Abstract
Intracellular Vitamin C (VC) is maintained at high levels in the developing brain by the activity of sodium-dependent VC transporter 2 (Svct2), suggesting specific VC functions in brain development. A role of VC as a cofactor for Fe(II)-2-oxoglutarate-dependent dioxygenases has recently been suggested. We show that VC supplementation in neural stem cell (NSC) cultures derived from embryonic midbrains greatly enhanced differentiation towards midbrain-type DA (mDA) neurons, the neuronal subtype associated with Parkinson’s disease. VC induced gain of 5-hydroxymethylcytosine (5hmC) and loss of H3K27m3 in DA phenotype gene promoters, which are catalyzed by Tet1 and Jmjd3, respectively. Consequently VC enhanced DA phenotype gene transcriptions in the progenitors by Nurr1, a transcription factor critical for mDA neuron development, to be more accessible to the gene promoters. Further mechanism studies including Tet1 and Jmjd3 knockdown/inhibition experiments revealed that both the 5hmC and H3K27m3 changes, specifically in the progenitor cells, are indispensible for the VC-mediated mDA neuron differentiation. We finally show that in Svct2 knockout mouse embryos, mDA neuron formation in the developing midbrain decreased along with the 5hmC/ H3k27m3 changes. These findings together indicate an epigenetic role of VC in midbrain DA neuron development.
Keywords: dopamine neuron, fetal midbrain, vitamin C, 5hmC, Jmjd3
INTRODUCTION
Dopamine (DA) neurons in the midbrain are a neuronal subtype with high physiologic and clinical importance. Midbrain DA (mDA) neurons play critical roles in the control of motor behaviors and emotions, and degeneration or dysfunction of these neurons underlies various neuropsychiatric disorders such as Parkinson’s disease (PD), schizophrenia, and drug addiction. mDA neurons arise from the ventral midbrain (VM) by the sequential and combinatory actions of the developmental transcription factors such as Nurr1, Foxa2, Pitx3, Lmx1a, Msx1, Neurogenin2, and Mash 1 [1]. Multipotent neural stem cells (NSCs) differentiate into mDA neurons by acquiring both neuronal and DA neuron-specific phenotypes. Among the factors essential for mDA neuron development, Nurr1, an orphan nuclear receptor, acts as a transcription factor responsible for the DA phenotype acquisition [2–4]. The Nurr1-mediated DA gene transcription is regulated by co-activators and repressors, in a stereotypical epigenetic control manner during the midbrain development [5–7].
Ions, chemicals, and other small molecules in intra- and extra-cellular milieu are regarded to be important in cellular functions and homeostasis. However, physiologic roles of those small molecules in the brain development are largely unknown. Vitamin C (L-ascorbic acid; VC) is an important micro-constituent participating in numerous cellular functions, such as scavenging oxygen free radicals and its roles as an enzyme cofactor, in most of tissues. VC concentration in brain is higher than in any other organ, and has multiple functions including anti-oxidant protection, neurotransmission modulation, myelin formation, and synaptic potentiation [8]. Brain VC levels are even greater during embryonic development [9], suggesting specific VC functions in brain development. Recent studies have revealed roles of VC in epigenetic regulation of stem cell behaviors. VC acts as a co-factor for the family of Fe(II)-2-oxoglutarate-dependent dioxygenases [10]. One important group of enzymes in this family is the ten-eleven-translocation 1–3 (Tet1–3) enzymes. These enzymes catalyze conversion of 5-methylcytosine (5mC) at CpG dinucleotides of DNA into5-hydroxymethylcytosine (5hmC). The VC-mediated activation of Tets activates gene expressions via elimination of the DNA methylation (demethylation; derepression) and possibly via indirectly inducing histone modifications [11–13]. 5hmC and Tets have important roles in epigenetic reprogramming, stem cell differentiation, regulation of tissue-specific gene expression, and transformation [14, 15]. In the CNS, Tet1 activity is involved in memory formation by positively regulating NSC proliferation in adult hippocampus [16] and the expression of CNS activity-dependent neuronal genes [17, 18]. Furthermore, 5hmC levels increase during neuronal differentiation in embryonic mouse brain [19]. Jumonji C (JmjC)-domain-containing histone demethylases (Jmjds) are another class of dioxygenases where activity is potentially modulated by VC. The Jmjd enzymes catalyze demethylation on lysine residues of histone proteins, and thereby modify local chromatin structures. VC facilitates the reprogramming of fibroblasts towards induced pluripotent stem cells via Jmjd-mediated histone demethylation [20]. The repressive mark histone 3 lysine 27 trimethylation (H3K27me3) is erased by Jmjd3 during epidermal differentiation [21]. Similarly, removal of H3K27me3 along with gain of 5hmC is manifest in neurogenic genes in the developing brain [19]. Furthermore, overexpression of Jmjd3 in NSCs was shown to activate a subset of neural differentiation genes [22].
Previous studies, including some from our own laboratory, have demonstrated that VC enhances DA neuronal yields from cultured stem cells [23–25], and VC is currently used as a common culture supplement in the study aiming at efficient DA neuron production. However, the mechanisms underlying these essential effects of VC remain unknown, despite their profound physiologic implications for nutrition during development. In the present study we extended the study of VC-mediated mDA neuron differentiation with a primary focus on the molecular mechanism and implications. Our findings suggest that intracellular VC acts as a micro-environmental cue to control DA neuron differentiation in developing VM via Tet1- and Jmjd3-mediated epigenetic regulation.
MATERIALS AND METHODS
Cell culture and chemicals
VMs of rat embryos (Sprague Dawley, SD) at embryonic day 12 (E12) were triturated and plated on 10-cm dishes or 24-well plates pre-coated with 15 µg/ml poly-L-ornithine (PLO; Sigma, St. Louis, MO)/1 µg/ml fibronectin (FN; Sigma) in serum-free N2 medium. NSCs were proliferated by the mitogenic action of basic fibroblast growth factor (bFGF, 20 ng/ml, R&D Systems, Minneapolis, MN). At 70–80% cell confluency (usually for 4–6 days), the expanded NSCs were induced to differentiate (unpassaged culture; P0) or subcultured into freshly prepared PLO/FN-coated surfaces (24-well plates) and then followed by subsequent bFGF-induced cell proliferation for another 2–4 days (passaged culture; P1). Differentiation of the expanded NSCs was induced by withdrawing the mitogen from the media (for 3–16 days). Cultures were maintained at 37°C in humidified 5% CO2 incubators. All the experiments, except those to compare P0 vs P1 cultures, were carried out using P1 cultures. Chemicals used are VC (0.1–200 µM), glutathione (reduced form, GSH; 200 µM), Vitamin E (alpha-tocopherol; 200 µM), docosahexanoic acid (200 µM), phloretin (10 µM), quercetin (10 µM), and GSK-J4 (0.25 µM, all from Sigma).
Immunoblot assays
For DNA dot blot analysis, total DNA was extracted, quantified. DNA (80 ng) was loaded on nitrocellulose membrane, air dried, and exposed in UV for 20 min. After blocking with by 5% BSA/TTBS at room temperature for 2 hours, the membrane was incubated in anti-5hmC (Active Motif) and anti-5mC (Abcam) antibodies at 4°C overnight. For protein blot, histones were acid-extracted from cell samples. 2µg of histone samples were electrophoresized with 15% SDS-PAGE gels, and the blotted membranes were incubated with anti-H3K4m3, H3K9m3, H3K27m3, H3K36m3 and H3 antibodies (all from Millipore). Positive bands were detected and captured by ChemiDocR (Bio-rad, Hercules, Ca), and intensities of the bands were quantified using Image J software (http://imagej.nih.gov/ij). Quantification was performed using average values from three independent experiments.
DA neuron functional assays
Presynaptic DA neuron functions were assessed by DA release and uptake assays in the differentiated rat E12 VM-NSC cultures. To attain a full maturation of DA neuron functionality, the VM-NSCs underwent a differentiation for 16 days. The cultures were incubated in fresh N2 containing 56 mM KCl (evoked) or not (basal release) for 30 min. The media were collected and applied to DA level determination using an ELISA kit (BA E-5300, LDN). DA uptake assay was conducted as described previously [26]. Briefly, cells were incubated with 50nM [3H] DA (5 µCi/mmol, Amersham) with or without 10 µM nomifensine (RBI) to determine nonspecific uptake level. Radioactivity from lysed cells was measured by liquid scintillation counter (MicroBeta TriLux ver. 4.4). DA uptake level was calculated by subtracting nonspecific uptake value (with nomifensine) from the uptake value without nomifensine.
Immunofluorescent staining and measurement of mean fluorescence intensity (MFI)
Cells or cryosectioned embryonic VM tissues were fixed with 4% paraformaldehyde, blocked for 40 min in a blocking solution (1% bovine serum albumin/0.3% Triton X-100 (or 0.1% saponin for DAT staining)), and incubated overnight at 4°C in the blocking solution containing the primary antibodies listed in Table S1. Secondary antibodies tagged with Alexa 488 (1:200, Invitrogen) and Cy3 (1:200, Jackson Immunoresearch Laboratories) were applied. Stained samples were mounted in Vectashield medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, West Grove, PA) and analyzed under an epifluorescence microscope (Leica, Wetzlar, Germany). For 5hmC staining, fixed cells were incubated with 2N HCl for 20min before the blocking reaction. To compare 5hmC, H3K27m3, and TH levels in individual cells of VC-treated and –untreated cultures (or SVCT2+/+, +/−, and −/− VM tissues), all the immunofluorescent staining procedures within the comparing samples was done in parallel in the identical experimental conditions, and intensities of the fluorescence (MFI) in the immunoreactive cells were assessed by Image JR software with the formula: MFI = Integrated Density – (Area of selected cell×Mean fluorescence of background readings).
Enzyme activity assays
TET and JMJD3 activities were measured using EpigenaseTM5mC hydroxylase TET activity assay and JMJD3/UTX demethylase activity assay colorimetric kits (Epigentek), respectively. Briefly, cells from two 10 cm-dishes were harvested after 4 days of differentiation with or without VC treatment. Cytosolic and nuclear fractions were obtained using EpiQuik nuclear extraction kit (Epigentek). Triplicate samples were applied to assays and final results were calculated from three independent cell cultures.
Plasmids and viral infection
The doxycycline-inducible Nurr1 (mouse, Genebank: GI 7305324) expression vector (pCURT-Nurr1) was prepared as described before [28]. The retrovirus vectors expressing rat-specific shTet1 (Gene ID 309902) and shJmjd3 (Gene ID 363630) were purchased from Origene (OriGene Technologies, Rockville, MD, www.origene.com). For the production of the expression viruses, the vectors were introduced into the 293gpg packaging cell line by transient transfection with Lipofectamine (Invitrogen). Three days later, supernatants were harvested, concentrated with PEG8000 and stored at -70°C until use. For viral transduction, NSCs were incubated with the viral supernatant containing polybrene (4 µg/ml, Sigma) for 2 hours.
Semi-quantitative PCR and real-time quantitative PCR (qPCR) analyses
RNA was extracted, reverse-transcribed into cDNA, amplified and applied to PCR analyses as previously described [6]. Primers used are listed in Table S2.
Chromatin immunoprecipitation (ChIP)-qPCR and 5-hydroxymethylated DNA immunoprecipitation (5hmC DIP)-qPCR assays
Chromatins or genomic DNA were sheared into an average 200–400 bp in length by sonication and immunoprecipitated with antibodies listed in Table S1. Immunoprecipitated DNA fragments were collected by magnetic beads (Invitrogen), purified, and subjected to real-time qPCR using primers specific to rat Th, Dat, Vmat2, Aadc, Tph2 and Gad67 promoter loci (Table S2). The comparative cycle threshold method was used for quantification. Data were normalized to values of the input DNA.
Th Promoter assay
The rat Th promoters with different sizes (0.27–6 kb) were cloned into a pGL3 (Promega) luciferase plasmid. The vector was transformed from Dam-Dcm- strains to prevent DNA methylation. The plasmid was then methylated by M.SssI CpG methylase (Zymo Research) and the methylation efficiency was confirmed by Msp I digestion. Methylated or unmethylated plasmids were co-transfected into cells with a β-galactosidase expression vector as a reference using Lipofectamine 2000 (Invitrogen). Two days after transfection, cell lysates were collected and subjected to luciferase assay (BD Bioscience). Luciferase activity was measured and normalized to β-galactosidase activity.
Bisulfite sequencing (BS) and Tet-assistant bisulfate sequencing (TAB-seq)
BS and TAB-seq were performed as described [29]. For BS, genomic DNA (1 µg) was modified with sodium bisulfite using the EZ DNA Methylation-Gold kit (Zymo Research). For TAB-Seq, genomic DNA (1µg) was glucosylated by β-glucosyltransferase and oxidized by Tet1 using the 5hmC TAB-Seq kit (WiseGene) and then modified by sodium bisulfite using EZ DNA Methylation-Gold kit. Th promoter region (from -575 to +131) was amplified by PCR using the primers 5’-tatgttttggaggggattttatgatagata-3’ and 5’-accactccaaccctaaataccaaac-3’. The PCR products were purified and cloned using the pGEM-T Easy Vector system (Promega). Twelve clones for each gene were randomly chosen for sequencing.
Histological assessment on the VM of Svct2 KO embryos
Svct2+/− mice are maintained as a colony on a C57Bl/6J background by crossing Svct2+/− with Svct2+/+ (wild-type) mice. To obtain homozygous Svct2−/− mice for experimental purposes, timed matings were set up between Svct2+/− males and females. Careful records were made of vaginal plugs and dam weights to determine embryonic ages. Pregnant females were euthanized at embryonic day 14.5 and the pups were removed by cesarean section, placed on ice, and euthanized by decapitation. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Leg and tail tissue were also removed from each embryo, rinsed with 1× PBS and used for DNA extraction for genotyping by polymerase chain reaction (Sigma Extract-N-Amp kit). For immunohistochemistry studies, whole heads were removed and fixed by immersion in fresh 4% PFA, prepared with 1× phosphate buffered saline (PBS), for 1 hour, at room temperature, with gentle rocking. Samples were then rinsed with 1× PBS and stored in 20% sucrose at 4 °C. Midbrain sections (15 µm thick) were subjected to TH, 5hmC or H3K27m3 immunohistochemistry as described above. The total number of the immunoreactive cells in the VM was counted. The Abercrombie correction factor [N = n X T/(T + D)], where N is the actual number of cells, n is the number of nuclear profiles, T is the section thickness (15 µm), and D is the average diameter of nuclei, was used to compensate for double counting in adjacent sections.
RESULTS
VC treatment enhances the generation of midbrain-type DA neurons with presynaptic functions from cultured VM-NSCs
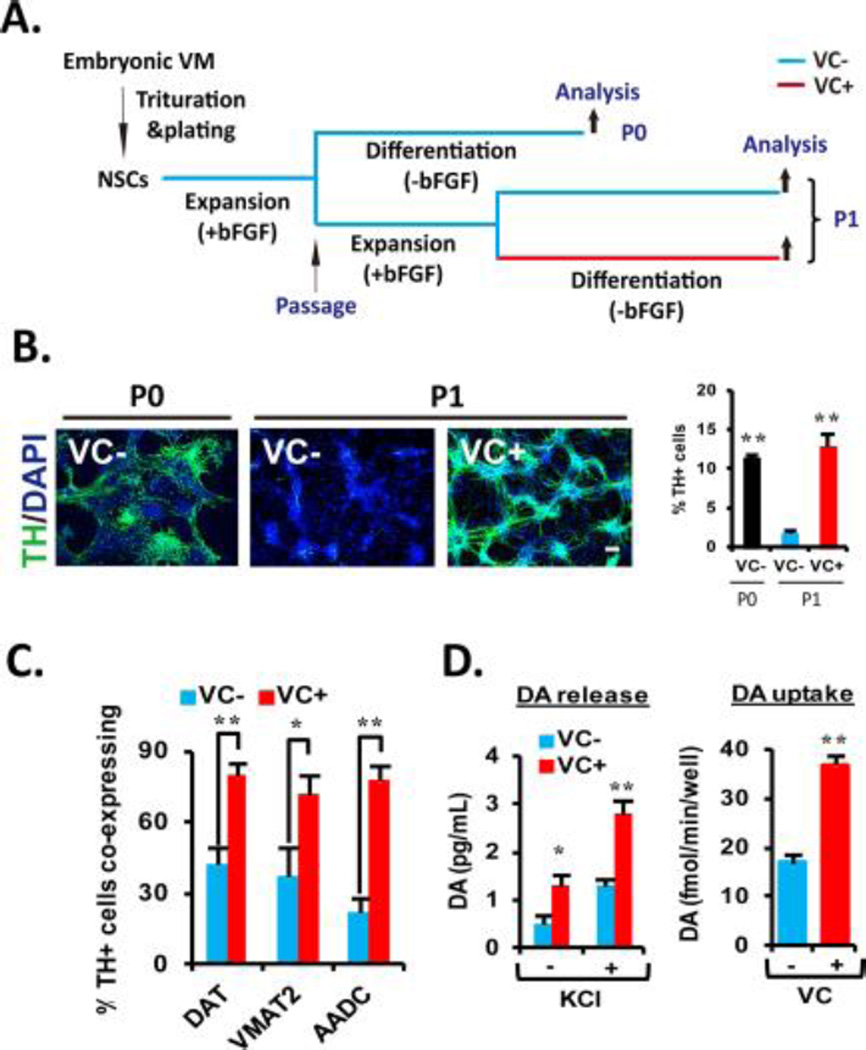
VM-NSCs are used as a useful bioassay system for the understanding of mDA neuron development [7, 27]. Furthermore, they can provide experimental and transplantable mDA neurons for the disorders associated with this neuronal subtype. VM-NSCs derived from early embryonic development (for example, rat embryonic day 12, E12) maintain their DA neurogenic potential and specific gene expression patterns after in vitro expansion for a short term (4–6 days) [29]. However, when an additional 4 days were introduced (including a cell passage; schematized in Fig. 1A), a great yield reduction of DA neurons occurred: percentage of cells expressing tyrosine hydroxylase (TH), the key enzyme for DA biosynthesis, were 11.42±0.52% in unpassaged (P0) cultures vs 1.78±0.45% in passaged (P1) cultures 5 days after differentiation (mean±SEM, n=3 independent cell cultures; Fig. 1B). Treatment with VC (100 µM) during differentiation of the P1 cultures greatly rescued TH+ cell yields to the level comparable with those of P0 cultures (12.91±1.64%; Fig. 1B). In addition to the loss of TH+ cell yield after passage, only small portion of TH+ cells (20–40%; Fig. 1C) were co-localized with other DA neuron-specific proteins including those involved in DA reuptake, homeostasis (DA transporter, DAT, and vesicular monoamine transporter 2, VMAT2) and DA biosynthesis (aromatic amino acid decarboxylase, AADC) in the differentiated P1 cultures. By contrast, the expressions of DAT, VMAT2, and AADC in the presence of VC were co-localized in majority of the TH+ neurons (>80%; Fig. 1C and Supporting information Fig. SA). The TH+ DA neurons differentiated in the presence of VC were the cells with authentic neuron shapes and the marker expressions specific for general neuron (TuJ1, Map2, HuC/D) and midbrain-type DA neuron (Nurr1, Foxa2, Pitx3) (Supporting information Fig. S1B). In the assays for pre-synaptic DA neuron functions, the differentiated P1 VM-NSC cultures treated with VC exhibited greater activities for DA neurotransmitter release and uptake (Fig. 1D). These findings, collectively, indicate that VC treatment is critical for the generation of midbrain-type DA neurons equipped with pre-synaptic neuronal functions for the purpose of research and transplantation in a large scale.
Figure 1. VC enhances the cultured VM-NSC differentiation toward midbrain-type DA neurons equipped with the phenotypical and functional maturities.
(A): Schematic of the experimental design. NSCs derived from rat embryonic VM tissues at E12 were expanded with bFGF for 6 days in vitro, and then followed by 6 days of differentiation in the absence of bFGF (unpassaged, P0). In other case, after P0 expansion, the cultures were passaged, and an additional cell expansion was induced for 4 days (passaged, P1). Differentiation of P1 cultures was induced in the identical conditions with the P0 differentiation. (B): VC treatment rescues cell passage-dependent loss of DA neurogenic potential in VM-NSC cultures. TH+ cell yields were determined in P0 and P1 cultures with or without VC (100 µM). Right: Graph depicting VC treatment effect on percentages of TH+ cells yielded during P1 differentiation. Values represent mean ± SEM; n = 3; *, p < .05 and **, p < .01, one-way ANOVA; this applies to all the experiments hereafter measuring percentage of respective cells. Scale bars are 20 µm in this study. Hereafter, all the following experiments were carried out in P1 cultures with or without VC treatment. (C): VC treatment enhances coexpression of the other DA phenotype genes DAT, VMAT2, and AADC in TH+ cells at D6. (D): Presynaptic DA neuron functions assessed by DA release (left) and uptake (right) assays. The assays were carried out at D16 in the cultures treated or untreated with VC (from D1 to D6). KCl was used to evoke depolarization; n = 4; *, p < .05 and **, p < .01. Abbreviations: bFGF, basic fibroblast growth factor; DA, dopamine; NSC, neural stem cell; TH, tyrosine hydroxylase; VC, vitamin C; VM, ventral midbrain.
VC enhances Nurr1-induced DA gene transcriptions by promoting Nurr1 protein recruitment to those gene promoters
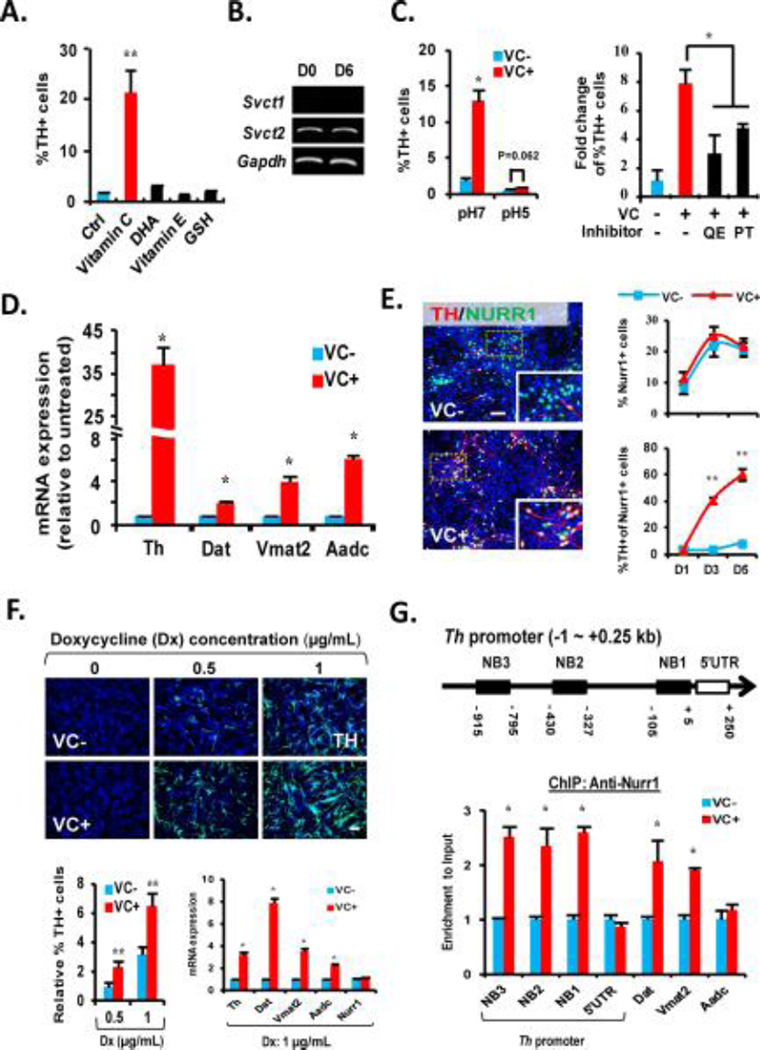
Based on anti-oxidant role of VC, the increase of DA neuron yield could be attained through VC-mediated cell survival. However, none of the antioxidants treated to the VM-NSC cultures, except VC, altered DA neuronal yield (Fig. 2A), excluding the possibility of anti-oxidant role of VC as the responsible mechanism. In developing brain, intracellular VC contents are much higher than in extracellular fluids by the action of the sodium-dependent VC transporter (SVCT), transporting VC into the intracellular space against the gradient in a sodium- and pH-dependent manner [30]. Svct2, but not Svct1, is expressed in VM-NSC cultures (Fig. 2B and [24]). Blocking SVCT2 action by lowering pH [31] or by specific SVCT2 inhibitors [32] abolished the VC effect on TH+ DA neuronal yield (Fig. 2C), indicating an intracellular action of VC on mDA neuron differentiation.
Figure 2. VC activates Nurr1-mediated dopamine (DA) gene transcriptions.
(A): TH+ cell yields at D6 in the ventral midbrain-neural stem cell (VM-NSC) cultures treated with the antioxidants indicated. (B): Semiquantitative PCR analysis exhibiting expression of Svct2, but not Svct1, in undifferentiated (D0) and differentiated (D6) VM-NSC cultures. (C): VC effect on TH+ DA neuronal yields was abolished by blocking SVCT2 activity with lowering pH (left) and with treatment of the SVCT2 inhibitors QE and PT. The inhibitors and vehicle (DMSO, control) were treated for 3 hours prior to VC treatment. (D): VC effect on the transcription of the DA phenotype genes Th, Vmat2, Dat, Aadc estimated by quantitative real-time PCR (qRT-PCR) analyses. n = 3 replicates; *, p < .05 throughout the panels for qRT-PCR analyses. (E): VC enhances TH expression in the Nurr1+ DA neuron progenitors and neurons (%TH+ of Nurr1+ cells, bottom right), without altering Nurr1+ cell number (%Nurr1+ cells of total DAPI+ cells, top right) in the differentiating VM-NSCs (D1–D5). Left: Representative images for TH+/Nurr1+ cells at D5. Insets, high-powered views of the boxed areas. (F): VC effect on TH+ cell yields (top and bottom left) and DA phenotype gene expressions (bottom right) induced by exogenous Nurr1 expression. NSCs derived from nondopaminergic embryonic cortex of rat at E14 were transduced with a doxycycline-inducible Nurr1 expression vector (pCURT-Nurr1), and the Nurr1 expression was induced by doxycycline (Dx) supplementation (0, 0.5, or 1 µg/ml). No TH+ cells were induced without Dx treatment. (G): Top, Schematic for the consensus Nurr1 binding sites (NB1, 2, 3) within −1 kb promoter region of rat Th gene. Bottom, Nurr1 protein enrichments on the consensus Nurr1 binding regions of Th and the other DA phenotype genes (right) assessed by ChIP-qPCR analyses. Abbreviations: DHA, docosahexaenoic acid; PT, phloretin; QE, quercetin; TH, tyrosine hydroxylase; VC, vitamin C. *, p < .05 and **, p < .01, one-way ANOVA. (G) Top, Schematic for the consensus Nurr1 binding sites (NB1,2,3) within -1 kb promoter region of rat Th gene. Bottom, Nurr1 protein enrichments on the consensus Nurr1 binding regions of Th and the other DA phenotype genes (right) assessed by ChIP-qPCR analyses.
Notably, the VC-mediated effect on DA neuron yield was accompanied by a remarkable increase of mRNA expression for the DA phenotype genes (Fig. 2D), indicating an action of VC at the gene transcriptional level. Nurr1, specifically expressed from the stage of mDA neuron progenitors to mature mDA neurons, is the most critical transcription factor to induce DA phenotype gene transcription during development [2–4]. Numbers of Nurr1+ progenitors in VC-treated cultures were indistinguishable from those of the untreated controls (Fig. 2E, top right). However, the proportion of Nurr1+ that were also positive for TH 5 days after differentiation was greatly increased by VC treatment (Fig. 2E, bottom right), suggesting that VC enhances Nurr1 action to induce DA gene transcription. To confirm the VC effect on the Nurr1-induced DA gene expression, we performed a gain-of-function study for Nurr1. NSCs derived from non-dopaminergic embryonic cortex, were transduced with a doxycycline-inducible Nurr1 expression vector, and Nurr1 expression was induced by doxycycline supplementation (data not shown). VC treatment markedly enhanced TH+ cell yield and DA neuron-specific gene expression induced by the forced expression of Nurr1, regardless of Nurr1 (doxycycline) levels (Fig. 2F).
Nurr1-mediated DA gene transcriptions during development are controlled by various co-activators and co-repressors, which epigenetically regulate Nurr1 protein binding to those gene promoters [5–7]. There are three consensus Nurr1 binding sites have been revealed within -1kb of rat Th promoter (Fig. 2G top and [33]). In chromatin immunoprecipitation (ChIP)-PCR analysis, VC treatment greatly promoted Nurr1 protein recruitment to the consensus Nurr1 binding sites of Th promoter (Fig. 2G, bottom). Increased Nurr1 enrichment was also manifest in the promoter regions for Dat and Vmat2, but not in Aadc (Fig. 2G, bottom). It has been reported that Aadc is regulated in its expression differently from the other DA phenotype genes and is less reliant on Nurr1 action [34], implying that the other mechanisms, but not that associated with Nurr1 recruitment control, are involved in VC-induced increase of Aadc expression.
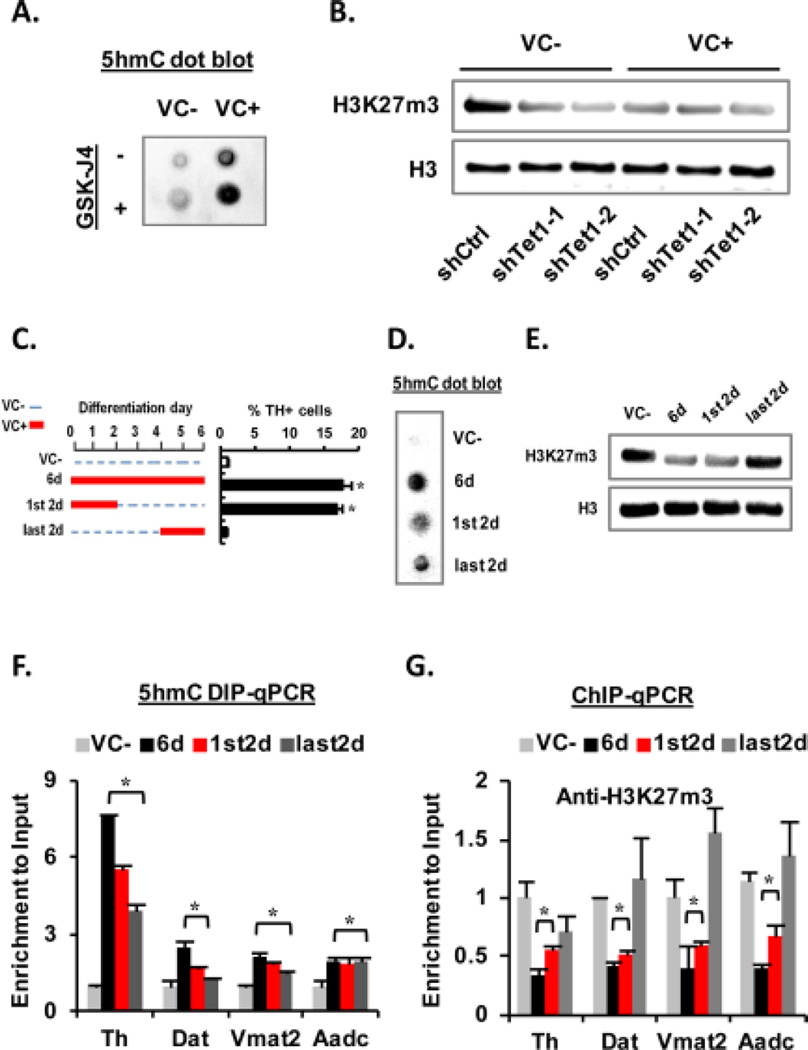
VC facilitates Tet1-mediated DNA hydroxymethylation in the DA neuron-specific genes
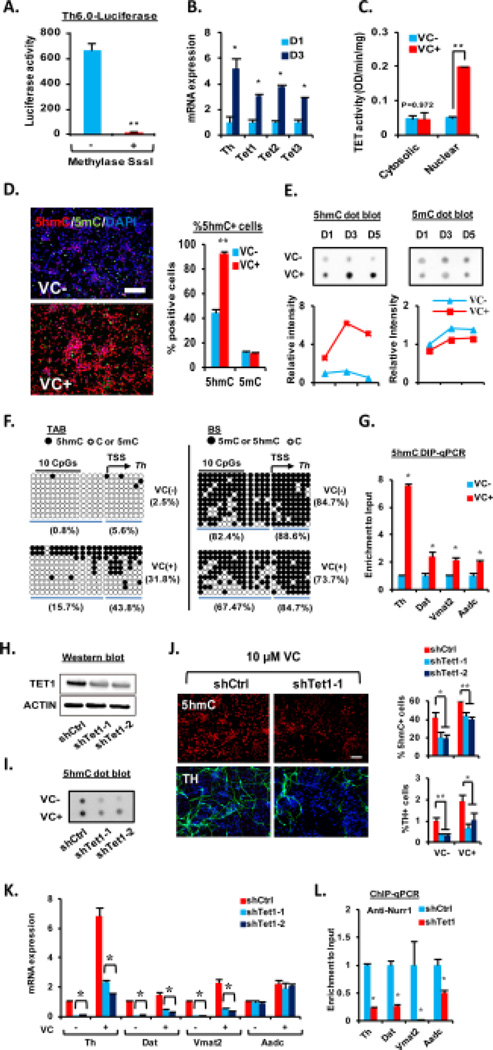
DNA methylation/demethylation is a common mode of epigenetic control of tissue-specific gene expression during development [35]. Luciferase activity driven by the Th promoters (from -6 kb to transcription start site (TSS)) in the VM-NSCs was almost completely abolished after DNA methylation reaction (Fig. 3A), suggesting DNA methylation is a crucial mechanism for silencing the DA gene expression. Based on VC as an activator for Fe(II)-2-oxoglutarate-dependent dioxygenase, we have questioned whether Tet-mediated DNA hydoxymethylation/demethylation is associated with the VC-induced activation of the DA gene transcriptions shown in Fig. 2D. All Tet1, 2, 3 mRNAs were expressed in cultured VM-NSCs and their expressions were increased during differentiation (Fig. 3B). VC treatment greatly enhanced Tet enzyme activity in the nuclear fractions of the cultured VM-NSCs (Fig. 3C). Consequently, global 5hmC levels (total levels in genomic DNAs) estimated by immunocytochemical (Fig. 3D) and immunoblotting (Fig. 3E) analyses were greatly increased by VC treatment, along with a subtle 5mC level decrease. Consistent with the previous report exhibiting 5hmC/5mC dynamics in proximal regions to TSS [19], luciferase assays on the Th promoters with sequential deletion of the distal fragments of the promoter indicated that the region as proximal as -0.27 kb to TSS is the target region, the promoter activity of which is sensitively silenced by DNA methylation (Supporting information Fig. S2). Based on this finding, we assessed 5hmC/5mC levels in a region of Th gene close to TSS by TAB-seq and BS. These analyses showed that 5hmC in the Th promoter region was more abundant in the VC-treated cultures, while 5mC (+5hmC) was slightly decreased (Fig. 3F). Increased 5hmC levels in the promoters of Th and the other DA genes (Dat, Vmat2, Aadc) in VC-treated VM-NSCs were further confirmed by 5hmC DIP-qPCR (Fig. 3G). TH+ DA neuronal yields increased in a VC concentration-dependent manner. The similar VC dose-dependent pattern was shown in the percentage of 5hmC+ cells (Supporting information Fig. S3A). VC treatment during the initial 2 days of differentiation was sufficient to enhance TH+ DA neuron yields to similar levels as the cultures treated with VC for whole 6 days of differentiation, along with an increase of %5hmC+ cells. By contrast VC treatment during the last 2 days (differentiation day 4–6) did not promote DA neuron yields (Supporting information Fig. S3A), although it also significantly increased %5hmC+ cells, indicating that only the 5-hydoxymethylation increase in the undifferentiated or differentiating progenitor stages is responsible for the DA neuron yield increase. Similar to the TH+ cell yield changes shown in Fig. 2A, only VC, but the other anti-oxidants, enhanced %5hmC+ cells (Supporting information Fig. S3C). In addition, inhibition of SVCT action completely abolished VC-induced increase of 5hmC+ cells (Supporting information Fig. S3D). These findings, collectively, indicate a close association between mDA neuron differentiation and DNA hydroxymethylation/demethylation mediated by VC. Tet1 protein expression was efficiently down-regulated by the shTet1 treatments (Fig. 3H). Along with a reduction of 5hmC protein and % 5hmC+ cells (Fig. 3I and 3J, top), the knocking-down of Tet1 markedly abolished VC (10 µM)-mediated increase of TH+ DA neuron yields (Fig. 3J, bottom) and DA gene mRNA expressions (except Aadc, Fig. 3K). Furthermore, shTet1 treatment in VC-treated cultures led to a robust reduction of Nurr1 protein enrichment in the DA gene promoters (Fig. 3L). In summary, these findings suggest that VC treatment promotes mDA neuron differentiation from cultured VM-NSCs in an epigenetic mechanism, in which VC increases accessibility of Nurr1 and/or other transcription factors to the gene promoters via Tet1-mediated DNA hydroxymethylation/demethylation.
Figure 3. Tet1-mediated DNA hydroxymethylation is involved in midbrain dopamine (DA) neuron differentiation activated by VC.
(A): Th-luciferase assay with the reporter plasmids containing rat Th promoter (6 kb) nonmethylated and methylated by in vitro methylation reaction using the CpG methyltransferase (M.Sssl). The ventral midbrain-neural stem cells (VM-NSCs) were transfected with the vectors at differentiation day 0 (D0) and the luciferase activity was determined at D2. n = 3; **, p < .01. (B): Changes of Tet1, 2, and 3 expressions during in vitro differentiation of VM-NSCs determined by qRT-PCR analysis. (C): Tet enzyme activity assays exhibiting Tet activity increase in nuclear fractions of VM-NSCs after 4 days of VC treatment. (D, E): Immunocytochemical (D) and dot blot (E) analyses showing VC effects on 5hmC and 5mC levels during differentiation of VM-NSCs. Left in (D) are representative images for 5hmC- and 5mC-immnoreactive cells at D3 in the VC-untreated and treated cultures. The global 5hmC levels were determined by percent immunoreactive cells in the cultures immunofluorescently stained against the 5hmC antibody. Graphs in (E) depict the intensities of the dots quantified using ImageJ software. (F): Tet-assisted bisulfite sequencing (TAB-seq) and bisulfite sequencing (BS) for DNA hydroxymethylation/methylation status in the Th gene regions close to the TSS (−14 to +10 CpGs). (G): 5hmC enrichments in the promoter regions of the DA phenotype genes were also assessed by 5hmC DIP-qPCR analyses at D5. (H–J): Knocking-down of Tet1 using the shRNA (shTet1-1 or -2) abolished VC-induced increase of TH+ DA neuron yields along with the 5hmC level reductions in the differentiating VM-NSC cultures. The Tet1 knockdown experiments were done in the absence or presence of 10 µM of VC. The efficiency of shTet1 was confirmed by Western blot against TET1 antibody (H); beta-actin was used as a reference. The global 5hmC level changes by shTet1-1 and -2 were further confirmed by dot-blot analysis (I). (K): qRT-PCR analysis exhibiting the shTet1 treatment effects on DA phenotype genes transcript levels. (L): Knockdown of Tet-1 decreases Nurr1 protein recruitment to the DA phenotype genes in ChIP-qPCR analyses. Abbreviations: TSS, transcription start site; VC, vitamin C. *, p < .05 and **, p < .01, one-way ANOVA.
Involvement of Jmjd3-mediated H3K27m3 demethylation in VC-induced DA neuron differentiation
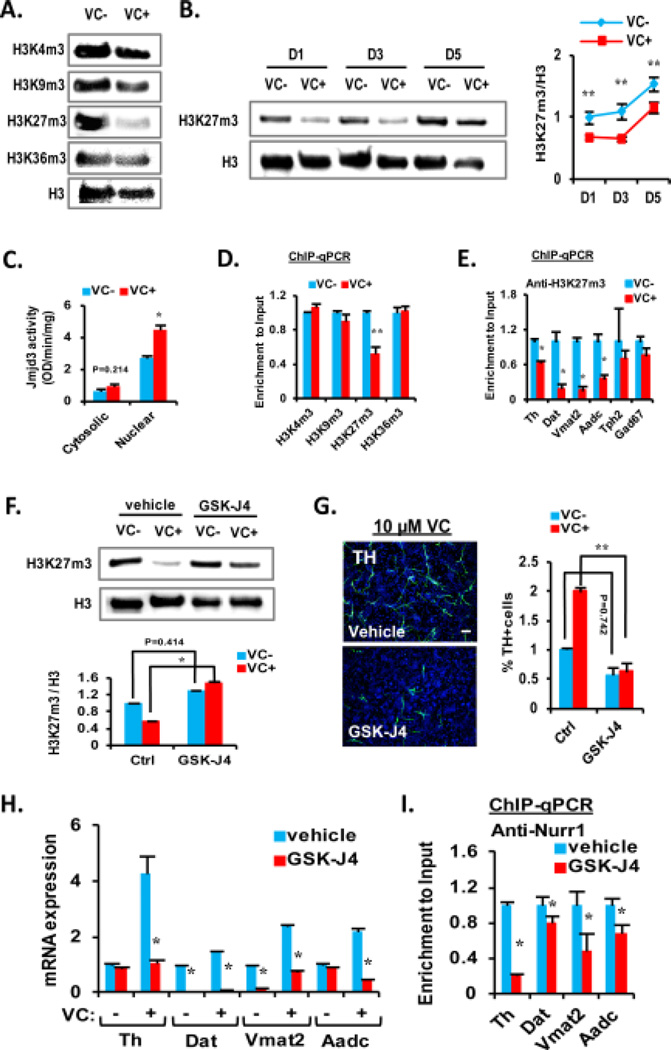
Histone demethylases are another group of Fe(II)-2-oxoglutarate-dependent dioxygenases, the activities of which are purported to be regulated by VC. VC-induced stem cell differentiation and reprogramming have been shown to be mediated via activation of the histone demethylase activities [6, 36, 37]. Among the histone lysine methylations tested, only H3K27m3, which has been reported as an important histone code regulating mDA neuron differentiation [6], was remarkably reduced in the VM-NSC cultures by VC treatment (Fig. 4A). The global H3k27m3 levels gradually increased during 5 days of differentiation. VC-mediated reduction of the histone mark was shown during the differentiation period (Fig. 4B), along with increased nuclear activity of Jmjd3, the enzyme catalyzing demethylation of H3K27m3 (Fig. 4C). ChIP-qPCR analyses showed that H3K27m3, but not the other methylated histone lysine marks, was significantly less abundant in the promoter regions of Th (Fig. 4D) and the other DA phenotype genes Dat, Vmat2, and Aadc in VC-treated cultures (Fig. 4E). No alteration of H3K27m3 enrichment was seen in the genes specific for serotonergic (Tph2) or GABAergic (GAD67) neurons by VC treatment (Fig. 4E). Along with increase in H3K27m3 levels (Fig. 4F), treatment of GSK-J4, a specific inhibitor for Jmjd3 [38], markedly abolished VC (10 µM)-induced increases in TH+ DA neuronal yields (Fig. 4G) and mRNA expressions of the DA genes (Fig. 4H). In addition, Nurr1 recruitments to the DA phenotype gene promoters were reduced by the Jmjd3 inhibitor treatment in VC-treated VM-NSC cultures (Fig. 4I), collectively suggesting that Jmjd3-dependent demethylation of H3K27m3 is another epigenetic mechanism for VC-induced mDA neuron differentiation.
Figure 4. VC reduces the repressive histone mark H3K27m3 on chromatins of dopamine (DA) phenotype genes through Jmjd3.
(A): Specific reduction of H3K27m3 but not other modifications by VC is shown as a representative Western blot. Histone 3 (H3) expressions were used as the loading control and the reference for quantification of the histone modifications in the blot analyses. (B): Protein expression pattern of H3K27m3 along differentiation. A representative Western blot is shown on the left. Quantification of intensity is shown on the right. (C): Colorimetric measurement showing nuclear-specific increase of Jmjd3 enzyme activity by VC with cytosolic or nuclear extracts from VC treated or non-treated samples. (D): ChIP-qPCR analysis of various histone modifications on Th promoter region from samples treated with or without VC. (E): Enrichments of H3K27m3 on promoters of DA phenotype genes are specifically decreased in the DA neuron-specific gene promoters, along with no significant change of those on promoters specific for serotonergic (Tph2) and GABAergic (Gad67) neurons. Data are presented relative to no VC treatment. (F, G): Pretreatment of the H3K27m3 inhibitor GSK-J4 abolishes VC-induced demethylation of H3K27m3 (F) and TH+ cells increase (G). VC (10 µM) was present in vehicle and GSK-J4 treated cultures. (H): qRT-PCR analysis for expression of DA phenotype genes altered by GSK-J4 pretreatment. (I): Decreased enrichments of Nurr1 on promoters of DA phenotype genes by GSK-J4 treatment. Abbreviations: TH, tyrosine hydroxylase; VC, vitamin C. *, p < .05 and **, p < .01, one-way ANOVA.
The 5hmC and H3K27m3 changes, independently induced by VC, are required during early stage of DA neuron differentiation
In addition to the link between 5hmC and histone marks in ES cells [13], a recent study specifically reported that gain of 5hmC co-occurs with loss of H3K27m3 in neuronal genes during neuronal differentiation [19]. Consistently, our study shows the same epigenetic mark changes in DA neuron-specific genes during the midbrain DA neuron differentiation induced by VC. These findings suggest that both the 5hmC and H3K27m3 changes may be linked in a positive regulatory loop, in which one activates the other epigenetic change. However, in contrast to our expectation, levels of 5hmC in VM-NSC cultures were rather increased by inhibition of Jmjd3 demethylases (Fig. 5A). In addition, knocking-down Tet1 reduced the H3K27m3 levels (Fig. 5B). Consistent with our finding, a recent report has shown a negative crosstalk between 5mC and H3K27m3 where loss of 5mC caused by Dnmt3a-knock out resulted in accumulation of H3K27m3 [12]. Thus we can conclude that VC induces 5hmC increase and H3K27m3 decrease independently by activating Tet1 and Jmjd3 activities, and the enzyme activation roles of VC were potent enough to induce the mark changes by overcoming the naively existing negative regulatory mode.
Figure 5. Both the 5hmC increase and H3K27m3 decrease, independently induced by VC, are required during early stage of dopamine (DA) neuron differentiation.
(A, B): A negative regulatory interaction between 5hmC and H3K27m3 is shown in respective inhibition analyses using GSK-J4 and shTet1. (C–G): Changes of 5hmC and H3K27m3 levels in differentiating ventral midbrain-neural stem cells by the VC treatments with different time periods. (C): Schematic for the VC treatment schedules along with their effects on TH+ cell yields. The epigenetic code changes in total genomic DNA (D, E) and in the DA neuron-specific genes (F, G) are shown. Abbreviation: VC, vitamin C. *, p < .05, one-way ANOVA.
Erasing either of the 5hmC and H3K27m3 changes robustly or completely abolished the VC effect on DA neuron yields (Fig. 3H–J, 4G), suggesting both of the epigenetic changes are required for DA phenotype expression during the midbrain development. When examined 6 days after differentiation of the VM-NSCs, VC treatment during the last 2 days caused increases of the 5hmC levels in total genomic DNA (Fig. 5D) and in the DA neuron-specific genes (Fig. 5F), but failed to induce DA neuron yield increase (Fig. 5C,right and Supporting information Fig. S3B). Interestingly, the H3K27m3 levels, however, were not altered by the last 2 days of VC treatment (Fig. 5E and 5G), further supporting the hypothesis that both the epigenetic changes are required for the DA neuron yield increase. By contrast, VC treatment during the first 2 days of differentiation resulted in a sufficient increase of DA neuron yields (Fig. 5C, right and Supporting information Fig. S3B) along with both the 5hmC and H3K27m3 level changes (Fig. 5E and 5G), although the epigenetic mark changes induced by VC slowly subside long after differentiation in the absence of VC (data not shown). These findings collectively indicate a developmental stage-dependent difference of VC effects and that the epigenetic code changes in early differentiation stage is critical.
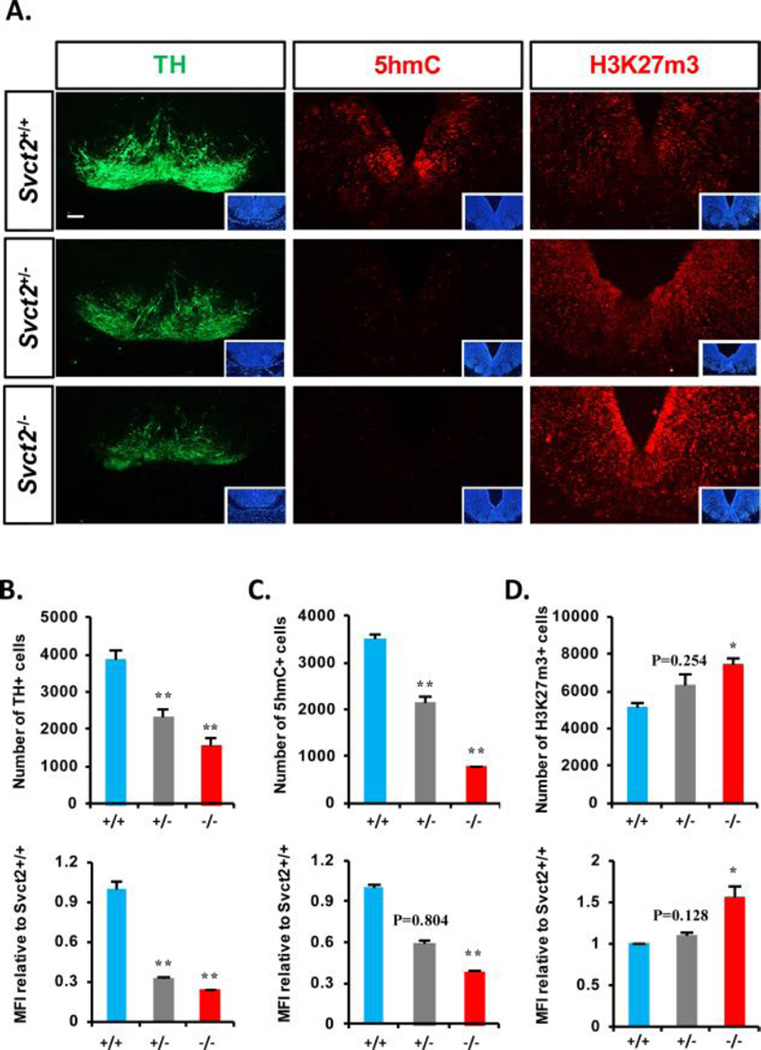
Reduced genesis of DA neurons in the midbrains of Svct2 KO mouse embryos
Based on our in vitro results, we assessed in vivo DA neuron formation in the developing VMs of the wild-type (WT, +/+), Svct2 KO heterozygote (+/−), and homozygote (−/−) mouse embryos. The midbrain sizes and anatomical structures were indistinguishable among the embryos. Histologic counting throughout the antero-posterior VM sections of the embryos at E14.5 showed a marked decrease of TH+ DA neuron formation in the KO embryos in a Svct2 dose-dependent manner (Fig. 6A, left and 6B). Notably, the percentage of 5hmC+ cells and 5hmC levels in individual cells were greatly reduced in the VM of the KO embryos (Fig. 6A, middle and 6C), indicating again that the physiologic 5hmC levels in undifferentiated NSCs/progenitors is critical for DA neuron generation in the developing midbrain. It was also observed that the percentage of 3HH3H3K27m3+ cells and staining intensity were significantly greater in the Svct2 KO VM than those in the WT embryos (Fig. 6A,right and 6D). These findings collectively provide a physiologic implication of the role of intracellular VC in DA neuron generation during the midbrain development in an epigenetic manner via the 5hmC/H3K27m3 marker changes.
Figure 6. Defect of midbrain dopamine neuron genesis in the midbrain of Svct2 KO embryos.
(A): Representative images of histological immunofluorescent staining of TH (left), 5hmC (middle), and H3K27m3 (right) in E14.5 ventral midbrain (VM) sections of Svct2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) embryos. Insets are DAPI staining of the section. (B–D): Measurements of TH+ (B), 5hmC+ (C), and H3K27m3+ (D) cell numbers and their individual intensities (MFI) in VM sections are shown from three embryos for each group (derived from two pregnant mothers, three SVCT2+/+, three +/−, two −/− embryos from one litter, and one SVCT2 −/− from the other litter). Statistics are all analyzed in comparison to Svct2+/+. *, p < .05; **, p < .01, n = 3 biological replicates. Abbreviations: MFI, mean fluorescence intensity; TH, tyrosine hydroxylase.
DISCUSSION
Given the fact that VC concentration is maintained at such high levels in the brain, and is conserved at the expense of other organs, and that these levels are greater still in the developing brain [8, 9], it has long been suggested that VC plays important roles in brain development. Specifically we and other groups have shown that DA neuron differentiation in vitro is greatly increased by VC supplementation in NSC cultures derived from embryonic midbrain [24, 25], suggesting VC action in midbrain DA neuron development. However, the mechanisms by which VC acts on the differentiation process remain to be clarified. In the meantime, DNA hydoxymethylation of methylated CpG sites (5hmC), catalyzed by Tet enzymes, recently opened up as a new horizon and as an important epigenetic signature to control biological activities in developing and adult tissues, including those of the CNS [39]. VC acts chemically as a cofactor for Fe(II)-2-oxoglutarate-dependent dioxygenases including those involved in epigenetic regulation, such as Tets and JmJd histone demethylases. Based on this proposed role for VC, recent several studies have shown the cellular functions of VC in ES cells and somatic cell reprogramming via promotion of Tet and Jhdm1a/1b enzyme activities, respectively [36, 40, 41]. Epigenetic gene expression control is suggested as an important mechanism for DA neuron development in the developing midbrain. Specifically, we have reported epigenetic mechanisms induced by extracellular high K+ concentration (membrane depolarization) [6] as well as intracellular transcription factors [7] in mDA neuron development. These findings collectively prompted us to test the extent of the epigenetic regulatory role of VC in midbrain DA neuron development.
In this study we show that Tet1, 2, and 3 are expressed in NSCs derived from embryonic midbrain. Addition of VC to the VM-NSC cultures enhanced Tet enzyme activity in a biochemical assay, and led to a robust increase of global and local 5hmC in the DA phenotype gene promoter regions. In ES cells, a 5hmC increase was followed by a substantial decrease of 5mC [40], indicating 5hmC as an intermediate to be eliminated ultimately by the base excision repair pathway (active demethylation). By contrast, only subtle decreases of 5mC was shown in the VC-treated VM-NSCs in our study, which is consistent with the previous report of 5hmC/5mC pattern in embryonic cortex [19] and postnatal neurodevelopment [43]. These data suggest that 5hmC may slowly be converted to cytosine during the maturation of the neurons or that 5hmC is a rather stable epigenetic marker [44–47]. As a stable marker, 5hmC is thought to activate gene transcription by repressing promoter methylation activity to inhibit accessibility of transcriptional activators. In addition, hydroxylation of 5mC may interfere with protein complexes that bind to 5mC, such as methyl-CpG binding proteins [48], or to CpG-rich DNA regions in general, such as the Polycomb complex, or may establish novel protein complexes at 5hmC sites, all of which ultimately lead to histone code alterations associated with open chromatin structures. We show that DNA hydroxymethylation promoted by VC is accompanied with demethylation of the inhibitory histone code H3K27m3, and a positive crosstalk between VC-induced 5hmC and H3k27m3 changes was expected. However, our Tet1 Knock-down/Jmjd3 activity inhibition experiments in the absence of VC revealed an interaction in the opposite direction. One plausible interpretation is a compensatory increase or decrease to maintain gene silencing associated with cell differentiation in NSCs until critical and potent differentiation-inducing stimuli, such as VC, is present at the correct time point. Regardless of the crosstalk, our loss-of-function studies in this study show that both the 5hmC and H3K27m3 changes are necessary for midbrain DA neuron differentiation induced by VC.
None of the early midbrain patterning and developmental gene expressions (Otx2, En1, Shh, Fgf8, Wnt1, Foxa2, Lmx1a, Msx1) was affected by VC treatment (data not shown). The yields of the other neuronal subtypes such as serotonergic and GABAergic neurons, known to be generated at the expense of DA neurons in the developing midbrain [49, 50], did not alter in the VC-treated VM-NSC cultures (data not shown), suggesting that VC-promoted Tet1/Jmjd3 activity is not associated with the transcriptional activation of the early developmental genes including those specific for cell fate determination. Instead, VC activated DA phenotype gene expression in late progenitor cells via Tet1/Jmjd3-mediated epigenetic control. Consistently, Tet roles in later progenitors, but not early NSC stages, were shown during in neurogenesis of the developing cortex [19] and adult hippocampus [16].
We finally showed that intracellular deficiency of VC in the VMs of Svct2-KO mouse embryos resulted in a reduced genesis of DA neurons in the midbrain along with loss of 5hmC and gain of H3K27m3 levels. Further in vivo studies analyzing the KO embryos at different time points are required to determine how the VC-deficiency and its related epigenetic code changes affect mDA neuron development. In addition, it would be interesting to know consequences of reduced mDA neuron genesis by the embryonic midbrain VC deficiency, especially to know if prenatal deficiency of VC could be a predisposing factor for PD and other mDA neuron-related disorders. In conclusion, we report for the first time the epigenetic mechanism of a role of VC in midbrain DA neuron development. With its significant physiologic implications, our study indicates a necessity of further studies on VC roles in tissue development and epigenetics.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Center (2008-0062190) and the Bio & Medical Technology Development Program (2010-0020232), funded by the National Research Foundation of Korea (NRF) of the Ministry of Education, Science and Technology (MEST), Republic of Korea, the research fund of Hanyang university (HY-2014) and NIH grant AG038739.
Footnotes
Author contributions:
Xi-Biao He: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Mirang Kim: Collection and/or assembly of data, data analysis and interpretation.
Seon-Young Kim: Collection and/or assembly of data, data analysis and interpretation.
Sang-Hoon Yi: Collection and/or assembly of data.
Yong-Hee Rhee: Collection and/or assembly of data.
Taeho Kim: Collection and/or assembly of data.
Eun-Hye Lee: Collection and/or assembly of data.
Chang-Hwan Park: Provision of study material, data analysis and interpretation.
Shilpy Dixit: Collection and/or assembly of data.
Fiona E. Harrison: Financial support, provision of study material, manuscript writing, final approval of manuscript.
Sang-Hun Lee: Conception and design, financial support, administrative support, provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Ang S-L. Transcriptional control of midbrain dopaminergic neuron development. [Accessed July 19, 2011];Development. 2006 133(18):3499–3506. doi: 10.1242/dev.02501. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16899537. [DOI] [PubMed] [Google Scholar]
- 2.Zetterström RH. Dopamine Neuron Agenesis in Nurr1-Deficient Mice. [Accessed March 20, 2013];Science. 1997 276(5310):248–250. doi: 10.1126/science.276.5310.248. (80-.). Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 3.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. U. S. A. 1998;95(7):4013–4018. doi: 10.1073/pnas.95.7.4013. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=19954&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le W, Conneely OM, Zou L, et al. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp. Neurol. 1999;159:451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs FMJ, Linden AJ a van der, Wang Y, et al. Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons. [Accessed July 19, 2011];Development. 2009 136(14):2363–2373. doi: 10.1242/dev.037556. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19515692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X-B, Yi S-H, Rhee Y-H, et al. Prolonged membrane depolarization enhances midbrain dopamine neuron differentiation via epigenetic histone modifications. [Accessed August 17, 2013];Stem cells. 2011 29(11):1861–1873. doi: 10.1002/stem.739. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21922608. [DOI] [PubMed] [Google Scholar]
- 7.Yi S-H, He X-B, Rhee Y-H, et al. Foxa2 acts as a co-activator potentiating expression of the Nurr1-induced DA phenotype via epigenetic regulation. [Accessed April 5, 2014];Development. 2014 141(4):761–772. doi: 10.1242/dev.095802. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24496614. [DOI] [PubMed] [Google Scholar]
- 8.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. [Accessed June 1, 2013];Free radic. Biol. Med. 2009 46(6):719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. Available at: http://dx.doi.org/10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kratzing CC, Kelly JD, Kratzing JE. Ascorbic acid in fetal rat brain. [Accessed June 16, 2014];J. Neurochem. 1985 44(5):1623–1624. doi: 10.1111/j.1471-4159.1985.tb08804.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3989554. [DOI] [PubMed] [Google Scholar]
- 10.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat. Chem. Biol. 2008;4(3):152–156. doi: 10.1038/nchembio0308-152. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18277970. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Wu H, Diep D, et al. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. [Accessed August 20, 2013];Cell. 2013 153(3):692–706. doi: 10.1016/j.cell.2013.04.002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23602152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Coskun V, Tao J, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. [Accessed February 28, 2013];Science. 2010 329(5990):444–448. doi: 10.1126/science.1190485. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3539760&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastor Wa, Pape UJ, Huang Y, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. [Accessed November 1, 2012];Nature. 2011 473(7347):394–397. doi: 10.1038/nature10102. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3124347&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cimmino L, Abdel-Wahab O, Levine RL, et al. TET family proteins and their role in stem cell differentiation and transformation. [Accessed March 1, 2012];Cell stem cell. 2011 9(3):193–204. doi: 10.1016/j.stem.2011.08.007. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3244690&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. [Accessed March 1, 2012];Nat. Rev. Genet. 2012 13(1):7–13. doi: 10.1038/nrg3080. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22083101. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R-R, Cui Q-Y, Murai K, et al. Tet1 Regulates Adult Hippocampal Neurogenesis and Cognition. [Accessed June 14, 2013];Cell stem cell. 2013:1–9. doi: 10.1016/j.stem.2013.05.006. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1934590913001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudenko A, Dawlaty M, Seo J, et al. Tet1 Is Critical for Neuronal Activity-Regulated Gene Expression and Memory Extinction. [Accessed October 29, 2013];Neuron. 2013 79(6):1109–1122. doi: 10.1016/j.neuron.2013.08.003. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0896627313007149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaas GA, Zhong C, Eason DE, et al. TET1 Controls CNS 5-Methylcytosine Hydroxylation, Active DNA Demethylation, Gene Transcription, and Memory Formation. [Accessed September 19, 2013];Neuron. 2013 79(6):1086–1093. doi: 10.1016/j.neuron.2013.08.032. Available at: http://www.sciencedirect.com/science/article/pii/S0896627313007915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn Ma, Qiu R, Wu X, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. [Accessed June 17, 2013];Cell Rep. 2013 3(2):291–300. doi: 10.1016/j.celrep.2013.01.011. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3582786&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. [Accessed July 17, 2011];Cell stem cell. 2010 6(1):71–79. doi: 10.1016/j.stem.2009.12.001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20036631. [DOI] [PubMed] [Google Scholar]
- 21.Sen GL, Webster DE, Barragan DI, et al. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. [Accessed November 22, 2013];Genes Dev. 2008 22(14):1865–1870. doi: 10.1101/gad.1673508. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2492733&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepsen K, Solum D, Zhou T, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. [Accessed August 16, 2011];Nature. 2007 450(7168):415–419. doi: 10.1038/nature06270. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17928865. [DOI] [PubMed] [Google Scholar]
- 23.Lee J-Y, Chang M-Y, Park C-H, et al. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J. Neurosci. Res. 2003;73(2):156–165. doi: 10.1002/jnr.10647. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12836158. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Studer L, McKay RD. Ascorbic acid increases the yield of dopaminergic neurons derived from basic fibroblast growth factor expanded mesencephalic precursors. J. Neurochem. 2001;76(1):307–311. doi: 10.1046/j.1471-4159.2001.00073.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11146004. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Lumelsky N, Studer L, et al. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 2000;18(6):675–679. doi: 10.1038/76536. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16766027. [DOI] [PubMed] [Google Scholar]
- 26.Rhee Y, Ko J, Chang M, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. [Accessed August 22, 2011];J. Clin. Invest. 2011 121(6):2326–2335. doi: 10.1172/JCI45794. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3104759&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. [Accessed July 23, 2014];Dev. Cell. 2010 18(4):533–543. doi: 10.1016/j.devcel.2010.02.013. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3325599&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park C-H, Lim M-S, Rhee Y-H, et al. In vitro generation of mature dopamine neurons by decreasing and delaying the expression of exogenous Nurr1. [Accessed February 3, 2014];Development. 2012 139(13):2447–2451. doi: 10.1242/dev.075978. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22627286. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Park Y-K, Kang T-W, et al. Dynamic changes in DNA methylation and hydroxymethylation when hES cells undergo differentiation toward a neuronal lineage. [Accessed November 12, 2013];Hum. Mol. Genet. 2013 428798119:1–11. doi: 10.1093/hmg/ddt453. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24087792. [DOI] [PubMed] [Google Scholar]
- 30.Tsukaguchi H, Tokui T, Mackenzie B, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399(6731):70–75. doi: 10.1038/19986. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10331392. [DOI] [PubMed] [Google Scholar]
- 31.Liang W, Johnson D, Jarvis S. Vitamin C transport systems of mammalian cells. [Accessed August 29, 2013];Mol. Membr. 2001 doi: 10.1080/09687680110033774. Available at: http://informahealthcare.com/doi/abs/10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 32.Gess B, Lohmann C, Halfter H, et al. Sodium-dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L-ascorbic acid into Schwann cells. [Accessed July 14, 2011];Glia. 2010 58(3):287–299. doi: 10.1002/glia.20923. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19672970. [DOI] [PubMed] [Google Scholar]
- 33.Kim K-S, Kim C-H, Hwang D-Y, et al. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. [Accessed December 16, 2011];J. Neurochem. 2003 85(3):622–634. doi: 10.1046/j.1471-4159.2003.01671.x. Available at: http://doi.wiley.com/10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 34.Smits SM, Ponnio T, Conneely OM, et al. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. [Accessed January 20, 2014];Eur. J. Neurosci. 2003 18(7):1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. Available at: http://doi.wiley.com/10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. [Accessed July 19, 2011];Nat. Rev. Neurosci. 2010 11(6):377–388. doi: 10.1038/nrn2810. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20485363. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Chen K, Zeng X, et al. The Histone Demethylases Jhdm1a/1b Enhance Somatic Cell Reprogramming in a Vitamin-C-Dependent Manner. [Accessed November 21, 2011];Cell stem cell. 2011 9(6):575–587. doi: 10.1016/j.stem.2011.10.005. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1934590911004851. [DOI] [PubMed] [Google Scholar]
- 37.Chen JJ, Liu H, Liu J, et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. [Accessed December 3, 2012];Nat. Genet. 2013 Dec;45:34–42. doi: 10.1038/ng.2491. Available at: http://www.nature.com/doifinder/10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- 38.Kruidenier L, Chung C, Cheng Z, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. [Accessed November 7, 2013];Nature. 2012 488(7411):404–408. doi: 10.1038/nature11262. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22842901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. [Accessed March 12, 2012];Science. 2009 324(5929):929–930. doi: 10.1126/science.1169786. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3263819&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blaschke K, Ebata KT, Karimi MM, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. [Accessed June 30, 2013];Nature. 2013:1–7. doi: 10.1038/nature12362. Available at: http://www.nature.com/doifinder/10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Guo L, Zhang L, et al. Vitamin C modulates TET1 function during somatic cell reprogramming. [Accessed October 28, 2013];Nat. Genet. 2013 Oct;:1–7. doi: 10.1038/ng.2807. Available at: http://www.nature.com/doifinder/10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 42.Heesbeen HJ van, Mesman S, Veenvliet JV, et al. Epigenetic mechanisms in the development and maintenance of dopaminergic neurons. [Accessed February 28, 2013];Development. 2013 140(6):1159–1169. doi: 10.1242/dev.089359. Available at: http://dev.biologists.org/cgi/doi/10.1242/dev.089359. [DOI] [PubMed] [Google Scholar]
- 43.Szulwach KE, Li X, Li Y, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. [Accessed November 20, 2013];Nat. Neurosci. 2011 14(12):1607–1616. doi: 10.1038/nn.2959. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3292193&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. [Accessed July 10, 2014];Science. 2011:5. doi: 10.1126/science.1212483. (80-.). Available at: http://www.sciencemag.org/content/334/6053/194.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin S-G, Jiang Y, Qiu R, et al. 5-Hydroxymethylcytosine Is Strongly Depleted in Human Cancers but Its Levels Do Not Correlate with IDH1 Mutations. Cancer Res. 2011;71:7360–7365. doi: 10.1158/0008-5472.CAN-11-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iqbal K, Jin S-G, Pfeifer GP, et al. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu T, Guo F, Yang H, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. [Accessed July 10, 2014];Nature. 2011:0–6. doi: 10.1038/nature10443. Available at: http://www.nature.com/nature/journal/v477/n7366/abs/nature10443.html. [DOI] [PubMed] [Google Scholar]
- 48.Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res. 2010;38(11):1–7. doi: 10.1093/nar/gkq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puelles E, Annino A, Tuorto F, et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. [Accessed July 28, 2011];Development. 2004 131(9):2037–2048. doi: 10.1242/dev.01107. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15105370. [DOI] [PubMed] [Google Scholar]
- 50.Ye W, Shimamura K, Rubenstein JL, et al. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93(5):755–766. doi: 10.1016/s0092-8674(00)81437-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9630220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.