Abstract
Objective
To assess the outcome of the drainage procedure used for treating a prostatic abscess, and to propose a treatment algorithm to reduce the morbidity and the need for re-treatment.
Patients and methods
We retrospectively reviewed patients who were admitted and received an interventional treatment for a prostatic abscess. All baseline relevant variables were reviewed. Details of the intervention, laboratory data, duration of hospital stay, follow-up data and re-admissions were recorded.
Results
A prostatic abscess was diagnosed in 42 patients; 30 were treated by transurethral deroofing and 12 by transrectal needle aspiration. The median (range) size of the abscess was 4.5 (2–23) mL and 2.7 (1.5–7.1) mL in the deroofing and aspiration groups, respectively (P = 0.2). In half of the cases multiple abscesses were evident on imaging before the intervention. The median (range) hospital stay after deroofing and aspiration was 2 (1–11) and 1 (1–19) days, respectively (P = 0.04). Perioperative complications occurred only in the deroofing group, in which two patients developed septic shock requiring intensive care (Clavien 4) and one developed epididymo-orchitis (Clavien 2). There were two late complications in the deroofing group, in which one patient developed a urethral stricture that required endoscopic urethrotomy (Clavien 3a) and one developed a urethral diverticulum and urinary incontinence that required diverticulectomy and a bulbo-urethral sling procedure (Clavien 3b). A urethro-rectal fistula developed after aspiration in one patient. Re-treatment for the abscess was indicated in two (7%) patients in the deroofing group, which was treated by aspiration.
Conclusion
Transrectal needle aspiration for a prostatic abscess, when done for properly selected cases, could minimise the morbidity of the drainage procedure.
Abbreviations: MIS, minimally invasive surgery
Keywords: Prostate, Abscess, Deroofing, Aspiration, Transrectal
Introduction
A prostatic abscess is an uncommon urological emergency but it is a serious infection of the prostate with a high mortality rate unless properly treated [1,2]. Patients with diabetes mellitus, renal insufficiency and immune suppression are particularly at risk. Urethral catheterisation, lower urinary tract instrumentation and a prostate biopsy are among the possible predisposing factors [3]. Several pathogens might be incriminated in the disease. Enterobacteriacae (particularly Escherichia coli) and Staphylococcus aureus are the commonest causative organisms [4]. Haematogenous spread from distant foci has also been reported. In these cases, organisms like Mycobacterium tuberculosis and Candida species might be found [5].
The clinical presentation varies depending on the severity of infection. A prostatic abscess is usually diagnosed when a patient with acute prostatitis fails to respond to medical treatment. The patient commonly presents with perineal, genital and suprapubic pain, exacerbating LUTS, and urinary retention might also occur. Constitutional symptoms (fever, rigors, malaise and anorexia) are frequently present. The prostate is intractably tender on a DRE. Fluctuation (a ‘boggy’ sensation) of the prostate on a DRE can establish the diagnosis [6]. TRUS and other cross-sectional imaging methods (pelvic CT or MRI) might be useful in the diagnosis, treatment and monitoring of the response to treatment [7]. Once liquefaction and abscess formation are diagnosed, several approaches have been described for drainage. Open perineal drainage, transurethral deroofing, transrectal needle aspiration or tube drainage [8,9] and percutaneous drainage [10] are the main therapeutic options. Transurethral holmium-laser deroofing of a prostatic abscess has been reported [11].
To the best of our knowledge, the available data do not support some treatments over others in any particular situation. Furthermore, the morbidity of different procedures was not sufficiently reported and the effect of different treatment approaches on voiding and sexual function is unknown. The need for secondary treatment for the abscess or for the underlying prostate pathology has not been assessed. The aim of the present study work was to clearly define a treatment algorithm based on a retrospective assessment of a single-institutional case series, and a review of the relevant reports on this topic, to reduce the morbidity and the need for re-treatment, all of which might help in counselling patients, particularly for a staged treatment plan.
Patients and methods
Using our electronic database we retrospectively reviewed all patients with a diagnosis of prostatic abscess between 2002 and 2012. All patients who were admitted and received an interventional treatment were included. The database analysis was approved by an internal review board. All relevant baseline variables were reviewed. Details of the intervention, laboratory data, duration of hospital stay, follow-up data and re-admissions were recorded. Patients were invited and interviewed to ascertain any medical treatment or secondary surgical procedures for the prostate.
Intervention
Once the diagnosis of a prostatic abscess was confirmed, broad-spectrum antimicrobial third generation cephalosporin was given after a midstream urine sample was taken for culture.
For transurethral deroofing, the procedure was performed under a spinal or general anaesthesia, based on an assessment by the anaesthesia team for the patient’s condition. Using 24–26 F resectoscope sheath with glycine 1.5% as an irrigant, transurethral resection started at the 5–7 o’clock position, deep enough to deroof the abscess (Fig. 1a). Based on the preoperative radiological assessment the resection might extend to the lateral lobes if they were involved. The specimen and the drained pus were collected and sent for pathological and microbiological assessment. After adequate haemostasis a 22 F urethral catheter was fixed.
Figure 1.
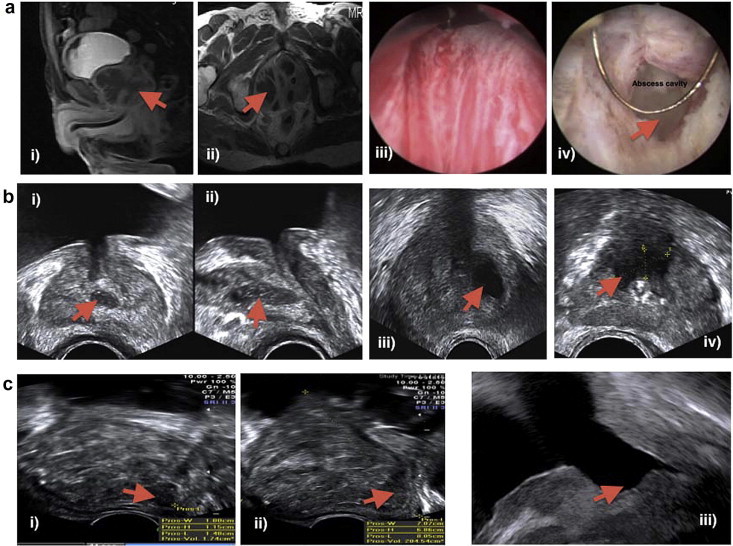
(a) MRI, Sagittal (i) and axial (ii) views after administration of intravenous contrast medium show enlarged right side of the prostate. The abscess appears multilocular with enhancing wall. (iii) A cystoscopic view of the abscess bulge at time of deroofing. (iv) A cystoscopic view of the abscess cavity during deroofing. (b) TRUS images showing hypoechoic areas with thick well-defined walls (abscesses) (c) (i) TRUS image (sagittal) showing a solitary posterior abscess in a giant prostate (>200 mL) 1c (ii) TRUS image (sagittal) after transrectal aspiration of the abscess in giant prostate (> 200 mL). (iii) TRUS image (sagittal) for the same case, 3 months after a subsequent laser procedure (holmium laser enucleation of the prostate).
For transrectal needle aspiration, a cleansing enema was applied when tolerable by the patient before the procedure, to eliminate faeces in the rectum. Initially patients were scanned in the lithotomy position and TRUS performed in both the transverse and the sagittal planes. The location of the abscess, as one or more hypoechoic areas with thick well-defined walls containing thick fluid, was ascertained (Fig. 1b). A 7-MHz transducer probe was used, with a focal range of 1–4 cm; a lower frequency was used for large glands. Local anaesthesia was often infiltrated before the drainage procedure. A peri-prostatic block was obtained by injecting 5 mL lidocaine 2% at the junction of the seminal vesicles and the prostate bilaterally. An 18-G long Chiba needle, which can be passed through the needle guide attached to the ultrasound probe, was most often used. The ultrasound unit provided the best visualisation of the needle path in the sagittal plane. Images were typically superimposed with a ruled needle path that corresponded to the needle guide of the TRUS unit. All detectable abscesses were aspirated completely and the aspirate was sent for pathological and microbiological assessment.
The patient was discharged from the hospital after being afebrile for 48 h and the blood leukocyte count was declining. The patient was advised to keep taking ciprofloxacin 500 mg twice daily until the next clinic visit at 2 weeks after discharge.
Outcome measures
The peri-procedure morbidity and the need for re-intervention for the abscess were reported according to the modified Clavien scale. Furthermore, the hospital stay, re-admissions and consecutive treatment received (secondary medical or surgical treatment for the prostate) were recorded.
The results are presented as a description of the variables with the percentage, median (range) and mean (SD). Statistical analysis comprised Fisher’s exact test and the chi-squared text for categorical variables, and the Mann–Whitney U-test for continuous variables, with P < 0.05 considered to indicate statistical significance in all tests.
Results
An admission for active interventional treatment of a prostatic abscess was recorded in 42 patients. The baseline demographic data and possible predisposing factors are shown in Table 1. Diabetes mellitus was present in 59% of patients and 24% were on immunosuppressive treatment secondary to a renal/liver transplant. There was a history of an indwelling urethral catheter at presentation, recent urethral instrumentation and recent prostate biopsies in 29%, 17% and 8% of patients, respectively.
Table 1.
The baseline variables in the two groups, and the peri-procedure and late outcomes.
| Mean (SD), median (range), n (%) | Transurethral deroofing | Transrectal needle aspiration | P |
|---|---|---|---|
| Baseline | |||
| Number of patients | 30 | 12 | |
| Age at intervention (years) | 49.4 (14) | 55.4 (15) | 0.2 |
| Body mass index, kg/m2 | 28.6 (5.0) | 28.4 (5.3) | 0.8 |
| Diabetes mellitus | 15 (50) | 4 | 0.5 |
| Patients with system failure: | 0.5 | ||
| End-stage kidney disease | 3 (10) | – | |
| Liver cell failure | 3 (10) | 1 | |
| Indwelling urethral catheter | 9 (30) | 3 | 1 |
| Systemic chemo/immunosuppressive therapy | 6 (20) | 4 | 0.79 |
| Recent urethral instrumentation | 4 (13) | 3 | 0.03 |
| Recent prostate needle biopsy | – | 1 | 0.25 |
| Presentation | 0.51 | ||
| Exacerbating LUTs | 12 (40) | 8 | |
| Acute urine retention | 9 (30) | 1 | |
| Indwelling catheter with systemic and | 9 (30) | 3 | |
| local symptoms | |||
| PSA at presentation (ng/mL) | 1.7 (0.1–4.7) | 4.4 (0.8–50) | 0.12 |
| Leukocyte count at presentation (/mL) | 12.1 (4.8–16.7) | 12.5 (5.2–29) | 0.63 |
| Positive urine culture at presentation | 12 (40) | 5 | 0.37 |
| TRUS/MRI prostate size (mL) | 53 (6.2–110) | 70 (21–106) | 0.5 |
| TRUS/MRI abscess size (mL) | 4.5 (2–23) | 2.7 (1.5–7.1) | 0.2 |
| Prostate size group | 1 | ||
| TRUS/MRI prostate size (< 80 mL) | 7 (23) | 3 | |
| TRUS/MRI prostate size (> 80 mL) | 23 (77) | 9 | |
| Location of the abscess in the prostate | 0.06 | ||
| Right lobe | 9 (30) | 1 | |
| Left lobe | 6 (20) | 5 | |
| Multiple sites | 15 (50) | 6 | |
| Peri-procedure and late outcomes | |||
| Catheter after procedure | <0.001 | ||
| Urethral catheter | 26 (87) | 2 | |
| Suprapubic catheter | 2 (7) | 2 | |
| Both | 2 (7) | – | |
| Hospital stay (days) | 2 (1–11) | 1 (1–19) | 0.04 |
| Need for re-treatment for the abscess | 2 (7) | – | 1 |
| Need for re-treatment for the prostate | 14 (47) | 6 | 0.4 |
| α-blockers | 12 (40) | 2 | |
| TURP | 2 (7) | 2 | |
| Holmium laser enucleation of the prostate | – | 1 | |
| Androgen deprivation for prostate cancer | – | 1 | |
| Complications: Clavien grade/treatment offered | 0.1 | ||
| Septic shock/IVa/Antimicrobial + inotropics | 2 (7) | – | |
| Urethral stricture/IIIa/endoscopic urethrotomy | 1 (3) | – | |
| Epididymo-orchitis/II/Lead subacetate | 1 (3) | – | |
| +NSAIDs + quinolones + CIC | |||
| Urethro-rectal fistula/IIIb/Repair | – | 1 | |
| Urethral diverticulum/IIIb/bulbourethral sling | 1 (3) | – |
CIC, clean intermittent catheterisation.
The median estimated size of the prostate was 53 (6.2–110) and 70 (21–106) mL in the deroofing and aspiration groups, respectively (P = 0.5). The median estimated size of the abscess was 4.5 (2–23) and 2.7 (1.5–7.1) mL in deroofing and aspiration groups, respectively (P = 0.2). In half of the patients there were multiple abscesses (more than one site) on imaging before the intervention (Table 1).
All patients who had deroofing were catheterised for a median of 6 (3–14) days and four who had TRUS-guided aspiration were catheterised for a median of 3 (1–7) days. There was a significantly shorter hospital stay in patients who were treated with TRUS-guided aspiration (Table 1). A suprapubic cystostomy tube was used to replace an indwelling urethral catheter in two patients in each group (Table 1).
No deaths were reported after either intervention. The peri-procedure complications (in the first 30 days) were only in the deroofing group, in which two patients developed septic shock necessitating intensive care (Clavien 4a) and one patient developed epididymo-orchitis (Clavien 2). There were two late complications in the deroofing group, in which one patient developed a urethral stricture that required endoscopic urethrotomy (Clavien 3a) and one developed a urethral diverticulum (Fig. 2) and urinary incontinence that required a diverticulectomy and bulbo-urethral sling procedure (Clavien 3b). A urethro-rectal fistula developed after aspiration in one patient (Clavien 3b) (Table 1).
Figure 2.
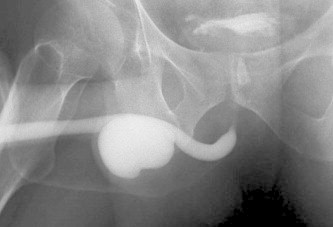
A retrograde urethrogram showing a urethral diverticulum.
Re-treatment for the abscess was indicated in two (7%) patients in the deroofing group, where TRUS-guided aspiration was used. In these patients the failure to control fever and an evident residual hypoechoic area by TRUS indicated aspiration.
The need for abscess re-treatment (both patients) and occurrence of complications (all five) consistently occurred in patients with an estimated prostate size of >80 mL (P = 1.0). Furthermore, there was a further need for re-treatment of the abscess (both patients) and more complications (four of five) in those with multiple abscess foci than in those with a single focus of abscess (P > 0.05).
There were no significant differences between the treatment groups in the need for auxiliary procedures to control residual LUTS (Table 1). One patient had a persistently high PSA level at 3 months after drainage and a subsequent prostatic biopsy showed underlying high-grade adenocarcinoma (Table 1).
Discussion
Unlike any other abscess in the body, prostatic abscess drainage is not the sole objective of the urologist. The goals of treatment are to lower the morbidity and mortality of the drainage procedure, reduce the need for re-treatment and to preserve normal urinary and sexual function, particularly in young motivated patients. In the present study we reviewed our experience with this rare urological emergency to devise an algorithm for treatment.
Our data showed that deroofing is not free of morbidity, and although it is effective for the immediate control of symptoms, the occasional need for the re-treatment of residual abscesses or the subsequent effect on urinary and sexual function invite other less-invasive treatment approaches.
Needle aspiration is a viable treatment option for deeply seated body abscesses, which could be used by different approaches [12]. In urological practice, needle aspiration has been used to treat renal, perirenal and pelvic abscesses. Unlike deroofing, the endpoint of TRUS-guided aspiration of a prostatic abscess is controlled by simultaneous US. Despite that drainage might be more complete with deroofing, the multiplicity of abscesses in a large prostate can be a limitation to the deroofing procedure.
Most of the published series are relatively small, outdated and reported before the era of minimally invasive surgery (MIS) and effective highly specific medical therapy for the prostate. Table 2 [5,8,9,13,14] summarises the outcome of drainage procedures reported previously. Re-treatment for the abscess was reported in 14–22% and re-treatment for BOO in 28% of patients. Unfortunately, the reporting is incomplete and the offered treatment was based on surgeon discretion and rarely on objective factors.
Table 2.
Summary of different contemporary case series of prostate abscess.
| Refs | N patients | Abscess size, mL | Abscess criteria | Prostate size, g | Treatment | Re-treatment for: |
Hospital stay, mean (range) days | |
|---|---|---|---|---|---|---|---|---|
| Abscess | BOO | |||||||
| [5] | 7 | – | – | – | 4 Perineal catheter drainage | – | – | 11.2 |
| 3 TUR deroofing | 14.2% | |||||||
| [13] | 6 | Mean 31.6 | Mean 93 | TRUS aspiration | ||||
| (17–65) | (42–162) | 16.7% | – | 1.6 (1–3) | ||||
| [8] | 7 | Mean diameter >1.5 cm | 28.5% Multifocal | – | TRUS-guided tube drain | – | 28.5% | 10 (7–17) |
| [14] | 11 | – | 3 Recurrent after aspiration | – | 7 TUR deroofing | – | – | |
| – | Posteriorly | 2 TRUS aspiration | – | – | – | |||
| Located | ||||||||
| – | Periprostatic | 2 Perineal drainage extension | – | – | – | |||
| [9] | 41 | Mean 3.87 (3–4) | – | Mean 59.4 (21–108) | 23 TUR deroofing | – | – | 10.2 (6–15) |
| Mean 4.04 (2.0–5.0) | – | Mean 41.6 (24–50) | 18 TRUS aspiration | 22.2% | – | 23.2 (18–34) | ||
TUR, transurethral.
We propose a treatment algorithm (Fig. 3) that identifies patients based on age, prostate size, abscess criteria and associating urinary tract anomalies. In the current algorithm, an age of 40–50 years was used as a threshold for the beginning of LUTS secondary to BPH. Despite the significantly different proportion of men having LUTS, a clear trend towards an increase in symptom scores with increasing age is reported in all population-based studies [15]. So in our algorithm, BOO is considered as an influential factor after that age. Furthermore, TRUS-guided aspiration is used as much as possible, aiming for deferred management of BOO (medical or MIS). When deroofing is indicated, especially for a multilocular abscess, a threshold for prostate size at 80 mL is identified, based on recommendations of most of the guidelines limiting the role of safe transurethral resection to this size [16,17]. In that situation, a limited deroofing or maximum possible aspiration is used, followed by a pre-planned deferred MIS. Fig. 1c shows an example of a 204-mL prostate with a solitary peripheral abscess where TRUS-guided aspiration was used, and 6 weeks later holmium laser enucleation of the prostate was performed.
Figure 3.
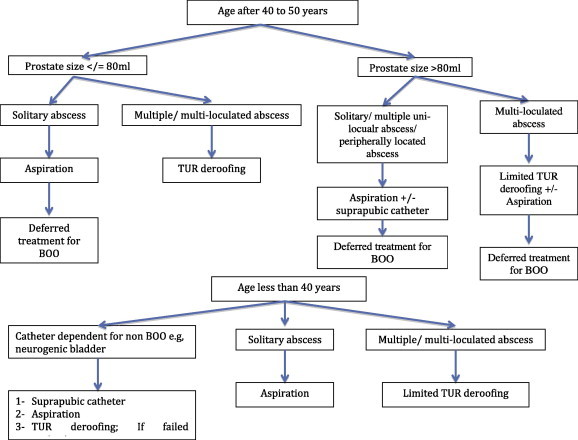
An algorithm for the treatment of prostatic abscess.
In younger patients the abscess criteria dictate the management plan, but suprapubic cystostomy remains an option for patients with indwelling catheters or those on regular catheterisation for causes other than BOO.
After reviewing the present case series we emphasise the need to keep assessing the PSA level during the follow-up visits until it reaches a nadir level, otherwise a prostate biopsy is highly recommended to avoid missing underlying prostate cancer. Furthermore, a staged management by needle aspiration of the abscess followed by a pre-planned definitive MIS treatment of the BOO is a reasonable option.
Limitations of the present study are inherent in any retrospective study. Furthermore, the situation affecting the choice of treatment approach was not addressed and the few patients included did not allow an analysis of predictors of the outcome.
In conclusion, transrectal needle aspiration and transurethral deroofing are viable, comparable treatment options for prostatic abscess. Needle aspiration, when done for properly selected cases, could minimise the morbidity of the drainage procedure.
Conflict of interest
None.
Source of funding
None.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Granados E.A., Caffaratti J., Farina L., Hocsman H. Prostatic abscess drainage: clinical-sonography correlation. Urol Int. 1992;48:358–361. [PubMed] [Google Scholar]
- 2.Ludwig M, Schroeder-Printzen I, Schiefer HG, Weidner W. Diagnosis and therapeutic management of 18 patients with prostatic abscess. Urology 199;53:340–5. [DOI] [PubMed]
- 3.Trauzzi S.J., Kay C.J., Kaufman D.G., Lowe F.C. Management of prostatic abscess in patients with human immunodeficiency syndrome. Urology. 1994;43:629–633. doi: 10.1016/0090-4295(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 4.Meares E.M., Jr. Prostatic abscess. J Urol. 1986;136:1281–1282. doi: 10.1016/s0022-5347(17)45313-4. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira P., Andrade J.A., Porto H.C., Filho J.E., Vinhaes A.F. Diagnosis and treatment of prostatic abscess. Int Braz J Urol. 2003;29:30–34. doi: 10.1590/s1677-55382003000100006. [DOI] [PubMed] [Google Scholar]
- 6.Barozzi L.P.P., Menchi I., De Matteis M., Canepari M. Prostatic abscess: diagnosis and treatment. AJR Am J Roentgenol. 1998;170:753–757. doi: 10.2214/ajr.170.3.9490969. [DOI] [PubMed] [Google Scholar]
- 7.Galosi A.B., Montironi R., Fabiani A., Lacetera V., Gallé G., Muzzonigro G. Cystic lesions of the prostate gland: an ultrasound classification with pathological correlation. J Urol. 2009;181:647–657. doi: 10.1016/j.juro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Aravantinos E., Kalogeras N., Zygoulakis N., Kakkas G., Anagnostou T., Melekos M. Ultrasound-guided transrectal placement of a drainage tube as therapeutic management of patients with prostatic abscess. J Endourol. 2008;22:1751–1754. doi: 10.1089/end.2008.0265. [DOI] [PubMed] [Google Scholar]
- 9.Jang K., Lee D.H., Lee S.H., Chung B.H. Treatment of prostatic abscess: case collection and comparison of treatment methods. Korean J Urol. 2012;53:860–864. doi: 10.4111/kju.2012.53.12.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basiri A., Javaherforooshzadeh A. Percutaneous drainage for treatment of prostate abscess. Urol J. 2010;7:278–280. [PubMed] [Google Scholar]
- 11.Shah H. Transurethral holmium laser deroofing of prostatic abscess: description of technique and early results. Abstract. J Urol. 2010;183:e128. [Google Scholar]
- 12.Ramesh J., Bang J.Y., Trevino J., Varadarajulu S. Comparison of outcomes between endoscopic ultrasound-guided transcolonic and transrectal drainage of abdominopelvic abscesses. J Gastroenterol Hepatol. 2013;28:620–625. doi: 10.1111/jgh.12081. [DOI] [PubMed] [Google Scholar]
- 13.Gögüs C., Ozden E., Karaboga R., Yagci C. The value of transrectal ultrasound guided needle aspiration in treatment of prostatic abscess. Eur J Radiol. 2004;52:94–98. doi: 10.1016/S0720-048X(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 14.El-Shazly M., El-Enzy N., El-Enzy K., Yordanov E., Hathout B., Allam A. Transurethral drainage of prostatic abscess: points of technique. Nephrourol Mon. 2012;4:458–461. doi: 10.5812/numonthly.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapple C.R. Lower urinary tract symptoms revisited. Eur Urol. 2009;56:21–23. doi: 10.1016/j.eururo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. Guidelines on the Management of Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign Prostatic Obstruction (BPO). European Association of Urology. Available at http://www.uroweb.org/gls/pdf/13_Male_LUTS_LR.pdf Accessed 17 September 2014.
- 17.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Guideline on the Management of Benign Prostatic Hyperplasia (BPH), 2012. Results of the Treatment Outcomes Analyses. American Urological Association. Available at http://www.auanet.org/common/pdf/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf Accessed 17 September 2014.


