hLECs cultured under the conditioned medium from coculture of PPMs and platelets demonstrated greater viability and proliferation compared to the culture medium from monoculture of platelets or PPMs. However, the enhanced capacity of hLECs was attenuated by blocking the podoplanin (*P < 0.05, **P < 0.01; n = 3 per experiment). BrdU, bromodeoxyuridine; hLECS, human lymphatic endothelial cells; LEC, lymphatic endothelial cells; NS, nonsignificant; O.D., optical density; WST-1, water soluble tetrazolium salts-1.
Human PPMs with platelets augment lymphatic vessel formation and wound healing in the nude mice
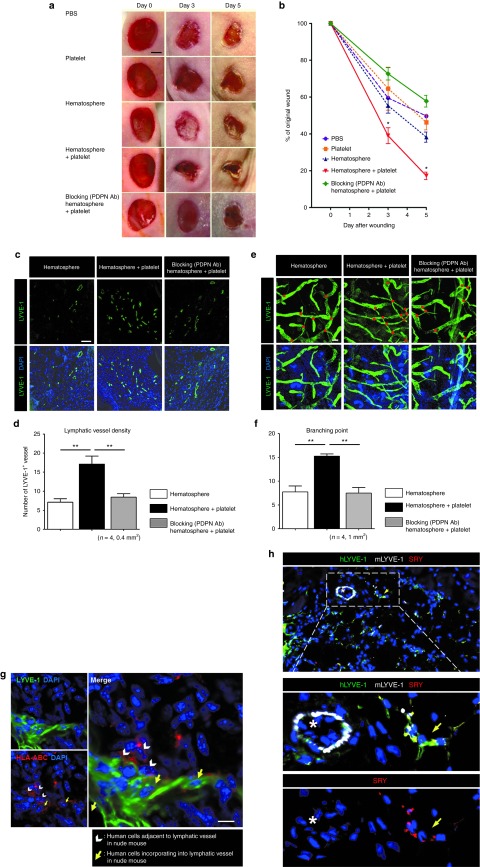
On the basis of these data that PPMs exposed to platelets are committed to lymphatic endothelial cell lineages and augment the lymphangiogenic capability of hLECs, we investigated the effect of human hematosphere–derived PPMs with platelets on the enhancement of lymphangiogenesis in the nude mouse dorsal skin and ear wound model. We compared the wound-healing efficacy of five different treatments by serial measurements of dorsal skin wound at days 0, 3, and 5 in nude mice: (i) phosphate-buffered saline, (ii) platelets, (iii) PPMs, (iv) PPMs with platelets, and (v) podoplanin-blocking antibody–pretreated PPMs with platelets. Local injection of PPMs with platelets remarkably facilitated wound healing as short period as 5 days, which was significantly different from the other treatments: phosphate-buffered saline, platelets, or PPMs. Such a facilitation of wound healing by PPMs with platelets was attenuated by podoplanin-blocking antibody (Figure 7a,b).
Figure 7.
Synergistic interaction of human podoplanin-positive monocytes (PPMs) and platelets led to lymphatic neovascularization and facilitated wound healing in nude mice. (a) Gross appearances of the wounds at the back of nude mice injected with the different cells at the indicated time points. Scale bar = 2 mm. (b) Diagram of the kinetics of wound closure in each group. Four mice were analyzed at each time point (*P < 0.05). (c–g) Mice that had received surgery for wound to back or ear skin were injected with the different cells, and the tissues were harvested at 7 days for immunohistochemistry. (c) In the confocal images of back skin from nude mice of indicated group, the LYVE-1-positive lymphatic vessels were more abundant after transplantation of platelets and PPMs than monocytes alone, which were reversed by blocking antibody against podoplanin. Scale bar = 100 µm. Blue fluorescence indicates DAPI. (d) Bar graph representing the quantification of lymphatic vessel number in the back skin, which was determined by scoring LYVE-1-positive vessels (**P < 0.01; n = 4 per experiment). (e) Whole-mount immunostaining of ear skin stained with antibodies against LYVE-1. Scale bar = 100 µm. Blue fluorescence indicates DAPI. Red dots denote branching points. LYVE-1-positive lymphatic vessels arborized more abundantly after transplantation of platelet and PPMs than monocytes alone, which was reversed by blocking antibody against podoplanin. (f) Bar graph representing the number of lymphatic vessels–branching points (**P < 0.01; n = 4 per experiment). (g) Whole-mount immunostaining of ear skin wound treated with PPMs and platelets. About half of these transplanted human cells were stained as red (HLA-ABC) and coexpressed LYVE-1 (green), suggesting that these cells transdifferentiated into lymphatic vessels in mice. Scale bar = 10 µm. Blue fluorescence indicates DAPI. (h) Confocal images of back skin from female nude mice treated with PPMs from human male volunteers. Transplanted human cells were stained with SRY (red) and hLYVE-1 (green), and mouse lymphatic vessel was stained with mLYVE-1 (white). Blue fluorescence indicates DAPI. DAPI, 4′,6-diamidino-2-phenylindole; HLA-ABC, human leukocyte antigen-ABC; hLYVE-1, human lymphatic vessel endothelial hyaluronan receptor-1; LYVE-1, lymphatic vessel endothelial hyaluronan receptor-1; mLYVE-1, mouse lymphatic vessel endothelial hyaluronan receptor-1; PBS, phosphate-buffered saline; PDPN Ab, podoplanin-blocking antibody; SRY, sex-determining region Y.
Histologic examination showed the increase of lymphatic vessel formation around dorsal skin wound in PPMs with platelets group. The number of LYVE-1-positive vessel was greater in PPMs with platelets group than that in PPM-only group, which was again attenuated by podoplanin-blocking antibody (Figure 7c,d). In addition, lymphatic vessel sprouting was more prominent by the treatment of PPMs with platelets in the whole-mount immunostaining of ear wound. The number of branching points of lymphatic vessel was significantly increased in PPMs with platelets than in PPMs alone, which was also diminished by podoplanin-blocking antibody (Figure 7e,f).
To trace injected human PPMs, we established the immunostaining of human-specific antibody, human leukocyte antigen-ABC and found that PPMs were either located adjacent to mouse lymphatic vessel or directly incorporated into lymphatic vasculature (Figure 7g). To further evaluate the origin of lymphatic cells in the recipient mouse, we performed additional in vivo experiment. We obtained PPMs from human male volunteers, which were then injected to female nude mouse. To trace the human cells, the dorsal wound tissue was labeled by SRY. Immunofluorescent staining of punch wound showed that SRY+hLYVE+ cells were found in the mouse lymphatic vessels (Figure 7h), which meant that human transplanted PPMs were directly incorporated into recipient lymphatic vessels. Together, these data demonstrated that human PPMs derived from hematosphere, with the aid of platelets, were effective in wound repair by stimulating lymphatic vessel formation and sprouting, which was dependent on podoplanin–CLEC-2 interaction.
Discussion
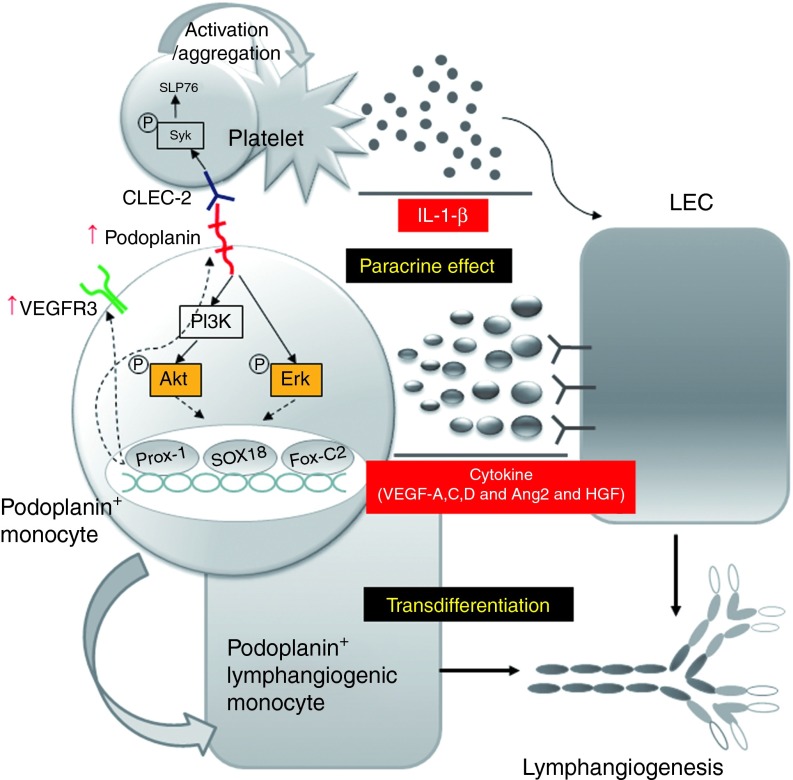
In this study, we have shown that (i) lymphatic endothelial cells could be remarkably expanded by the short period of aggregation culture (hematosphere) of human peripheral blood monocytes, which are easily obtained from a peripheral vein, (ii) reciprocal stimulation between human PPMs and platelets trigger the lymphangiogenic signaling through the activation of the podoplanin/CLEC-2 axis, (iii) the combination of PPMs and platelets leads to a marked increase of the secretion of various lymphangiogenic cytokines, leading to activation of migration and proliferation of hLECs, and finally enhanced lymphangiogenesis around skin wound with facilitated healing (Figure 8).
Figure 8.
Schematic diagram of lymphangiogenesis by the synergistic interaction between PPMs and platelets through podoplanin/CLEC-2 interaction. When the hematosphere is generated from a suspension culture of human peripheral blood monocytes under high cell density, there is a huge expansion of PPMs in hematospheres for several days. These cells express lymphatic endothelial cell–specific markers, such as Prox-1, Sox-18, FoxC-2, and VEGFR-3. When podoplanin on these monocytes is stimulated by CLEC-2 on platelets, the interaction of CLEC-2 on platelets and podoplanin on monocytes turns on Akt or Erk signaling pathways that provoke two changes on monocytes: (i) the enhanced expression of Prox-1, Sox-18, FoxC-2, and VEGFR-3 and the transdifferentiation to lymphatic endothelial cells and (ii) the enhanced secretion of lymphangiogenic cytokines, such as VEGF-A,C,D, angiopoietin-2, and HGF. Meanwhile, the interaction of CLEC-2 on platelets and podoplanin on monocytes stimulates platelets, leading to morphologic changes and secretion of lymphangiogenic cytokine, IL-1-β. Overall, these interactions facilitate lymphatic neovascularization and augment wound healing. CLEC-2, C-type lectin–like receptor-2; IL, interleukin; LEC, lymphatic endothelial cells; PPMs, podoplanin-positive monocytes; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Aggregation culture of human monocytes generates lymphangiogenic PPMs in lymphangiogenic culture condition
Our culture method to generate lymphatic endothelial cells from circulating monocytes shares the common keyword, ‘aggregation', with the previous mouse report where they induced transdifferentiation of mouse bone marrow macrophages into LECs through aggregation.6 On closer examination of these human peripheral blood monocytes aggregates, we discovered interesting evidence that human monocytes constituting these aggregates gradually increased the expression of lymphatic endothelial cell markers, such as podoplanin and VEGFR-3. Gene expression analysis showed that monocyte aggregates expressed Prox-1 and Sox-18 at day 5. Prox-1 is a master control gene in the program specifying lymphatic endothelial cell fate,21 and Sox-18 is known to induce development of the lymphatic vasculature.22
We postulated that microenvironment inside hematosphere has unique stem cell niche components that have sufficient factors to induce transdifferentiation of monocytes into lymphatic endothelial cells. In spite of the possibilities that murine myeloid cell could transdifferentiate into lymphatic cells being previously suggested,8,9,10 to our knowledge, there has been no definite report depicting the transdifferentiation of human peripheral monocyte into lymphatic cells.
Podoplanin and VEGFR-3-positive monocytes differentiate into lymphangiogenic population
Podoplanin and VEGFR-3 are the distinct markers of lymphatic cells and not expressed by the endothelial cells of blood vessels.13 Previous studies showed that VEGFR-3 and CD133 in human stem-progenitor cells9 or mouse bone marrow–derived podoplanin-positive cells are critical for lymphangiogenesis.10 Our analysis suggested that in human peripheral blood monocytes, VEGFR-3high/podoplanin-positive population showed stronger lymphangiogenic potential than VEGFR-3low/podoplanin-negative population. The mRNA expression of lymphangiogenic genes, such as VEGF-C, Prox-1, Sox-18, and FoxC-2, was higher in VEGFR-3high/podoplanin-positive cells than that in VEGFR-3low/podoplanin-negative cells. On the basis of these results, we suggest that double positivity for both podoplanin and VEGFR-3 might be better marker of lymphangiogenic cell in human circulation system than conventional VEGFR-3 positivity.
To clarify the role of CD14 and podoplanin, PBMC and hematosphere were sorted according to their CD14 and podoplanin expression status. CD14+Pod+ cells from hematosphere enhanced the induction of lymphangiogenic cytokines and wound healing than CD14+Pod− cells from hematosphere (Supplementary Figures S9 and S10). Interestingly, CD14+ cells from hematosphere had similar potency with CD14+Pod+ cells from hematospheres. Considering the fact that CD14+ cells mostly express podoplanin in the hematosphere, these results indicated that podoplanin could be a pivotal marker for lymphangiogenic cell in hematosphere.
Reciprocal stimulation between podoplanin of monocytes and CLEC-2 molecules of platelets
Uhrin et al.23 suggested that the interaction of endothelial podoplanin of the developing lymph sac with blood platelets might be important for lymphangiogenesis. With previous studies describing the interaction of cell surface marker podoplanin with platelet-associated molecule CLEC-2,24 we decided to further investigate whether lymphangiogenesis could be augmented by interaction of PPMs with platelets and obtained these following observations.
First, platelets augmented the expression of lymphangiogenic genes in PPMs, suggesting that platelets might increase the lymphangiogenic potency of PPMs. In this process, CLEC-2 on platelet interacted with podoplanin and turned on the Akt and Erk pathways in monocytes, which is known to play a critical role in regulation of lymphocyte migration.25,26,27
Second, PPMs activated platelets, leading to a significant morphological change of platelets and induction of IL-1-β, which was known as a potent lymphangiogenic cytokine.20 Recombinant podoplanin also activated platelets and induced IL-1-β. With previous studies suggesting that anucleate platelets induce a signal-dependent pre-mRNA splicing of IL-1-β28 and other genes29 when external stimuli are given,30 we proved that both recombinant human podoplanin and PPMs upregulate IL-1-β by interaction with CLEC-2 on platelets.
Third, podoplanin-blocking antibody attenuated the reciprocal effects between PPMs and platelets. On the basis of these data, we assumed that the podoplanin might be a key molecule in the interaction between PPMs and platelets and that the reciprocal effects between PPMs and platelets might play an important role in lymphangiogenesis.
Therapeutic lymphangiogenesis by synergistic interaction between human PPMs and platelets: a promising strategy in treating lymphatic vessel diseases
To examine the role of PPMs with platelets on the lymphangiogenesis, we performed in vitro and in vivo experiments. The supernatant of PPMs cocultured with platelets significantly enhanced the migration and proliferation of hLECs, compared to supernatant of either cell alone. We investigated whether combination of human PPMs and platelets augments lymphatic neovascularization in nude mice skin wound model. We confirmed that platelets play an important role to enhance lymphangiogenesis and facilitate wound repair, because treatment effect by PPMs alone was less potent than that of combination of PPMs plus platelets. In the histologic examination, the therapeutic effects were displayed in several ways: (i) increased number of lymphatic vessels, (ii) enhanced maturation or arborization of lymphatic vessels, and (iii) direct incorporation of the transplanted cells to lymphatic vessels. PPMs may trigger lymphangiogenesis through two ways: direct incorporation into lymphatic vessels or paracrine factors released from the interaction between monocytes and platelets. Intriguingly, the interaction of monocytes and platelets has been described as a physiologically occurring phenomenon termed as monocyte–platelet aggregation.31
In addition, we investigated whether PPMs and platelets increase angiogenesis and improve wound healing. Blood vessel area was higher in PPMs with platelets than in PPMs or platelets only. However, the increase of blood vessel area was not attenuated by podoplanin-blocking antibody (Supplementary Figure S11) while wound healing was attenuated by blocking antibody. This result suggested that lymphangiogenesis by podoplanin/CLEC-2 axis might be important for wound healing in our study irrespective of angiogenesis.
In conclusion, PPMs have a potential of lymphangiogenesis, which can be augmented by platelets through interaction of podoplanin on monocytes and CLEC-2 on platelets. We found an easy and practical way to expand human PPMs through hematosphere culture that is a high-density aggregation suspension culture of human peripheral blood monocytes. Therefore, combined cell therapy using PPMs and platelets might become a promising way to induce lymphatic neovascularization and facilitate wound healing.
Materials and Methods
Preparation of human hematospheres and platelets. The study was approved by the Institutional Review Board of Seoul National University Hospital (H-0905-033-281). Written and informed consent was obtained from all volunteers before enrollment in the study. We prepared human PBMCs from healthy donors (mean age 27.1 ± 2.5). On the basis of a history taking, systemic review and physical examination, all blood donors were examined by physicians. Their baseline characteristics were described in Supplementary Table S1. Isolation of PBMCs was performed by Ficoll-PaqueTM PLUS (GE Healthcare, Piscataway, NJ) according to the instructions, and PBMCs were washed five times with phosphate-buffered saline to completely remove remaining debris. PBMCs suspended in StemSpan H3000 (StemCell Technologies, Vancouver, Canada) or mTeSR (StemCell Technologies) were cultured at 3–5 × 106 cells/ml on HydrocellTM ultralow attach surface (NUNC, Roskilde, Denmark). One milliliter of fresh medium was added every second day without media change. Isolated mononuclear cells started to aggregate after 1 day of culture, and these aggregates were termed as hematospheres. The number of hematospheres increased during 5 days of culture, with nonincorporated single cells dispersed in the periphery.
Platelets were isolated as previously described.30 Human blood was drawn into acid-citrate-dextrose (ACD)-coated vacuum tube and centrifuged (200g for 20 minutes) to obtain platelet-rich plasma. Platelet-rich plasma was centrifuged (500g for 20 minutes) in the presence of 100 nmol/l prostaglandin E1 (PGE-1) (Sigma, St Louis, MO) and washed with pipes/saline/glucose containing 100 nmol/l of PGE-1. The isolated platelets were either quiescent or allowed to adhere to immobilized human fibrinogen (Sigma) in the presence of thrombin (Sigma) or rh-podoplanin (ProSpec Bio, Rehovot, Israel).
hLECs (PromoCell, Heidelberg, Germany) were maintained in EBM-2 supplemented with cytokine cocktail (SingleQuots; Lonza, Basel, Switzerland).
Acquisition of conditioned media and measurement of cytokine concentration. After 2 days of cell culture, conditioned medium was acquired from each group by removing cellular debris via centrifuging and were stored at −70 °C until the analysis. The concentration of VEGF-A, HGF, and interferon-γ were analyzed by Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's protocol. Anti-VEGF-A, HGF, and interferon-γ antibodies were purchased from Bio-Rad Laboratories.
Scratch wound assay. Scratch wound assay was performed as described previously.32 hLECs were grown in 35-mm dishes to a confluent monolayer. The hLEC monolayer was scraped in a straight line with a 200-µl pipet tip. After wounding, monolayers were immediately washed and incubated with each conditioned medium. After 20 hours, migrated cell number from wound edge to point of cell migration was measured.
Viability assay. Cell viability was carried out as previously described.32 For assessment of viability, 1 × 104 hLECs were seeded to each well of 96-well plate in EBM-2 with 1% FBS. Then, the cultured media were changed with each conditioned medium. After 48 hours of incubation, reagent water soluble tetrazolium salts-1 (WST-1; Roche Molecular Biochemicals, Basel, Switzerland) was added with cell culture medium as 20 µl/well. The cells were incubated for 2–4 hours in the same incubator. Absorbance was measured using a microplate reader (SpectraMax 190; Molecular Devices, Sunnyvale, CA) at 450 nm.
Proliferation assay. hLECs were seeded on 96-well plates at a concentration of 1 × 104 cells/well in EBM-2 with 5% FBS and stabilized for 12 hours. After overnight culture in EBM-2 with 1% FBS, hLECs were incubated in each conditioned medium. Then, the cells were treated with bromodeoxyuridine for 24 hours. Incorporated bromodeoxyuridine was detected with anti-bromodeoxyuridine monoclonal antibodies conjugated with peroxidase. Sample absorbance was analyzed using a microplate reader (SpectraMax 190) at 450 nm.
Matrigel tube formation. In vitro tube formation was evaluated using the Matrigel plate as described previously.33 Matrigel (Becton Dickinson Labware, San Jose, CA) basement membrane matrix was added to four-well chamber slide. After 30 minutes of incubation at 37 °C, each cells were seeded in EBM-2 with 5% FBS. Twelve hours later, four representative fields were taken.
Skin and ear wound model. All animal experiments were performed after receiving approval of the Institutional Animal Care and Use Committee in Seoul National University Hospital and complied with the National Research Council “Guidelines for the Care and Use of Laboratory Animals”. Wounds were created in nude mice 8–10 weeks of age as described previously.34 Full-thickness and excisional skin wounds were created on the backs of the mice using 6-mm skin biopsy punches. Ear wounds were created by using ear punch with 2-mm diameter. Each cells were administered intradermal around the wounds, and wounds were covered with a sterile transparent occlusive dressing (Tegaderm; 3M Health Care, Loughborough, UK). To neutralize the interaction between podoplanin and CLEC-2, antipodoplanin monoclonal antibody (eBioscience, San Diego, CA) was used. Wound areas were measured, and digital photographs were taken for 0, 3, 5, and 7 days. Seven days later, the wounds were carefully excised and fixed in 4% paraformaldehyde and embedded in paraffin or optimal cutting temperature compound.
Image acquisition and analysis. Images were obtained by an Olympus IX2 inverted fluorescence microscope (Olympus, Shinjuku, Japan) equipped with an Olympus DP50 CF CCD camera and analySIS 5.0 software (Olympus). Confocal images were obtained by Zeiss LSM-710 META confocal microscope and ZEN 2009 analysis software (Zeiss, Oberkochen, Germany).
Statistical analysis. All data were presented as means ± SEM. The statistical significance of difference between two groups was evaluated with an unpaired t-test, and the significance between three groups was analyzed with one-way analysis of variance followed by Bonferroni's method using the Prism 4 program (GraphPad, La Jolla, CA). Probability values less than 0.05 were considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Flow cytometry analysis of hematospheres cultured 5 days in various culture media. Figure S2. Flow cytometry analysis of hematospheres using different clones of podoplanin antibodies Figure S3. Fluorescence-associated cell sorter analysis of VEGFR-3 and LYVE-1 in podoplanin-positive cells. Figure S4. Composition of 5-day cultured hematospheres. Figure S5. Expression of LYVE-1 on the presence of podoplanin among CD14+ monocytes. Figure S6. Immunofluorescence analysis of dissociated single cells from hematosphere. Figure S7. Platelets stimulation in the presence of rh-podoplanin on immobilized fibrinogen. Figure S8. Significant paracrine factor in supernatant of PPMs and platelets. Figure S9. Enhancement of lymphangiogenic cytokines in CD14+Pod+ cells from hematosphere. Figure S10. Facilitated wound healing in CD14+Pod+ cells from hematoshere. Figure S11. Blood vessel area analysis by podoplanin-blocking antibody. Table S1. Blood donor information Table S2. Information of primers
Acknowledgments
This study was supported by grants from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Education, Science, and Technology (MEST), Republic of Korea (2010-0020258), from the SNUH Research Fund (04-2013-0940), from the Innovative Research Institute for Cell Therapy (A062260), and from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea [HI12C0199].
Supplementary Material
References
- Olszewski W. On the pathomechanism of development of postsurgical lymphedema. Lymphology. 1973;6:35–51. [PubMed] [Google Scholar]
- Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol. 2008;52:799–806. doi: 10.1016/j.jacc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, et al. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Park J, Lee SE, Yoon CH, Jang JH, Yang JM, et al. Human peripheral blood-born hematosphere as a niche for hematopoietic stem cell expansion. Cell Res. 2011;21:987–990. doi: 10.1038/cr.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Choi JI, Yun JY, Yoon CH, Jang JH, Im SG, et al. Highly angiogenic CXCR4(+)CD31(+) monocyte subset derived from 3D culture of human peripheral blood. Biomaterials. 2013;34:1929–1941. doi: 10.1016/j.biomaterials.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- Watari K, Nakao S, Fotovati A, Basaki Y, Hosoi F, Bereczky B, et al. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Suzuki-Inoue K, Fuller GL, García A, Eble JA, Pöhlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X, et al. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin Cancer Res. 2012;18:5387–5398. doi: 10.1158/1078-0432.CCR-12-0708. [DOI] [PubMed] [Google Scholar]
- Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, et al. Akt/Protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am J Pathol. 2010;177:2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, et al. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–1537. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Saaristo A, Tammela T, Farkkila A, Kärkkäinen M, Suominen E, Yla-Herttuala S, et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169:1080–1087. doi: 10.2353/ajpath.2006.051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.