The stable retroviral integration of therapeutic transgene cassettes into patients' hematopoietic stem and progenitor cells enables gene therapy for various disorders of the blood and immune systems.1,2,3,4 However, adverse events related to insertional mutagenesis were observed in the first clinical gene therapy trials.5,6 These adverse events were caused mainly by the insertional activation of proto-oncogenes, such as LMO2 and MDS/EVI1, which occurred as a consequence of the retroviral integration preferences and the strength of the enhancer of the first-generation long terminal repeat (LTR)-driven gammaretroviral vectors. Two concepts have been proposed to achieve a “safer” integration profile. The first is targeted integration into genomic “safe harbors” by designer nucleases. Sadelain et al. defined safe harbors as chromosomal sites where transgenes can be stably and reliably expressed without adversely affecting endogenous gene structure or expression.7 The second concept is directing integration away from potentially harmful chromosomal sites, such as promoters of proto-oncogenes, by exploiting retroviral vectors with a more random integration pattern or, alternatively, by interfering with the integration and chromatin-tethering processes during retroviral integration (Figure 1).
Figure 1.
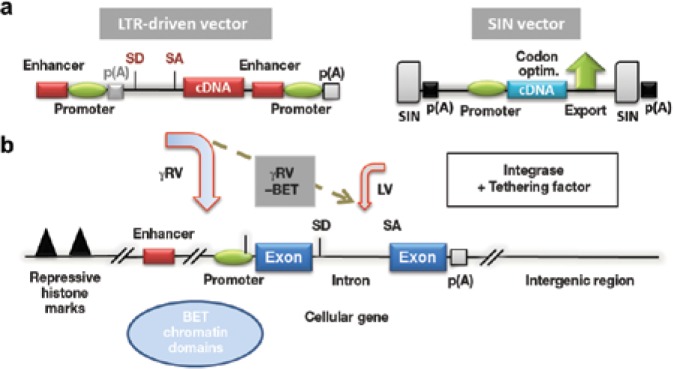
Strategies to prevent insertional mutagenesis. (a) Toward a safer vector architecture: from a long-terminal repeat (LTR)-driven vector to a self-inactivating (SIN) vector with a more physiological internal promoter. (b) Retroviral integration as a concerted action of retroviral integrase and host cell tethering factor. Integration-site preferences of murine leukemia virus (MLV)-based gammaretroviral (γRV) and HIV1-based lentiviral (LV) vectors. γRV integrations cluster with BET chromatin domains and are associated with promoter/enhancer sequences. LV vectors integrate preferentially into actively transcribed genes. BET-independent (−BET) γRV integrate less in proximity to transcriptional start sites and promoter/enhancer regions. cDNA, complementary DNA; SD/SA, splice sites; p(A), polyA site. Modified from artwork courtesy of Chris Baum.
In a paper in Molecular Therapy—Nucleic Acids, El Ashkar et al. from Rik Gijsbers's group in Leuven, Belgium,8 describe a promising new way to direct the integration profile of gammaretroviral murine leukemia virus (MLV)-based vectors away from their natural preference for transcriptional start sites (TSSs), CpG-rich islands, and promoter/enhancer regions.9,10 Previous work by the authors,11 as well as work from other groups,12,13 identified the bromodomain and extraterminal (BET) family of proteins as the cellular tethering factors for MLV integrase (IN). El Ashkar and colleagues determined the specific amino acids and regions in IN that are responsible for binding to BET-proteins.8 Deletion of the 20 C-terminal amino acids of IN or introduction of a single-point mutant (INW390A) led to the creation of MLV-based vectors that no longer exhibited a typical MLV integration profile with a preference for promoter/enhancer regions and that associated less frequently with proto-oncogene TSSs. Their comparison of BET-independent MLV vector integration–site selection to a set of (epi) genetic modifications (distance to TSS, DNase I hypersensitivity sites, CpG islands, association to specific histone codes) showed that overall integration is distributed more randomly and is less frequently associated with markers of active chromatin. Accordingly, gene expression from these vectors was slightly decreased. The authors demonstrated that viral titers produced from the INW390A construct were similar to those produced from wild-type vectors, whereas the C-terminal IN truncation led to slightly reduced (one-sixth the production and twice the integration defect) viral titers. Importantly, titers are still in a range likely to be in accordance with the needs of gammaretroviral vector production for clinical gene therapy trials.
The current work by Gijsbers's group also extends previous studies aimed at deciphering the tethering mechanism and demonstrates some parallels to the HIV IN tethering factor lens epithelium–derived growth factor (LEDGF). LEDGF also binds HIV IN, protects it from proteasomal degradation, and tethers the lentiviral preintegration complex to the chromatin.14,15,16 As in the BET-independent MLV IN setting, lentiviral preference for integration into active genes is decreased, arguing for a shift in integration preference.
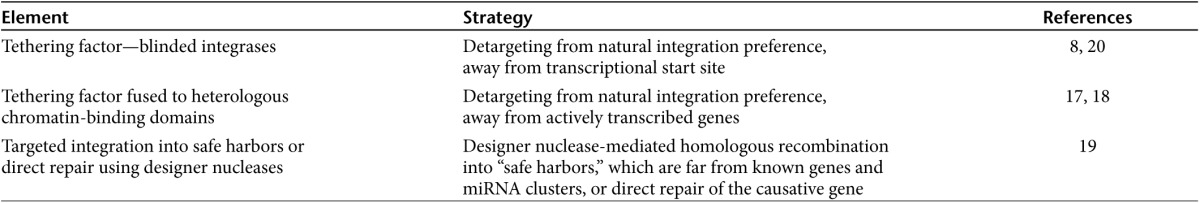
Another strategy to shift integration preference of retroviruses entails substitution of the chromatin-interacting binding domain of the tethering factor with histone-binding domains,17,18 as demonstrated for LEDGF and the histone-binding CBX1. Proof of principle was demonstrated as LV vectors integrated outside of genes, near regions occupied by CBX1. Ideally, however, this strategy requires abrogation of endogenous LEDGF and overexpression of the chimeric LEDGF-CBX1 fusion, which is very demanding from a practical standpoint. Therefore, direct IN changes as discussed here represent a more elegant and straightforward approach. In addition, complementing retroviral/lentiviral vector approaches with new developments to target stable integration sites should also be carefully considered (see Table 1). An example is the use of designer nucleases and homologous recombination of a donor sequence to reach “safe harbors” or to specifically repair defective genes as recently demonstrated in hematopoietic stem cells.19
Table 1. Strategies to influence integration-site selection.
Interestingly, Aiyer and co-workers20 recently used solution nuclear magnetic resonance and protein interaction studies to demonstrate that the C-terminal tail peptide region of MLV IN is important for the interaction with BET proteins. It is reassuring that they also observed that disruption of MLV IN-BET interaction through truncation mutations affected the global targeting profile of MLV vectors.
What is required to translate these findings into the (pre)clinical arena? First, the new BET-independent MLV vectors should be tested in relevant preclinical safety and bone marrow/hematopoietic stem cell transplantation assays to validate their performance and to provide proof of concept for improved safety. Second, BET-independent MLV packaging constructs should be combined with next-generation gammaretroviral self-inactivating vectors, given that their vector architecture with weaker, more physiological internal promoters (representing weaker cis-activators of neighboring genes) will probably lead to a further increase in safety.
In summary, the findings reported by El Ashkar et al. will help achieve better control of integration-site preferences of retroviral vectors and, together with improvements in vector design, will increase the safety of gammaretroviral vectors for gene therapy.
References
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L.et al. (2009Gene therapy for immunodeficiency due to adenosine deaminase deficiency N Engl J Med 360447–458. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP.et al. (2002Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy N Engl J Med 3461185–1193. [DOI] [PubMed] [Google Scholar]
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J.et al. (2004Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector Lancet 3642181–2187. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U.et al. (2006Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1 Nat Med 12401–409. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A.et al. (2008Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1 J Clin Invest 1183132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A.et al. (2010Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease Nat Med 16198–204. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Papapetrou EP., and, Bushman FD. Safe harbours for the integration of new DNA in the human genome. Nat Rev Cancer. 2012;12:51–58. doi: 10.1038/nrc3179. [DOI] [PubMed] [Google Scholar]
- El Ashkar S.et al. (2014BET-independent MLV-based vectors target away from promoters and regulatory elements Mol Ther Nucleic Acids 20143e179doi:10.1038/mtna.2014.33. Published online 29 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D, Crise B, Li Y, Princler G, Lum N, Stewart C.et al. (2007Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses J Virol 816731–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC.et al. (2004Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences PLoS Biol 2E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rijck J, de Kogel C, Demeulemeester J, Vets S, El Ashkar S, Malani N.et al. (2013The BET family of proteins targets moloney murine leukemia virus integration near transcription start sites Cell Rep 5886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Larue RC, Plumb MR, Malani N, Male F, Slaughter A.et al. (2013BET proteins promote efficient murine leukemia virus integration at transcription start sites Proc Natl Acad Sci USA 11012036–12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SS, Maetzig T, Maertens GN, Sharif A, Rothe M, Weidner-Glunde M.et al. (2013Bromo- and extraterminal domain chromatin regulators serve as cofactors for murine leukemia virus integration J Virol 8712721–12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan AM., and, Poeschla EM. Chromatin tethering and retroviral integration: recent discoveries and parallels with DNA viruses. Biochim Biophys Acta. 2010;1799:182–191. doi: 10.1016/j.bbagrm.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ F., and, Debyser Z. The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy. Virology. 2013;435:102–109. doi: 10.1016/j.virol.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Ciuffi A., and, Bushman FD. Retroviral DNA integration: HIV and the role of LEDGF/p75. Trends Genet. 2006;22:388–395. doi: 10.1016/j.tig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Gijsbers R, Ronen K, Vets S, Malani N, De Rijck J, McNeely M.et al. (2010LEDGF hybrids efficiently retarget lentiviral integration into heterochromatin Mol Ther 18552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vets S, De Rijck J, Brendel C, Grez M, Bushman F, Debyser Z.et al. (2013Transient expression of an LEDGF/p75 chimera retargets lentivector integration and functionally rescues in a model for X-CGD Mol Ther Nucleic Acids 2e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese P, Schiroli G, Escobar G, Di Tomaso T, Firrito C, Calabria A.et al. (2014Targeted genome editing in human repopulating haematopoietic stem cells Nature 510235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyer S, Swapna GV, Malani N, Aramini JM, Schneider WM, Plumb MR.et al. (2014Altering murine leukemia virus integration through disruption of the integrase and BET protein family interaction Nucleic Acids Res 425917–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]


