Abstract
Background
To assess the effects of single polycyclic aromatic hydrocarbons (PAHs) on solid tumor initiation, and investigate their roles in immune response regulation.
Material/Methods
Mice (100) were randomly divided into 5 groups (n=20) to be intraperitoneally injected with 10 daily doses of DMSO (control), anthracene (50 mg/kg), benzo-(a)-pyrene (10 mg/kg), benzo-(a)-pyrene (20 mg/kg), and benzo-[G, H, I])-perylene (5 mg/kg), respectively. Three months later, serum IL-2 and IL-6 levels were assessed by ELISA; liver, kidney, stomach and lung tissues were subjected to histopathological examinations.
Results
Liver cancer incidences after benzo-[G, H, I]-perylene, benzo-(a)-pyrene (10 mg/kg), benzo-(a)-pyrene (20 mg/kg), and anthracene were 21.1, 26.3, 35.3, and 27.8%, respectively; 21.1, 0, 41.2, and 0% showed stomach cancer, respectively; 0, 0, 11.8 and 0% displayed kidney cancer, respectively. The occurrences of precancerous liver lesions for benzo-[G, H, I]-perylene, benzo-(a)-pyrene (10 mg/kg), benzo-(a)-pyrene (20 mg/kg) and anthracene groups, respectively, were 68.4, 73.7, 64.7, and 55.6%; 78.9, 68.4, 29.4, and 27.8% showed precancerous stomach lesions, while 42.1, 47.4, 58.8, and 33.3% had precancerous kidney lesions; respectively. No obvious lung lesions were found in any group. Serum IL-2 and IL-6 levels in treatment groups were significantly lower compared with control values (P<0.01).
Conclusions
PAHs induce cancer and precancerous lesions in the liver, stomach, and kidney. Benzo (a) pyrene initiates gastric cancer in a dose-dependent manner, but does not induce precancerous lung lesions. Lower IL-2 and IL-6 levels in treatment groups compared with controls suggest that PAHs cause overt immune inhibition.
MeSH Keywords: Bay-Region, Polycyclic Aromatic Hydrocarbon; Hydrocarbons; Mice, 129 Strain; Carcinoma, Acinar Cell; Immunologic Factors
Background
Polycyclic aromatic hydrocarbons (PAHs) were the first discovered environmental pollutants and carcinogens; as such, they were suggested to be controlled with high priority by the United States Environmental Protection Agency (USEPA). PAHs are widely found in the air, food and drinking water. The metabolic end product of PAHs in the human body is 7,8-dihydrodiol-9.10-epoxide benzo [a] pyrene (BPDE), which has the ability to covalently bind to the nucleophilic sites (outside amino terminals of guanine) of DNA to form PAH-DNA adducts, thus inducing DNA damage, gene mutations and carcinogenesis, processes related to tumor formation. These findings indicate that PAHs are indirect carcinogens. Benzo (a) pyrene is considered a PAH representative, as a chemical carcinogen with high carcinogenicity. On the other hand, PAHs are important environmental pollutants that can suppress the immune function. Studies by Szczeklik et al. have shown that exposure to PAHs results in immune inhibition, with reduced serum IgG and IgA levels and increased IgE and IgM amounts in coke oven workers. IL-2 is a T cell growth factor, which enhances the cytotoxic activity of T cells; IL-6 is a multi-effect cytokine produced by endothelial cells, monocytes/macrophages, and lymphoid cells. IL-6 promotes B cell proliferation and differentiation, inducing antibody secretion. However, the effect of single PAHs such as anthracene, benzo (a) pyrene, and benzo [G, H, I] perylene, on IL- 2 and IL-6 production/secretion is still largely unknown.
Material and Methods
Material
A total of 120 SPF Kun Ming mice (Gansu College Experimental Animal Center, SCXK (Gan) 2011-0001-0001331) at 1:1 sex ratio (20±2g) were housed in a standard rodent room (temperature 18–29ºC, diurnal temperature ≤3ºC and relative humidity of 40–70%). Anthracene, benzo (a) pyrene and benzo [G, H, I] perylene, were purchased from AccuStandard (USA). Dimethyl sulfoxide (DMSO) and ether were purchased from Shanghai No. 1 Biochemical Pharmaceutical Co., Ltd (China).
The animal equipment was purchased from Lanzhou City medical Devices Corporation (China). IL-2 and IL-6 ELISA assay kits were purchased from R&D Company, Inc. (USA).
Methods
Establishment of animal models
The animal models were established as described previously. Briefly, 20 mice were used for preliminary experiments to determine suitable dosages for PAHs (EU 2005 standard). Then, 100 mice (1:1 sex ratio, 20±2 g) were randomly divided into five groups (n=20) to be intraperitoneally injected with 10 daily doses of DMSO (control), anthracene (50 mg/kg), benzo-(a)-pyrene (10 mg/kg), benzo-(a)-pyrene (20 mg/kg) and benzo-[G, H, I])-perylene (5 mg/kg), respectively. All test articles were administered in 0.1 mL DMSO/gram body weight. Animals were examined once daily for three months.
Sample collection and assessment
After anesthesia with ether, mouse blood was collected from the femoral artery and Verify. Then, serum IL-2 and IL-6 levels were determined following the manufacturer’s instructions. Moreover, liver, spleen, kidney, pancreas, stomach and lung were collected from each animal upon euthanasia. Then, the tissue samples were fixed with formalin, conventionally embedded and sliced. After hematoxylin and eosin (H&E) staining, slides were analyzed by microscopy.
Statistical analysis
Statistical analysis was carried out using the SPSS 11.5 software (SPSS, USA). P<0.05 was considered statistically significant.
Results
Tumorigenic effects of PAHs
During the study, 7 mice died due to toxicity. Cancer incidences for the various groups are summarized in Table 1 and Figures 1–3. The incidences of precancerous lesions were significantly higher in treatment groups compared with control animals (P<0.05). Treatment with benzo (a) pyrene at 20 mg/kg significantly increased the incidences of cancer and precancerous lesions in the stomach compared with the lower dose of 10 mg/kg. No obvious damages were observed in the lung for either treatment groups.
Table 1.
Incidences of cancer and precancerous lesions.
| n | Liver | Gastric | Kidney | ||||
|---|---|---|---|---|---|---|---|
| Cancer (%) | Pre-cancer (%) | Cancer (%) | Pre-cancer (%) | Cancer (%) | Pre-cancer (%) | ||
| Control group | 20 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Benzo(GHI)perylene 5 mg/kg | 19 | 4 (21.1) | 13 (68.4) | 4 (21.1) | 15 (78.9) | 0 (0.0) | 8 (42.1) |
| Benzo(a)pyrene 10 mg/kg | 19 | 5 (26.3) | 14 (73.7) | 0 (0.0) | 13 (68.4) | 0 (0.0) | 9 (47.4) |
| Benzo(a)pyrene 20 mg/kg | 17 | 6 (35.3) | 11 (64.7) | 7 (41.2) | 5 (29.4) | 2 (11.8) | 10 (58.8) |
| Anthracene 50 mg/kg | 18 | 5 (27.8) | 10 (55.6) | 0 (0.0) | 5 (27.8) | 0 (0.0) | 6 (33.3) |
Figure 1.
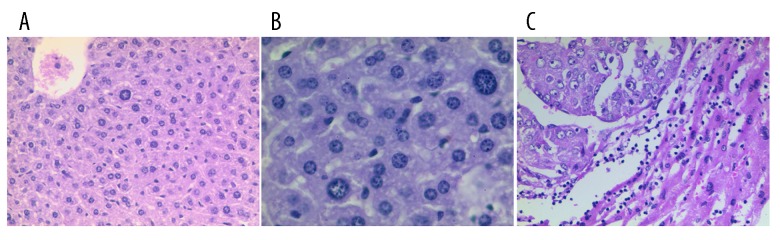
H&E staining of the liver. (A) Controls (×200), (B) precancerous lesions (×200), and (C) hepatocellular carcinoma (×200).
Figure 2.
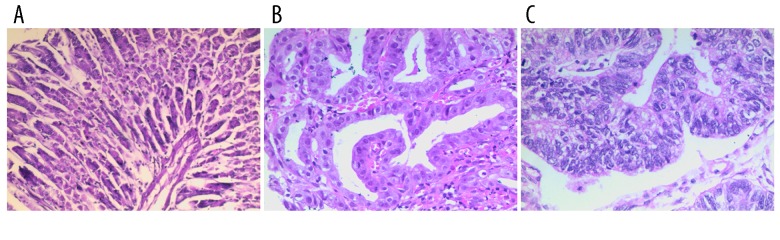
H&E staining of the stomach. (A) Controls (×200), (B) precancerous lesions (×200), and (C) gastric carcinoma (×200).
Figure 3.
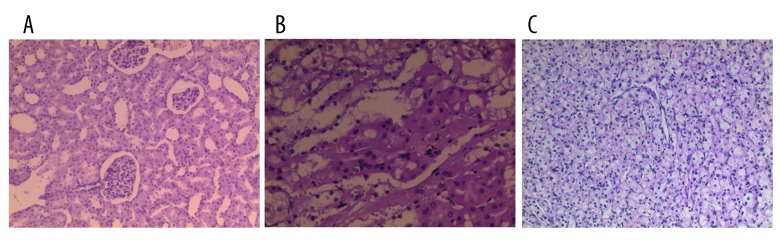
H&E staining of the kidney. (A) Controls (×100), (B) precancerous lesions (×100), and (C) renal carcinoma (×100).
Effect of PAHs on IL-2 and IL-6 levels
Table 2 summarizes the serum levels of IL-2 and IL-6 in each group. They were significantly lower in the treatment groups compared with control values (p<0.05); no significant differences were obtained among treatment groups (p>0.05).
Table 2.
IL-6 and IL-2 levels in different groups.
| Group | n | IL-2 (ng/L) | Il-6 (pg/ml) |
|---|---|---|---|
| Control group | 20 | 360±15.92 | 32.65±5.32 |
| Benzo(GHI)perylene 5 mg/kg | 19 | 137.26±15.89 | 23.63±2.72 |
| Benzo(a)pyrene 10 mg/kg | 19 | 92.59±6.12* | 21.78±4.62 |
| Benzo(a)pyrene 20 mg/kg | 17 | 83.84±5.89* | 24.54±9.89 |
| Anthracene 50 mg/kg | 18 | 154.49±5.23 | 23.03±5.56 |
VS Control group, P<0.01.
Discussion
PAHs mainly originate from the incomplete combustion of organics, and are easily produced by human activities. PAHs were the first discovered environmental pollutants and carcinogens. Following entry in the body, most PAHs produce a variety of intermediates and end products after mixed function oxidase metabolism. Some of their metabolites can covalently bind to DNA and form PAH-DNA adducts, thus inducing DNA damage and gene mutations, and even promoting carcinogenesis. Benzo(a)pyrene (BaP) is a pro-carcinogen, which can cause DNA damage: BaP is oxidized by cytochrome P450 enzymes (such as CYPIA1) in the microsomes of liver and lung cells to produce epoxides, which are then hydrolyzed into dihydroxy compounds by epoxide hydrolases. Dihydroxy compounds are further oxidized by cytochrome P450 enzymes to produce dihydroxy epoxides, which bind to DNA of target cells and form DNA adducts, thus causing DNA damage, and initiating mutations and the carcinogenic process. BaP can produce more than 10 metabolites under catalysis by P450 enzymes. Trans-BPDE (anti-BPDE) is widely accepted as the end metabolic carcinogen of BaP metabolism. Anti-BPDE has a pro-electronic group, which covalently binds to nucleophilic sites (outside amino terminals of guanine) of DNA as well as transcription factors, thus inducing DNA damage and causing G-T and G-A mutations; this promotes the activation of oncogenes or inhibits tumor suppressors, and initiates mutations and carcinogenesis.
In this study, we found that treatment with single PAHs, including anthracene, benzo (a) pyrene and benzo [G, H, I] perylene, induces significant damage in mouse liver, stomach and kidney. Cancer incidences in the mouse liver were 21.1% (4/19), 26.3% (5/19), 35.3% (6/17) and 27.8% (5/18), respectively, in the benzo [G, H, I] perylene, benzo (a) pyrene (10 mg/kg), benzo (a) pyrene (20 mg/kg) and anthracene groups; the occurrence rates of precancerous lesions in the liver were 68.4% (13/19), 73.7% (14/19), 64.7% (11/17) and 55.6% (10/18), respectively. Stomach cancer incidences were, respectively, 21.1% (4/19), 0% (0/19), 41.2% (7/17) and 0% (0/18), while occurrence rates of precancerous lesions of 55.6% (10/18), 78.9% (15/19), 68.4% (13/19), and 29.4% (5/17), respectively, were obtained after treatment with benzo [G, H, I] perylene (5 mg/kg), benzo (a) pyrene (10 mg/kg), benzo (a) pyrene (20 mg/kg) and anthracene. Kidney cancer incidences of 0% (0/19), 0% (0/19), 11.8% (2/17) and 0% (0/18), and precancerous lesion occurrence rates of 27.8% (5/18), 42.1% (8/19), 47.4% (9/19) and 58.8% (10/17), respectively, were obtained in the benzo [G, H, I] perylene (5 mg/kg), benzo (a) pyrene (10 mg/kg), benzo (a) pyrene (20 mg/kg) and anthracene groups. These findings indicated significantly higher occurrences of precancerous lesions in treatment groups compared with control values. Treatment with benzo (a) pyrene at 20 mg/kg induced significantly higher stomach damage compared with the lower dose of 10 mg/kg. No obvious damage to lung and pancreas was found in any groups, suggesting different invasion approaches and targeted organs for PAHs.
Yang et al. showed that PAH carcinogenicity is enhanced with the increase of ring numbers. In the current study, although the carcinogenicity of benzo [G, H, I] perylene and anthracene was not higher than that of benzo (a) pyrene, benzo [G, H, I] perylene was used at 5 mg/kg, since a higher dose (10 mg/kg) was lethal to mice; meanwhile, anthracene was used at 50 mg/kg. Thus, the carcinogenicity order for these PAHs is: anthracene < benzo (a) pyrene < benzo [G, H, I] perylene. The chemical structures of benzo [G, H, I] perylene, benzo (a) pyrene and anthracene present six, five and three rings, respectively, which is consistent with our findings regarding the carcinogenicity order.
PAHs are important environmental contaminants that exhibit immune inhibition effect. Burchiel et al. have shown that PAH is able to affect human T cell and B lymphocyte properties, and induce monocyte damage. A study by Jedrychoski et al. showed that exposure to PAHs of pregnant women can induce impaired immune function in the fetus, and increase the risk of respiratory diseases in neonates and young children. In addition, Detmar et al. found that long-term exposure to PAHs may induce high abortion rates in women. Moreover, Heudorf et al. conducted an investigation for one year on children exposed to benzopyrene, and found 15, 10, 20, 15, 25, 42 and 60% children with elbow eczema, rubella, sneezing and runny nose, nose bleeding, difficulty breathing, dry cough, and frequent colds, respectively.
IL-2 is a T cell growth factor produced by activated T cells. It promotes NK cell proliferation and maintains NK cell long-term growth. In addition, IL-2 promotes the expression of IL-2R in B cells, increases B cell proliferation and produces immunoglobulins, and stimulates macrophages to enhance their phagocytic capacities. IL-6, a multi-effect cytokine, is mainly produced by endothelial cells, monocytes/macrophages, and lymphoid cells. It promotes B cell differentiation and proliferation, and induces antibody secretion in B cells. Furthermore, IL-6 can directly or indirectly enhance NK cell cytotoxicity and T cell tumoricidal activity.
Conclusions
Here, we showed that IL-2 and IL-6 levels in treatment groups were significantly lower than control values (P<0.01), suggesting an inhibition of the immune function by PAHs. However, further studies are warranted to reveal the underlying mechanisms of these effects.
Footnotes
Source of support: This work was supported by the Natural Science Foundation of Gansu Province, China (Grant No. 145RJZA235) and the Fundamental Research Funds for the Universities in Gansu Province
Reference
- 1.Arnould JP, Kubiak R, Belowski J, et al. Detection of benzo[a]pyrene-DNA adducts in leukocytes of coke oven workers. Pathol Biol (Paris) 2000;48:548–53. [PubMed] [Google Scholar]
- 2.Bansal S, Leu AN, Gonzalez FJ, et al. Mitochondrial targeting of cytochrome P450 (CYP) 1B1 and its role in polycyclic aromatic hydrocarbon-induced mitochondrial dysfunction. J Biol Chem. 2014;289:9936–51. doi: 10.1074/jbc.M113.525659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8(3):444–72. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 4.Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- 5.Detmar J, Rabaglino T, Taniuchi Y, et al. Embryonic loss due to exposure to polycyclic aromatic hydrocarbons is mediated by Bax. Apoptosis. 2006;11:1413–25. doi: 10.1007/s10495-006-8442-3. [DOI] [PubMed] [Google Scholar]
- 6.Georgiadis P, Kovacs K, Kaila S, et al. Development and validation of a direct sandwich chemiluminescence immunoassay for measuring DNA adducts of benzo[a]pyrene and other polycyclic aromatic hydrocarbons. Mutagenesis. 2012;27:589–97. doi: 10.1093/mutage/ges024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heudorf U, Schumann M, Angerer J, Exner M. Dermal and bronchial symptoms in children: are they caused by PAH containing parquet glue or by passive smoking? Int Arch Occup Environ Health. 2005;78:655–62. doi: 10.1007/s00420-005-0007-1. [DOI] [PubMed] [Google Scholar]
- 8.Hodek P, Koblihova J, Kizek R, et al. The relationship between DNA adduct formation by benzo[a]pyrene and expression of its activation enzyme cytochrome P450 1A1 in rat. Environ Toxicol Pharmacol. 2013;36:989–96. doi: 10.1016/j.etap.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Huc L, Rissel M, Solhaug A, et al. Multiple apoptotic pathways induced by p53-dependent acidification in benzo[a]pyrene-exposed hepatic F258 cells. J Cell Physiol. 2006;208:527–37. doi: 10.1002/jcp.20686. [DOI] [PubMed] [Google Scholar]
- 10.Indra R, Moserova M, Sulc M, et al. Oxidation of carcinogenic benzo[a]pyrene by human and rat cytochrome P450 1A1 and its influencing by cytochrome b5 – a comparative study. Neuro Endocrinol Lett. 2013;34(Suppl 2):55–63. [PubMed] [Google Scholar]
- 11.James MO, Kleinow KM, Zhang Y, et al. Increased toxicity of benzo(a)pyrene-7,8-dihydrodiol in the presence of polychlorobiphenylols. Mar Environ Res. 2004;58:343–46. doi: 10.1016/j.marenvres.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 12.Jedrychowski W, Galas A, Pac A, et al. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–82. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Nishida T, Ito Y, et al. Correlations of programmed death 1 expression and serum IL-6 level with exhaustion of cytomegalovirus-specific T cells after allogeneic hematopoietic stem cell transplantation. Cell Immunol. 2014;288:53–59. doi: 10.1016/j.cellimm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Ko CB, Park C, et al. p38 MAP kinase regulates benzo(a)pyrene-induced apoptosis through the regulation of p53 activation. Arch Biochem Biophys. 2005;444:121–29. doi: 10.1016/j.abb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Firozi PF, Wang LE, et al. Sensitivity to DNA damage induced by benzo(a)pyrene diol epoxide and risk of lung cancer: a case-control analysis. Cancer Res. 2001;61:1445–50. [PubMed] [Google Scholar]
- 16.Pratt MM, King LC, Adams LD, et al. Assessment of multiple types of DNA damage in human placentas from smoking and nonsmoking women in the Czech Republic. Environ Mol Mutagen. 2011;52:58–68. doi: 10.1002/em.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivani R, Nagarajan B. A prognostic insight on in vivo expression of interleukin-6 in uterine cervical cancer. Int J Gynecol Cancer. 2003;13:331–39. doi: 10.1046/j.1525-1438.2003.13197.x. [DOI] [PubMed] [Google Scholar]
- 18.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–32. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 19.Uno S, Sakurai K, Nebert DW, Makishima M. Protective role of cytochrome P450 1A1 (CYP1A1) against benzo[a]pyrene-induced toxicity in mouse aorta. Toxicology. 2014;316:34–42. doi: 10.1016/j.tox.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Wei LH, Kuo ML, Chen CA, et al. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20:5799–809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- 21.Zitvogel L, Kroemer G. Cytokines reinstate NK cell-mediated cancer immunosurveillance. J Clin Invest. 2014;124:4687–89. doi: 10.1172/JCI78531. [DOI] [PMC free article] [PubMed] [Google Scholar]


