Abstract
Tissue engineering has become an essential tool in the development of regenerative medicine. We have developed cell sheet–based techniques for use in regenerative medicine that have already been successfully used in clinical applications. Native corneal epithelium is produced from limbal stem cells located in the transition zone between the cornea and the bulbar conjunctiva. Limbal stem cell deficiency (LSCD) is a severe defect of the limbal stem cells leading to vision loss due to conjunctival epithelial invasion and neovascularization. Rabbit LSCD models were treated with transplantable autologous oral mucosal epithelial cell (OEC) sheets fabricated on temperature-responsive cell culture surfaces, after which, the ocular surfaces were clear and smooth with no observable defects. The central part of the reconstructed ocular surface was scraped and wounded, after which proliferating epithelial cells covered the scraped area within a few days. The ocular surfaces were clear and smooth even after repeated scrapings and consisted of only OECs or heterogeneously mixed with corneal epithelial cells. This study demonstrates that transplanted cell sheets containing oral mucosal epithelial stem cells could reconstruct the ocular surface to maintain cornea homeostasis; moreover, they provide an ideal microenvironment to support the proliferation of remaining native limbal stem cells.
Introduction
Regenerative medicine is an attractive new therapy for the restoration of lost tissues or function. Induced pluripotent stem cells1 and other stem cells offer the potential to expand regenerative medicine by using them in tissue engineering for the fabrication and delivery of tissue-like structures. We have developed cell sheet–based techniques for regenerative medicine and successfully applied them in clinical settings for treatment of cornea,2 heart,3 esophagus,4 knee cartridge,5,6 and periodontal tissue.7,8
Native corneal epithelium turn over every 2 weeks and are maintained by the corneal epithelial stem cells localized in the limbus, which is the transitional zone between the cornea and the conjunctiva.9,10,11,12 When the corneal epithelial stem cells are severely damaged, the peripheral conjunctival epithelium invades with angiogenesis, resulting in a corneal opacification leading to severe visual loss, known as limbal stem cell deficiency (LSCD).13 Here, we treated LSCD with transplantable autologous oral mucosal epithelial cell (OEC) sheets fabricated on temperature-responsive cell culture surfaces.2
In cardiac regeneration, after ectopic transplantation of skeletal myoblast sheets in nonhomologous use, paracrine effects should be the major mode of action, since skeletal myoblasts never differentiate into cardiac myocytes.3 However, each cell sheet is transplanted for homologous use in the cases of esophagus,4 knee cartridge,5,6 and periodontal tissue.7,8 Therefore, transplanted cells can contribute to promotion of wound healing and tissue regeneration as a cell source, in addition to its paracrine effects. In the case of corneal regeneration, after ectopic transplantation of OEC sheets in nonhomologous use, two possibilities for the mode of action can be considered. One possibility is that epithelial stem cells contained in the transplanted OEC sheets might regenerate the ocular surface, since buccal mucosal epithelial cells and corneal epithelial cells are not the same but closely similar from the viewpoint of the absence of keratinization. The other possibility is that paracrine factors from transplanted OECs stimulate the growth of residual corneal epithelial stem cells. The major objective of this study is to reveal the mode of action in the treatment of LSCD by transplantation of autologous OEC sheets using repeated wound-healing assay in a rabbit LSCD model.
Results
Transplantation of fabricated autologous OEC sheets
LSCD models were prepared on three rabbits (rabbits nos. 1–3) by surgically removing the corneal and limbal epithelium with n-heptanol treatment.14 After 5 weeks, the ocular surface was covered with conjunctival tissue having neovascularization, and corneal opacification was observed. Fabricated autologous OEC sheets were transplanted onto the ocular surfaces after surgically removing the pannus (Figure 1).15 Cytokeratin 4 (K4) and cytokeratin 13 (K13), conjunctival epithelium markers,15,16 and mucin 5 (Muc5), a goblet cell marker,17 expressing cells were observed in the pannus of all three LSCD models by immunohistochemistry (Supplementary Figure S1; Supplementary Materials and Methods). Fabricated OEC sheets (Figure 2a,b) comprised three to five stratified layers containing small cuboidal cells in the basal layer and squamous epithelium on the apical side (Figure 2c). K4, a differential mucosal epithelial cell marker,15,18,19,20 was detected in the suprabasal to superficial cell layers, except for the basal cell layer, and cytokeratin 14 (K14), a basal cell marker,20,21 was found in the basal and suprabasal cell layers. p63, a putative stem/progenitor cell marker,22 was detected in the basal cell layer. These localizations were the same as those of normal oral mucosa (Figure 2d).
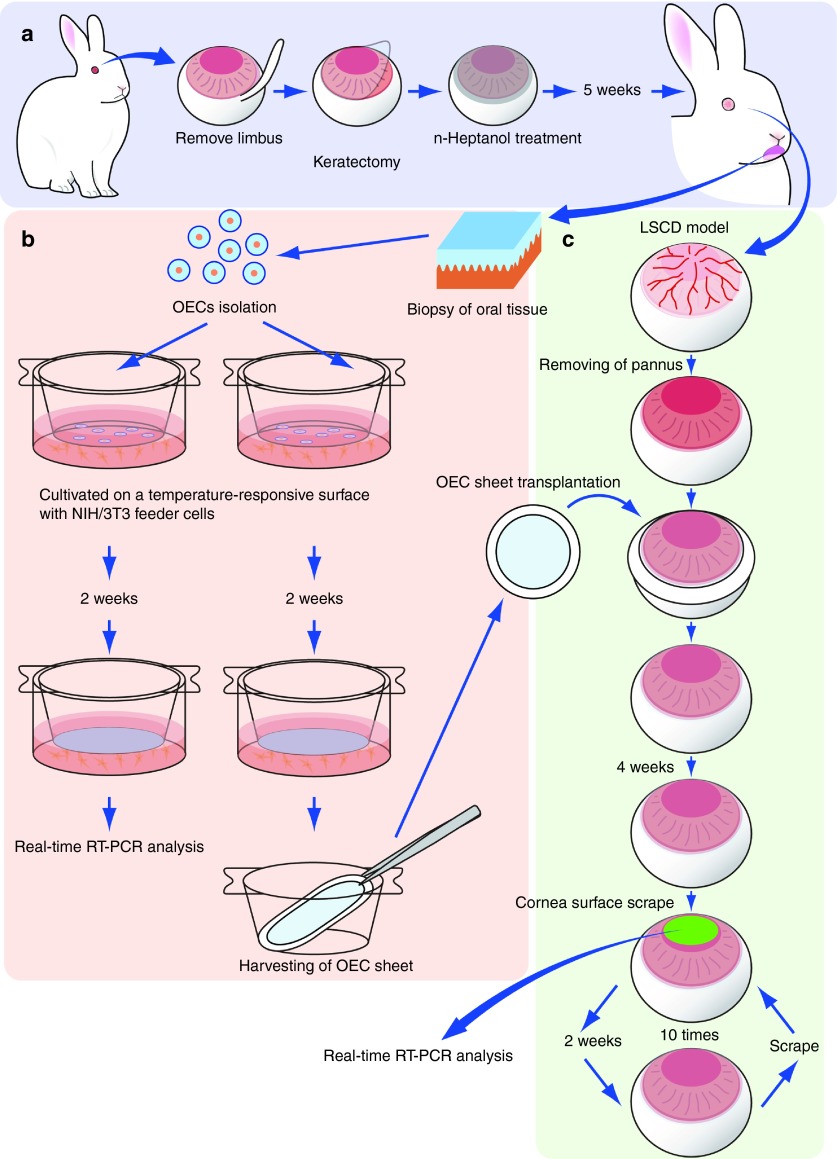
Figure 1.
Investigation of cultivated autologous oral mucosal epithelial cell (OEC) sheets transplantation into a rabbit limbal stem cell deficiency (LSCD) model. (a) Rabbit LSCD model was prepared by n-heptanol treatment after keratectomy. (b) An autologous OEC sheet was fabricated on a temperature-responsive cell culture surface with mitomycin-C–treated NIH/3T3 feeder layer and harvested by reducing temperature without enzymatic treatment. (c) OEC sheet was transplanted onto the ocular surface of a rabbit LSCD model after removing pannus. Four weeks after transplantation, the central part of reconstructed ocular surface (5 mm in diameter) was physically scraped once every 2 weeks, a total of 10 times over 24 weeks, and the scraped specimens were analyzed by real-time RT-PCR.
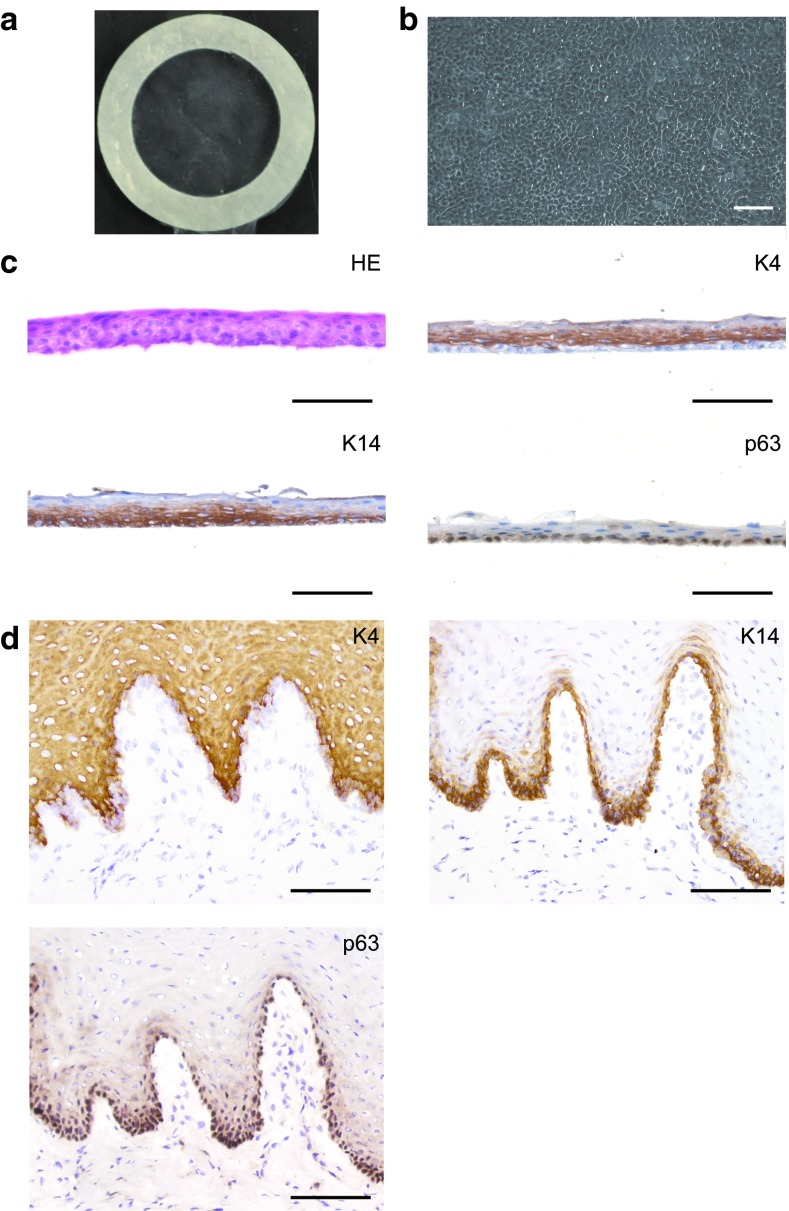
Figure 2.
Fabrication of autologous oral mucosal epithelial cell sheets using temperature-responsive cell culture surfaces. (a,b) An oral mucosal epithelial cell (OEC) sheet was harvested intact by reducing the temperature and using a supporting membrane ring with outer and inner diameters of 20 and 13 mm, respectively. The OEC sheet consisted of cobblestone epithelial cells morphology similar to cornea epithelial cells. (c) OEC sheet consisted of three to five layers, and K4 expression was detected in all cell layers, except the basal layer. K14, a basal cell marker, and p63, a putative stem/progenitor cell marker, expressions were observed in the basal cell layer. (d) In normal rabbit oral mucosal tissue, K4 expression was observed from the suprabasal layer to the superficial layer, except for the basal cell layer. K14 was observed from the basal layer to the suprabasal layer, and p63 was observed in the basal layer. These results suggested that the fabricated OEC sheets contained putative stem/progenitor cells. Bars = 100 µm.
After OEC sheet transplantation, fluorescein dye did not permeate into the corneal stroma (Figure 3), indicating that the ocular surfaces were completely covered without defect by the OEC sheets. Reconstructed ocular surfaces were clear and smooth 1 week after the transplantation with no observable defects. Although a small amount of neovascularization was observed in the peripheral cornea, the central cornea was clear with no fluorescein dye penetration 4 weeks after the transplantation, suggesting that the transplanted OEC sheets contributed to the maintenance of cornea transparency. The central part of the transplanted ocular surfaces (5 mm in diameter) was then physically scraped and subjected to RNA extraction. Fluorescein staining revealed that corneal stroma was completely reexposed by the scraping. Epithelial cells migrated from the edge of the wound area and covered the newly scraped area within a few days, as shown by the reduction of the fluorescein-stained area. Two weeks after the first scraping, the transparency of the ocular surfaces was retained, and no fluorescein staining was observed. The central part of ocular surfaces (5 mm in diameter) was physically scraped again. Proliferating epithelial cells covered the scraped area within a few days, and no penetration of fluorescein was observed even after the second scraping. Similarly, the transplanted ocular surfaces were scraped every 2 weeks, repeated 10 times, and then, the rabbits were sacrificed 2 weeks after the last scraping. From the sacrificed rabbits, all the ocular surfaces were scraped again and subjected to RNA extraction and histological analysis. The first scrape was performed 4 weeks after the transplantation, and the total follow-up period was 24 weeks. Every time, the scraped area was reepithelized within a few days and remained unchanged; moreover, the transparency of the ocular surfaces was confirmed in all three rabbits at 24 weeks before sacrifice (Figure 3; Supplementary Figures S2–S5).
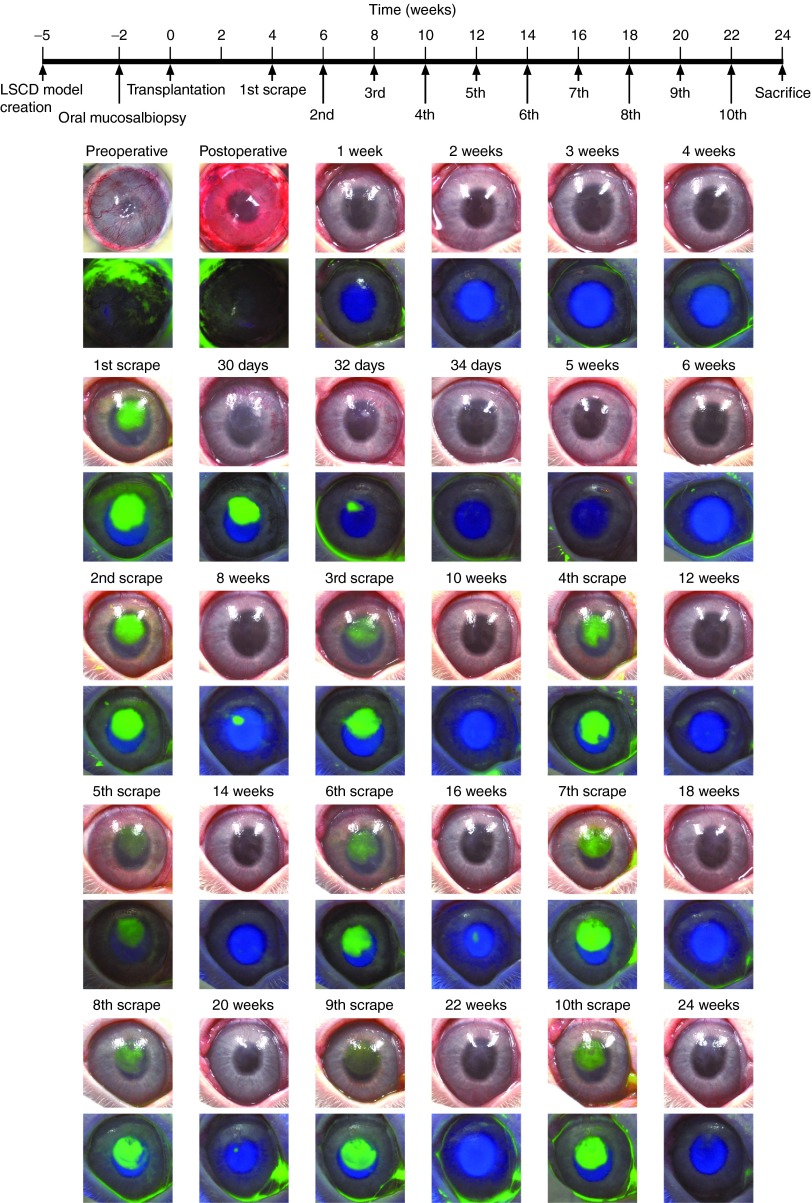
Figure 3.
Repeated wound-healing assay of proliferative and differentiation potential of transplanted oral mucosal epithelial cell (OEC) sheets. Neovascularization and opacification were observed in the rabbit limbal stem cell deficiency (LSCD) model 5 weeks after the surgery. An OEC sheet was transplanted onto the ocular surface after the removal of pannus without defects. Ocular surface was then reconstructed and confirmed to remain clear for 4 weeks, and then, the center of transplanted corneal surface was scraped to create a wound. Fluorescein staining revealed that the corneal stroma was completely reexposed. Proliferating epithelial cells covered the scraped area within a few days allowing the ocular surface to recover which was then confirmed to be stable with no evidence of fluorescein staining. Two weeks after the first scraping, the central part of healed ocular surface was physically scraped again. Epithelial cells migrated and covered the scraped area again after the second scraping. Similarly, the transplanted OEC sheet ocular surface was scraped every 2 weeks to a total of 10 times, and reepithelialization was observed after every scraping.
Real-time RT-PCR analysis
Gene expressions of human OECs were compared with those of human cornea, limbus, and conjunctival epithelial cells to confirm that the expressions were suitable as OEC markers. Microarray analysis of gene expression revealed that cytokeratin 6 (K6) was significantly highly expressed in oral mucosa, while faint or no expression of K6 was observed in cornea, limbus, and conjunctiva (Figure 4a). Significantly high expression of K6 in OECs was also confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) (Figure 4b). Immunohistochemistry using anti–K6 antibody also showed that K6 was expressed in the suprabasal to superficial epithelial cell layers of normal human oral mucosal epithelium, while normal human cornea, limbus, and conjunctiva epithelium were faintly stained with K6 antibody (Figure 4c). As a result, K6 was chosen as the specific marker of OECs to identify OEC sheet–derived epithelial cells on the transplanted ocular surfaces.
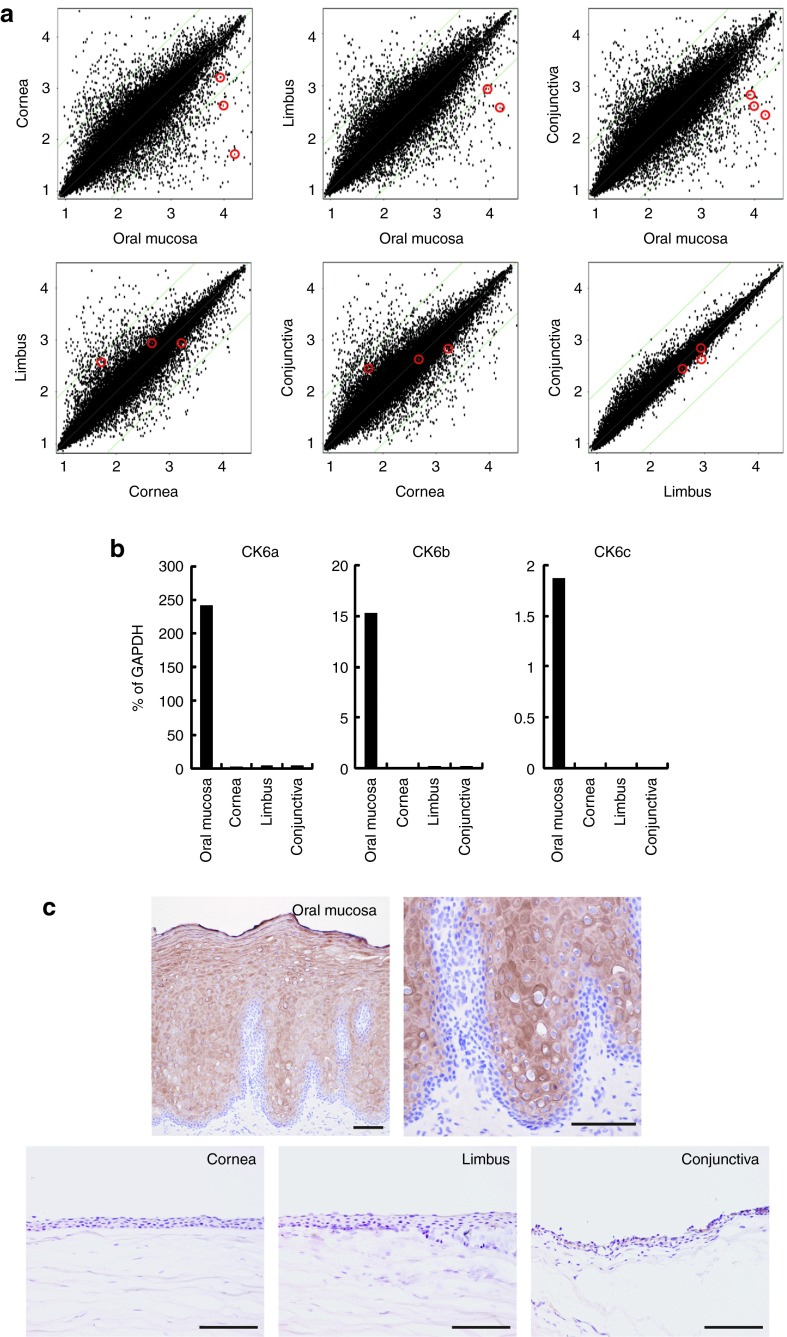
Figure 4.
Comparison analysis between native human oral mucosal, corneal, limbal, and conjunctival epithelia. (a) The global gene-expression patterns were compared between native human oral mucosal, corneal, limbal, and conjunctival epithelial cells with DNA microarrays. Red circles indicated the keratin 6 (K6) gene expression level of three of six isoforms. (b) K6 expression was validated by real-time RT-PCR analysis. K6 gene expressions of oral mucosal epithelium were significantly higher than that in corneal, limbal, and conjunctival epithelium. (c) In native human oral mucosal tissue, K6 expressions were observed from the suprabasal to the superficial layer, except in the basal layer. However, in native human cornea tissue, K6 expression was not observed in cornea, limbal, or the conjunctival region. K6 was chosen as a specific marker for oral mucosal epithelium to identify OEC sheets transplanted onto the ocular surfaces. Bars = 100 µm.
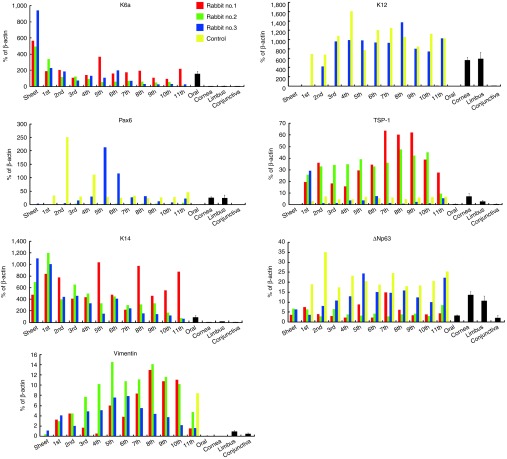
A significantly high expression of K6 was detected in normal rabbit oral mucosal epithelium and OEC sheets, while K6 was faintly expressed in normal rabbit cornea, limbus, and conjunctiva (Figure 5). K6 expression was detected in all three rabbits during the total follow-up period of 24 weeks, while it was not detected in the control rabbit that had a normal cornea with scrapings. The expressions of a corneal epithelium–specific keratin 12 (K12)23 and a homeobox transcription factor essential for the development and function of eye, Pax6,24 were only higher in the native rabbit cornea and limbus as well as the control rabbit. Interestingly, rabbit no. 3 also expressed Pax6, although neither rabbit no. 1 nor rabbit no. 2 expressed these genes. The expression of thrombospondin 1 (TSP-1), an antiangiogenic factor contributing to corneal avascularity,25 was detected in normal cornea and limbus, while it was hardly detected in oral mucosal epithelium, conjunctiva epithelium, or OEC sheets. TSP-1 expression was detected in the ocular surfaces of all the rabbits, although it was lower in rabbit no. 3 and the control rabbit. K14 expression was detected only in native oral epithelium, native limbus, and all the transplanted rabbits. Continuous expression of ΔNp63, a putative stem/progenitor marker,22,26 was detected in all the tissues of the native and the transplant model, as well as in the ocular surfaces of the control. Vimentin expression was detected in normal limbal and conjunctival epithelia and two of the OEC sheets (rabbits nos. 2 and 3). Interestingly, vimentin expression was upregulated in all three rabbits after transplantation. The expression was detected in all rabbits for the entire follow-up period of 24 weeks, while it was not detected in the control rabbit, except for the final scraping in which the entire corneal surface was removed instead of just the central corneal surface.
Figure 5.
Real-time RT-PCR analysis of the phenotype of transplanted oral mucosal epithelial cell (OEC) sheets. In rabbit nos. 1 and 2, cytokerain 6 (K6), an OEC marker, was detected, but neither of the corneal epithelial cell markers, cytokeratn 12 (K12) nor Pax6, were detected. In rabbit no. 3, K6, K12, and Pax6 were all detected, suggesting that the ocular surface was a heterogeneous mix of transplanted OEC sheets and corneal epithelial cells. Cytokeratin 14 (K14), a basal marker, and ΔNp63, a putative stem/progenitor marker, were detected during the 24-week follow-up period in all three rabbits. Vimentin, a mesenchymal marker, was detected in the transplanted OEC sheets, whereas cultivated OEC sheets and normal OECs only expressed these markers slightly. The red bars indicate the mRNA expression in rabbit no. 1; the green bars, rabbit no. 2; and the blue bars, rabbit no. 3; the yellow bars represent the control which was a normal cornea treated with 10 scrapings. Black bars indicate the mRNA expressions of oral mucosal, corneal, limbal, and conjunctival epithelial cells obtained from normal rabbit.
Immunohistology
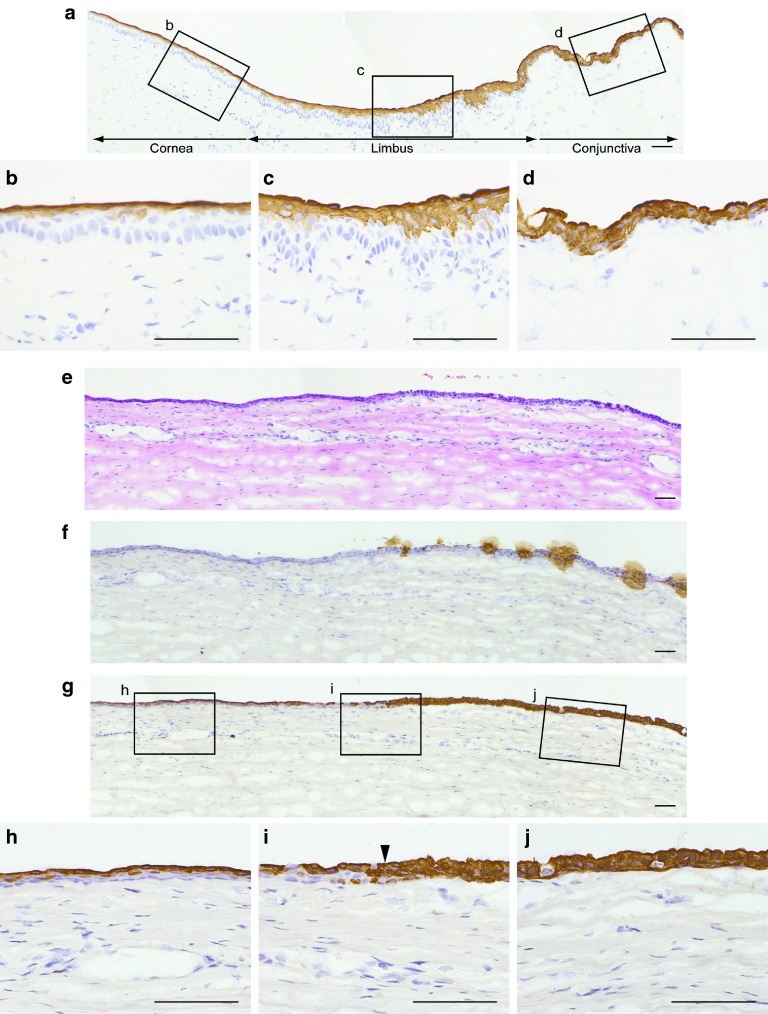
In the normal rabbit ocular surface, K4 was expressed in all conjunctival epithelial cell layers, while it was only expressed in the superficial layers of the limbal and peripheral corneal epithelia (Figure 6a–d). The transplant rabbits were sacrificed after 10 scrapings, and the ocular surfaces were subjected to histological analysis. Each of three serial sections was stained with hematoxylin–eosin, anti-Muc5, or anti-K4 antibody (Figure 6e–j). Reconstructed epithelia were thinner than native cornea and comprised three to four epithelial cell layers, while epithelial cell layers showed slightly increased thickness through the corneal periphery to the limbus and conjunctiva (Figure 6e). Muc5 was used to identify the conjunctival epithelial invasion into the cornea region.27 In transplanted ocular surfaces, Muc5-expressing cells were observed in the periphery of the cornea and in the conjunctiva (Figure 6f). K4 was expressed in the superficial layer of the reconstructed corneal epithelium, which did not express Muc5, as observed in normal central corneal and limbal epithelia (Figure 6g,h). On the contrary, all of the peripheral epithelial layers of the reconstructed cornea and conjunctiva expressed K4 (Figure 6g,i,j). The same expression pattern was obtained in all three rabbits.
Figure 6.
Investigation of the survival of transplanted oral mucosal epithelial cell (OEC) sheets by histological analysis. (a–d) In the normal rabbit ocular surface, K4 was expressed in all conjunctival epithelial cell layers, while only the superficial layer of the limbal and corneal epithelia expressed K4. The transplant rabbits were sacrificed after 10 scrapings, and the ocular surfaces were subjected to histological analysis. (e) Reconstructed epithelia were thinner than native cornea and were comprised three to four epithelial cell layers, while the epithelial cell layer was slightly thicker through the corneal periphery to the limbus and conjunctiva. (f) Mucin 5 (Muc5), a goblet cell marker, was used to identify conjunctival epithelial invasion into the cornea region. In transplanted ocular surfaces, Muc5-expressing cells were observed at the periphery of the cornea as well as the conjunctiva. (g–j) Interestingly, K4 was expressed in the superficial layer of ocular surface which did not express Muc5. These K4 expression patterns were similar to that of the transitional zone between the cornea and bulbar conjunctiva. Bars = 50 µm.
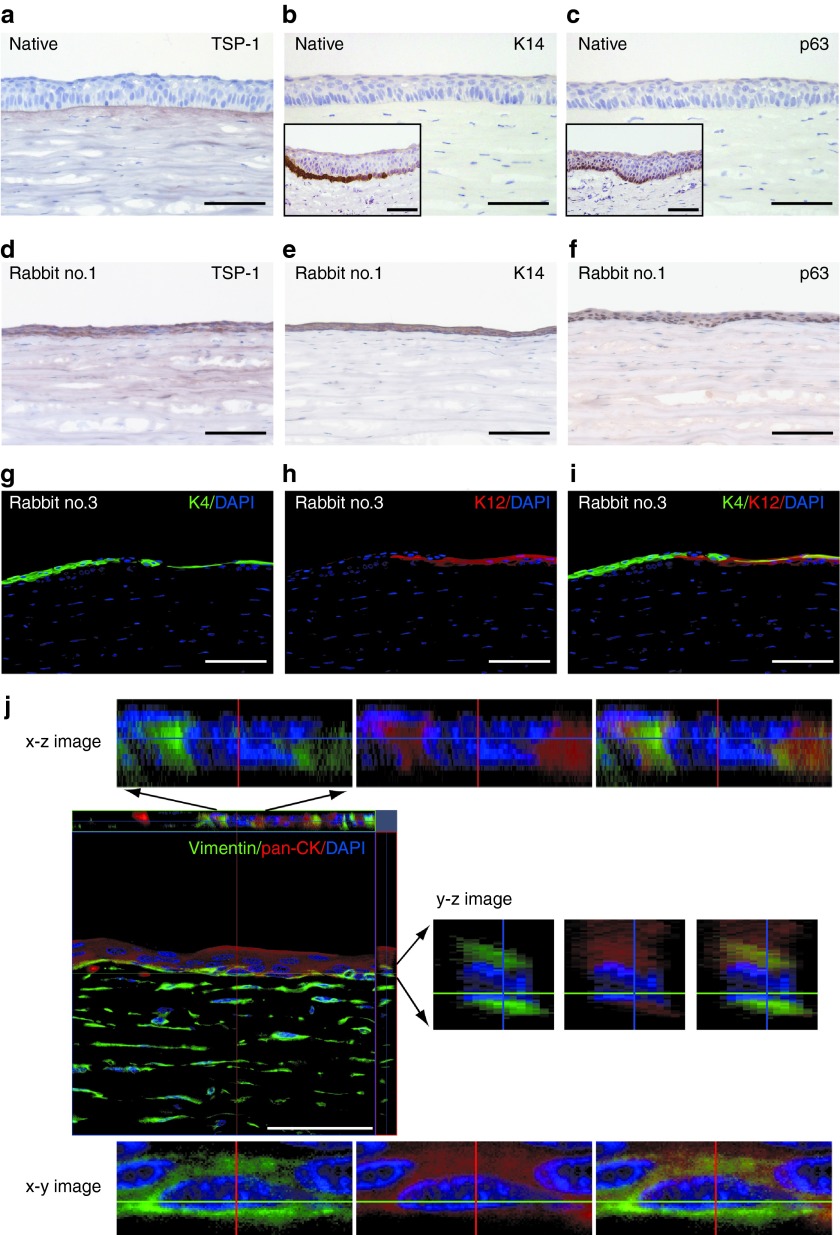
TSP-1 was deposited in the basement membrane and the upper part of the stroma beneath the basement membrane in normal rabbit cornea (Figure 7a). TSP-1 expression was detected in the epithelial cytoplasm and the upper part of the stroma in transplanted rabbits, while it was not detected in normal corneal epithelium (Figure 7a, d). K14, a basal epithelial cell marker, and p63, a putative stem/progenitor cell marker, were not detected in normal central cornea epithelium, while limbal basal epithelial cells were stained with K14 and p63 antibodies (Figure 7b,c). However, all the epithelial layers of the transplanted corneal surfaces were intensely stained with the antibodies (Figure 7e,f). These expression patterns of TSP-1, K14, and p63 were observed in all three rabbits. In line with the results of the gene expression analysis, K12 was detected only in the central reconstructed cornea of rabbit no. 3 (Figure 7g). The central ocular epithelium of rabbit no. 3 was a chimera of K12-positive and K4-positive cells (Figure 7g–i). Immunostaining of serial sections revealed that some cells were K12 and K4 double positive as the superficial layers of the peripheral native cornea and limbus.
Figure 7.
Characterization of transplanted oral mucosal epithelial cell (OEC) sheets by immunohistochemical analysis. (a) In normal cornea, thrombospondin 1 (TSP-1) expression was observed in the epithelial basement membrane and the upper part of the stroma beneath the basement membrane, while TSP-1 expression was not detected in normal corneal epithelium. (d) However, in transplanted ocular surfaces, TSP-1 expression was observed in all epithelial layers and diffused in the corneal stroma. (b,c) Cytokeratin 14 (K14) and p63 were not detected in normal central cornea epithelium, while limbal basal epithelial cells expressed K14 and p63 (inset). (e,f) However, K14 and p63 were observed in all the epithelial layers of the transplanted ocular surfaces. (g–i) In one of the rabbits (rabbit no. 3), K4-expressed transplanted OEC sheets and K12-expressed cornea epithelial cells were observed in a heterogeneous pattern. (j) Vimentin, a mesenchymal cell marker, was co-expressed in the basal layer of the cytokerain-expressed epithelial cells, suggesting that the basal cells of transplanted OEC sheets might induce epithelial–msenchymal transition. Bars = 100 µm.
In all three transplant rabbits, the basal epithelial layer of the central ocular surface contained vimentin-positive cells (Figure 7j). Interestingly, double immunofluorescent staining and confocal laser scanning microscopy revealed that these vimentin-positive cells were also pan-cytokeratin (pan-CK) positive. However, vimentin-positive keratocytes in the corneal stroma were pan-CK negative.
Discussion
This study reported that (i) the corneal surface of a rabbit LSCD model was successfully reconstructed by transplantation of cultivated autologous OEC sheets fabricated on a temperature-responsive culture surface and (ii) gene expression analysis and immunostaining after repeated scraping of the central ocular surfaces revealed two different types of ocular reconstruction. In two of the three rabbits (rabbit nos. 1 and 2), neither K12 nor Pax6 was detected over the entire period (Figure 5), suggesting that the ocular surfaces were reconstructed with only the transplanted OEC sheets. On the other hand, in the other rabbit (rabbit no. 3), the ocular surface expressed not only K6 but also K12 and Pax6 after the second scraping (Figure 5), implying that the ocular surface was reconstructed with K6-positive cells derived from the transplanted OEC sheet and K12- and Pax6-positive corneal epithelial cells derived from residual limbal epithelial stem cells. Since neither K12 nor Pax6 were expressed in the ocular surface of rabbit no. 3 at the first scraping, the ocular surface was initially reconstructed only with the transplanted OEC sheet. Since the expressions of K6, K12, and Pax6 were detected after the second scraping, the ocular surface appeared to be composed of two kinds of epithelial cells. Double immunostaining of K4 and K12 also revealed that the central reconstructed ocular surface of rabbit no. 3 was a chimera of K4-positive cells and K12-positive cells. However, the possibility of transdifferentiation of OECs to corneal epithelial cells cannot be excluded, although we previously reported that K12 expression was not detected in the ocular surface of a rabbit LSCD model with transplanted OEC sheets.15 If the K12- and Pax6-positive epithelial cells were derived from limbal epithelial stem cells, transplantation of OEC sheets likely provided an ideal environment for corneal epithelial cells derived from residual limbal epithelial stem cells in the LSCD model by reducing inflammation and neovascularization. Rapid epithelial cell proliferation for wound healing after scraping of the central ocular surface would evoke proliferation and differentiation of any residual limbal epithelial stem cells. The condition of the ocular surfaces such as the severity of inflammation, neovascularization, opacity, and conjunctival epithelium invasion was shown to be varied among human LSCD patients.28,29 In some patients, limbal epithelial stem cells might remain in the limbus but could not differentiate into corneal epithelial cells due to the lack of an appropriate environment. Limbal allografts are reported to be most successful in cases that show only recipient DNA in the reconstructed ocular surface, suggesting that paracrine factors from allogeneic transplantation stimulate proliferation of residual stem cells of the patient.30 For patients who do have residual stem cells, the primary role of transplanted OEC sheets would be to provide an appropriate environment for the proliferation of any residual limbal epithelial stem cells.
K14 and ΔNp63 were constantly expressed in the ocular surfaces of transplanted rabbits during the entire follow-up period, and in the fabricated OEC sheets (Figures 2c and 5), suggesting the presence of epithelial basal and putative stem/progenitor cells. In immunohistochemical analysis, OEC sheet–transplanted ocular surfaces were covered with approximately three layers of similar sized epithelial cells (Figure 7e,f), while the native cornea epithelial cell layer had cuboidal basal cells and flat cells at the upper cell layer (Figure 7b,c). Since K14 and p63 staining were observed in all cell layers of the transplanted OEC sheet, the transplanted OECs were considered to be in a growth phase induced by the physical scraping. Although the localization of epithelial stem cells in transplanted ocular surfaces still needs to be elucidated, the transplanted stem cells could be maintained, and it appears that the stem cell niche would be reconstructed.
Although ΔNp63α,22,26 C/EBPδ,31 ABCG2,32 cytokeratin 15 (K15),33,34 and others have been reported as a putative valuable candidates for epithelial stem cell markers, genuine epithelial stem cells have not been identified. Since stem cells have a replication competence and pluripotency,35,36 clonal analysis has been often utilized to examine the proliferative capacity and the differentiation potential of epithelial stem cells in vitro37 and ex vivo.38 In this study, the cornea reconstruction of the rabbit LSCD model was successfully observed for a total follow-up period of 24 weeks, even when scraping of central cornea was performed every 2 weeks (Figure 3 and Supplementary Figures S2–S4). This observation should provide strong evidence for the presence of epithelial stem cells on the ocular surface.
K6 expression is induced in hyperpfoliferative epithelium during wound hearing even in epidermis which lacks its expression in the normal condition, and the expression level returns to normal after healing is completed.39 In this study, K6 expression was not observed in the central corneal surface of the control rabbit 2 weeks after every scraping. K6 expression in OECs was significantly higher than that in cornea and conjunctival epithelial cells of native human or rabbit. Therefore, K6 was chosen as the marker for OECs. In the wound-healing process, the K6 expression level of corneal epithelial cells might increase just after scraping, but this has not been examined. In this study, K6 expression was hardly detected in the central corneal surface of the control rabbit 2 weeks after scraping. Therefore, the elevated K6 expression due to hyperproliferation was considered to have returned to normal levels at 2 weeks after scraping. This phenomenon is also reported in a mouse skin model.39 Moreover, it showed that the wound healing was completed within 2 weeks after every scraping. Fluorescein staining results also showed complete wound healing within 2 weeks after every scraping. However, K6 expression was observed in the central ocular surface of all three transplanted rabbits during the 24-week follow-up period (Figure 5). Furthermore, transplanted OEC sheet survival was investigated not only by K6 expression but also by K12 and Pax6 and corneal epithelial cell marker expression (Figure 5). This might be indirect evidence that epithelial stem cells contained in the transplanted OEC sheets could survive on the transplanted ocular surface during the investigation period.
It was reported that survival of a functional ocular surface decreases progressively until ~6 months after OEC sheet transplantation in LSCD patients.40 Kaplan–Meier analysis of the stability of ocular surfaces with transplanted OEC sheets revealed an early decline over the first 6 months, remaining comparatively stable thereafter.40 Therefore, the follow-up period in this study would be relevant to applications in clinical settings.
TSP-1 was deposited only at the basement membrane and the underlying stroma in normal rabbit cornea (Figure 7a), while it was detected in the epithelia and the upper part of the stroma in transplant rabbits (Figure 7a,d). Although TSP-1 is known as an antiangiogenic factor,41,42 it has also been reported that TSP-1 expression is induced in wound healing to support the proliferation of cornea epithelium.43 Furthermore, in the investigation into the manner of cultivated oral mucosal epithelial gene expression, TSP-1 expression is reported to increase during the culture period.44 In this study, TSP-1 was either faint or hardly expressed in both normal oral mucosa epithelium and fabricated OEC sheets before transplantation (Figure 5). Therefore, the significant upregulation of TSP-1 in transplanted ocular surfaces would be due to the wound healing. Interestingly, the high expression of TSP-1 was maintained in rabbit nos. 1 and 2 for a long period, while it returned to the normal corneal level in rabbit no. 3 after the second scraping. This expression pattern might resemble K6 expression.
In two of the three OEC sheets, a small amount of vimentin expression was detected before transplantation (Figure 5). The upregulation of vimentin expression was observed in reconstructed ocular surfaces after the transplantation of OEC sheets in all three rabbits, and the expression was detected during the entire follow-up period (Figures 5 and 7j). Double immunostaining and confocal laser scanning microscopy revealed that vimentin-positive cells were pan-CK positive in ocular surface epithelial cells. Vimentin is one of the mesenchymal cell-specific markers.45 Several vimentin-expressing cells including dendritic cells, melanocytes, and Langerhans cells are localized only in the limbal and conjunctival epithelia.46 Furthermore, some basal epithelia express vimentin in the limbal region, but the central corneal epithelium does not contain vimentin-expressing cells.46 In real-time RT-PCR analysis of native rabbit epithelium, vimentin expression was detected only in the limbal and conjunctival epithelium and was hardly detected in the cornea and oral epithelium (Figure 5). Since vimentin expression was not detected in the central corneal epithelium of the scraped control rabbit, the vimentin- and pan-CK double-positive cells would have been derived from the transplanted OEC sheets. Previously, Tseng's group47,48 reported that some basal epithelial cells in the limbus were vimentin- and pan-CK double positive. By the epithelial–mesenchymal transition, epithelial cells obtain mesenchymal phenotypes and increase their cell motility.49 It was also shown that epithelial–mesenchymal transition has a crucial role in gastrulation, tumor metastasis, and wound healing.49 Tseng's group50 also reported that limbal epithelial cells showed epithelial–mesenchymal transition and migrated to the stroma in organ culture with airlifting. Although, in this study, it is unclear whether the double-positive cells were the result of epithelial–mesenchymal transition or not, this is the first finding of the presence of vimentin- and pan-CK-double positive cells in central ocular surfaces.
This study successfully demonstrated that fabricated autologous OEC sheets had the ability to reconstruct LSCD ocular surfaces and maintain ocular surface homeostasis for a long period. The regulation of the fate of stem cells and the constitution of a stem cell niche should be elucidated to establish an effective therapy for reconstructing the ocular surface. In addition, the origin and role of pan-CK- and vimentin-double positive cells should also be investigated.
Materials and Methods
Autologous OEC sheet transplantation in a rabbit LSCD model. New Zealand White rabbits were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and with an experimental procedure approved by the Animal Care and Use Committee at the Tokyo Women's Medical University. To fabricate this rabbit LSCD model, the entire cornea epithelium including limbus and conjunctival tissue from the transitional zone between the cornea and the conjunctiva by 3 mm was completely removed surgically under anesthesia to expose the stroma. After keratectomy, the ocular surface was treated with the topical application of 1-n-heptanol for 5 minutes. An antibiotic (0.3% ofloxaxin; Santen, Osaka, Japan) and steroid (0.1% betamethasone; Shionogi, Osaka, Japan) were applied once a day for 1 week. Three weeks after surgery, a 5-by-10 mm specimen of oral mucosal biopsy was obtained from the interior buccal mucosa. After treatment with dispase II (Godo Shusei, Tokyo, Japan) at 37 °C for 1 hour, OEC layers were isolated and trypsinized for 20 minutes at 37 °C to produce single cell suspensions. The suspended OECs were cultivated on a temperature-responsive cell-culture insert (UpCell; CellSeed, Tokyo, Japan) at an initial cell density of 1.2 × 105 cells/cm2 with mitomycin C-treated (Wako, Osaka, Japan) NIH/3T3 feeder cells. The culture medium was a mixture of Dulbecco's modified Eagle's medium (Sigma, St Louis, MO) and Ham's F-12 (Sigma) at a ratio of 3:1 supplemented with 10% fetal bovine serum (Japan Bioserum, Hiroshima, Japan), 0.4 µg/ml hydrocortisone (Wako), 2 nmol/l triiodothyronine (Wako), 1 nmol/l cholera toxin (Wako), 5 µg/ml insulin (Life Technologies, Carlsbad, CA), 5 µg/ml transferring (Life Technologies), 10 ng/ml epidermal growth factor (Life Technologies), 100 U/ml penicillin (Life Technologies), and 100 µg/ml streptomycin (Life Technologies). Approximately 14 days later, the cell sheets were harvested by reducing the temperature. Five weeks after the preparation of LSCD model, the conjunctival and subconjunctival scar tissue of the model was removed to reexpose the corneal stroma. A harvested autologous OEC sheet with the supporting membrane ring was placed directly onto the exposed transparent stromal bed. For protection, the ocular surface was covered with a contact lens, and a tarsorrhaphy was performed. An antibiotic (0.3% ofloxacin) and steroid (0.1% betamethasone) were applied locally once a day for 1 week.
Follow-up and clonal analysis of transplanted cell sheets in vivo. One week after transplantation, the ocular surfaces were carefully observed and stained with fluorescein dye to determine if there were any defects. Four weeks after transplantation, the central ocular surface was scraped circularly (5 mm in diameter) by a scalpel under local anesthesia and stained with fluorescein dye to confirm that the corneal stroma was reexposed. The wounded ocular surface was covered with a contact lens, and an antibiotic (0.3% ofoxacin) and steroid (0.1% betamethasone) were applied locally once every few days. Two weeks after the artificial wounding, reepithelialization was identified by fluorescein staining and again re-scraped circularly. This 2-week procedure was then repeated 10 times for a total follow-up period of 24 weeks.
Microarray analysis. Human oral mucosal tissues were obtained in compliance with institutional review board regulations, informed consent regulations, and the tenets of the Declaration of Helsinki. An oral mucosal biopsy was obtained from a healthy volunteer, and the epithelial layers were carefully removed after treatment with dispase I (1,000 PU/ml; EIDIA, Tokyo, Japan) at 37 °C for 1 hour. Human conjunctiva and limbal tissues were isolated from whole human globes (Northwest Lions Eye Bank, Seattle, WA) using scissors, and the 8.0-mm-diameter portions of the central corneas were obtained by trephination. Excised tissues were incubated individually by treatment with 2.4 units/ml dispase II (Life Technologies) at 37 °C for 1 hour. Total RNA was extracted using an RNeasy Total RNA Kit (Qiagen, Hilden, Germany) from isolated human oral mucosal, corneal, limbal, and conjunctival epithelium. For microarray analysis, cDNA hybridization to Human Genome U133 Plus2.0 (47,000 probe sets, transcription products) was performed by RNA amplification procedure with a GeneChip 3′ IVT Express Kit (Affymetrix, Santa Clara, CA). The expression data were analyzed by GeneChip Operating Software using standard protocols.
Real-time PCR analysis. Total RNA was extracted from normal human oral mucosal, corneal, limbal, and conjunctival epithelial cells, and a piece of transplanted epithelium was obtained by scraping. Real-time PCR analysis was performed in the standard manner. Primer pairs were designed by Primer Express Software v2.0 (Applied BioSystems) to detect the rabbit mRNA expressions of K6a, K14, ΔNp63, TSP-1 and β-actin (Supplementary Table S1). All assays were performed in duplicate for individual samples.
Histological analysis. Normal human oral mucosa, human corneoscleral rims, and transplanted OEC sheets on rabbit cornea tissues were embedded in an optimum cutting temperature compound and processed into 5-µm frozen sections. Tissue sections were stained with mouse monoclonal anti-K6 (1:100 dilution; Lab Vision, Fremont, CA), mouse monoclonal anti-K4 (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-K13 (1:100 dilution; Abcam, Cambridge, UK), mouse monoclonal anti-Muc 5 (1:100 dilution; Life Technologies), mouse monoclonal anti-K14 (1:50 dilution; Santa Cruz Biotechnology), mouse monoclonal anti-p63 (1:100 dilution; Santa Cruz Biotechnology), and mouse monoclonal anti-TSP-1 (1:50 dilution; Abcam). For double immunofluorescence staining, tissue sections were stained with anti-K4, goat polyclonal anti-K12 (1:100 dilution; Santa Cruz Biotechnology), mouse monoclonal anti-pan-CK (1:20 dilution; Abcam), and mouse monoclonal anti-vimentin antibody (Santa Cruz Biotechnology). These sections were observed by a confocal laser-scanning microscope (LSM-510, Carl Zeiss, Jena, Germany).
SUPPLEMENTARY MATERIAL Table S1. Primer pairs and probes for rabbit mRNA expression analysis by real-time RT-PCR. Figure S1. Evaluation of rabbit limbal stem cell deficiency model by immunohistochemical analyses of pannus. Figure S2. Time course images of transplanted ocular surface with repeated wound healing (rabbit 1). Figure S3. Time course images of transplanted ocular surface with repeated wound healing (rabbit 2). Figure S4. Time course images of transplanted ocular surface with repeated wound healing (rabbit 3). Figure S5. Time course images of normal corneal surface with repeated wound healing (the control). Materials and Methods
Acknowledgments
The authors thank Norio Ueno, Ryo Takagi, and Daisuke Murakami (Tokyo Women's Medical University) for their useful comments and technical criticisms. This study was supported by the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology “Cell Sheet Tissue Engineering Center” and the Global COE program, the Multidisciplinary Education and Research Center for Regenerative Medicine, from the Ministry of Education, Culture, Sports, Science and Technology, Japan. T.O. is a director of the board of Cell Seed. M.Y. had been a science consultant of Cell Seed until 2011. T.O. and M.Y. are stake holders of Cell Seed and are inventors of cell sheet–related patent. Tokyo Women's University is collaborating with Cell Seed and receiving research fund from the company. We thank Allan Nisbet for his useful comments and editing assistance.
Supplementary Material
Evaluation of rabbit limbal stem cell deficiency model by immunohistochemical analyses of pannus.
Time course images of transplanted ocular surface with repeated wound healing (rabbit 1).
Time course images of transplanted ocular surface with repeated wound healing (rabbit 2).
Time course images of transplanted ocular surface with repeated wound healing (rabbit 3).
Time course images of normal corneal surface with repeated wound healing (the control).
Primer pairs and probes for rabbit mRNA expression analysis by real-time RT-PCR.
References
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, et al. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, et al. 2012Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets Gastroenterologyepub ahead of print). [DOI] [PubMed]
- Ito S, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, et al. Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials. 2012;33:5278–5286. doi: 10.1016/j.biomaterials.2012.03.073. [DOI] [PubMed] [Google Scholar]
- Hamahashi K, Sato M, Yamato M, Kokubo M, Mitani G, Ito S, et al. 2012Studies of the humoral factors produced by layered chondrocyte sheets J Tissue Eng Regen Medepub ahead of print). [DOI] [PubMed]
- Iwata T, Yamato M, Tsuchioka H, Takagi R, Mukobata S, Washio K, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30:2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, et al. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37:1088–1099. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3 Pt 2:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Ti SE, Anderson D, Touhami A, Kim C, Tseng SC. Factors affecting outcome following transplantation of ex vivo expanded limbal epithelium on amniotic membrane for total limbal deficiency in rabbits. Invest Ophthalmol Vis Sci. 2002;43:2584–2592. [PubMed] [Google Scholar]
- Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, et al. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46:1632–1639. doi: 10.1167/iovs.04-0813. [DOI] [PubMed] [Google Scholar]
- Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, Nguyen CV, Deng SX. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis. 2011;17:1652–1661. [PMC free article] [PubMed] [Google Scholar]
- Tanioka H, Kawasaki S, Yamasaki K, Ang LP, Koizumi N, Nakamura T, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 2006;47:3820–3827. doi: 10.1167/iovs.06-0293. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–116. doi: 10.1167/iovs.02-0195. [DOI] [PubMed] [Google Scholar]
- Murakami D, Yamato M, Nishida K, Ohki T, Takagi R, Yang J, et al. Fabrication of transplantable human oral mucosal epithelial cell sheets using temperature-responsive culture inserts without feeder layer cells. J Artif Organs. 2006;9:185–191. doi: 10.1007/s10047-006-0342-3. [DOI] [PubMed] [Google Scholar]
- Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D. The phenotype of limbal epithelial stem cells. Invest Ophthalmol Vis Sci. 2007;48:144–156. doi: 10.1167/iovs.06-0346. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci. 1998;39:2554–2561. [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Different roles for Pax6 in the optic vesicle and facial epithelium mediate early morphogenesis of the murine eye. Development. 2000;127:945–956. doi: 10.1242/dev.127.5.945. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch Ophthalmol. 2007;125:1337–1344. doi: 10.1001/archopht.125.10.1337. [DOI] [PubMed] [Google Scholar]
- Saito T, Nishida K, Sugiyama H, Yamato M, Maeda N, Okano T, et al. Abnormal keratocytes and stromal inflammation in chronic phase of severe ocular surface diseases with stem cell deficiency. Br J Ophthalmol. 2008;92:404–410. doi: 10.1136/bjo.2007.127738. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Nishida K, Sotozono C, Quantock AJ, Kinoshita S. Conjunctival inflammation in the chronic phase of Stevens-Johnson syndrome. Br J Ophthalmol. 2000;84:1191–1193. doi: 10.1136/bjo.84.10.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, De Luca M, Arsenijevic Y. Towards therapeutic application of ocular stem cells. Semin Cell Dev Biol. 2007;18:805–818. doi: 10.1016/j.semcdb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol. 2007;177:1037–1049. doi: 10.1083/jcb.200703003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Porter RM, Lunny DP, Ogden PH, Morley SM, McLean WH, Evans A, et al. K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab Invest. 2000;80:1701–1710. doi: 10.1038/labinvest.3780180. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- Lajtha LG. Stem cell concepts. Differentiation. 1979;14:23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notara M, Schrader S, Daniels JT. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng Part A. 2011;17:741–750. doi: 10.1089/ten.TEA.2010.0343. [DOI] [PubMed] [Google Scholar]
- Inada R, Matsuki M, Yamada K, Morishima Y, Shen SC, Kuramoto N, et al. Facilitated wound healing by activation of the Transglutaminase 1 gene. Am J Pathol. 2000;157:1875–1882. doi: 10.1016/S0002-9440(10)64826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118:1524–1530. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Sekiyama E, Nakamura T, Cooper LJ, Kawasaki S, Hamuro J, Fullwood NJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006;47:1352–1358. doi: 10.1167/iovs.05-1305. [DOI] [PubMed] [Google Scholar]
- Sekiyama E, Nakamura T, Kawasaki S, Sogabe H, Kinoshita S. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp Eye Res. 2006;83:741–746. doi: 10.1016/j.exer.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Uno K, Hayashi H, Kuroki M, Uchida H, Yamauchi Y, Kuroki M, et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004;315:928–934. doi: 10.1016/j.bbrc.2004.01.146. [DOI] [PubMed] [Google Scholar]
- Alaminos M, Garzón I, Sánchez-Quevedo MC, Moreu G, González-Andrades M, Fernández-Montoya A, et al. Time-course study of histological and genetic patterns of differentiation in human engineered oral mucosa. J Tissue Eng Regen Med. 2007;1:350–359. doi: 10.1002/term.38. [DOI] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Osborn M, Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci USA. 1978;75:5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548. doi: 10.1089/ten.tec.2010.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012;53:5686–5697. doi: 10.1167/iovs.12-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of rabbit limbal stem cell deficiency model by immunohistochemical analyses of pannus.
Time course images of transplanted ocular surface with repeated wound healing (rabbit 1).
Time course images of transplanted ocular surface with repeated wound healing (rabbit 2).
Time course images of transplanted ocular surface with repeated wound healing (rabbit 3).
Time course images of normal corneal surface with repeated wound healing (the control).
Primer pairs and probes for rabbit mRNA expression analysis by real-time RT-PCR.