Abstract
Background
The third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3) provides an opportunity to explore high-risk human papillomavirus (HR-HPV), and uptake of cervical screening and HPV vaccination in the general population.
Methods
Natsal-3, a probability sample survey of men and women aged 16-74, resident in Britain, interviewed 8869 women in 2010-12. We explored risk factors for HR-HPV (in urine from 2569 sexually-experienced women aged 16-44), non-attendance for cervical screening in the past 5 years and non-completion of HPV catch-up vaccination.
Results
HR-HPV was associated with increasing numbers of lifetime partners, younger age, increasing area-level deprivation and smoking. Screening non-attendance was associated with younger and older age, increasing area-level deprivation (age-adjusted odds ratio 1.91, 95% confidence interval, 1.48 to 2.47 for living in most vs. least deprived two quintiles), Asian/Asian British ethnicity (1.96, 1.32 to 2.90), smoking (1.97, 1.57 to 2.47) and reporting no partner in the past 5 years (2.45, 1.67 to 3.61 vs. 1 partner) but not with HR-HPV (1.35, 0.79 to 2.31). Lower uptake of HPV catch-up vaccination was associated with increasing area-level deprivation, non-white ethnicity, smoking and increasing lifetime partners.
Conclusions
Socio-economic markers and smoking were associated with HR-HPV positivity, non-attendance for cervical screening and non-completion of catch-up HPV vaccination.
Impact
The cervical screening programme needs to engage those missing HPV catch-up vaccination to avoid a potential widening of cervical cancer disparities in these cohorts. As some screening non-attenders are at low-risk for HR-HPV, tailored approaches may be appropriate to increase screening among higher-risk women.
Keywords: human papillomavirus, prevalence, cervical screening, human papillomavirus vaccination, inequalities
Introduction
In over 99% of cases, cervical cancer is associated with persistent infection with one or more high-risk human papilloma virus (HR-HPV) genotypes (1). Every year in Britain approximately 2,900 women are diagnosed with cervical cancer (2) and it is the most common cancer in women under 35 years (3). Worldwide the burden of cervical cancer varies substantially and 85% of cases occur in low-to-middle income countries (4). In many high-income countries, including Britain, incidence and mortality have decreased over the past few decades, since the introduction of cervical cancer screening programmes (5). In Britain, cervical screening uptake is high (around 80%) (6) but cervical cancer incidence and mortality are higher in more deprived areas (7,8). The two recent Cancer Reform Strategies (2011 and 2007) (9,10) have highlighted the need to reduce these inequalities. Understanding the burden of HR-HPV prevalence and uptake of cervical cancer prevention programmes (HPV immunisation and cervical screening) will help address this aim.
In Britain, there have been two recent notable changes in cervical cancer control. First, since 1996, increases in cervical cancer incidence have been seen in women aged 20-29 years (11), among whom screening uptake is lower and declining (12). Changes in both smoking and sexual behaviour may be contributing to the upward trend (11). Second, in September 2008, the UK introduced a school-based HPV immunisation programme against HPV-16/18 (the types associated with over 70% of cervical cancers) for girls aged 12 years which has achieved a fairly uniformly high uptake (>80% from 2008-12) (13). A catch-up programme was implemented in schools and general practice over the first few years for girls aged up to 18 years. Coverage in these catch-up cohorts was lower and more variable (13) and showed some tendency to be lower in more deprived areas (14)(15)(16). We have already reported that Britain’s third National Survey of Sexual Attitudes and Lifestyles (Natsal-3) found that women with more partners and those living in more deprived areas were less likely to complete the catch-up immunisation schedule (17).
If non-participation in cervical screening and HPV immunisation is not independent or participation is lower amongst individuals at risk of HR-HPV infection, their effectiveness may be limited. Natsal-3 provides an opportunity, unique in Britain, to explore individual-level data on participation in cervical screening and HPV immunisation in relation to detailed demographic characteristics, sexual behaviours and the presence of HR-HPV and to explore overlap between risk factors for HR-HPV infection and participation in prevention programmes and thus to inform the provision of future services.
Materials and Methods
Participants & procedure
Natsal-3 is a stratified probability sample survey of 8869 women and 6293 men aged 16-74 years, resident in Britain. The overall response rate was 57.7%. Interviews were carried out between September 2010 and August 2012. Participants were interviewed using computer-assisted personal interviewing with computer-assisted self-interview (CASI) for the more sensitive questions. Details of the methods have been published previously (18,19).
Natsal-3 included questions on socio-demographic characteristics, including educational level and occupation, allowing derivation of the National Statistics Socio-economic Classification (NS-SEC). Area-level deprivation was determined from postcodes using the Index of Multiple Deprivation (IMD) (20), a multi-dimensional measure of deprivation.
Women who reported some sexual experience (although not necessarily a sexual partner) were routed into the CASI section of the questionnaire (N=8538) where cervical screening and HPV immunisation questions were asked. Women aged 26 years and over at interview (N=5614) were asked “When did you last have a cervical smear test?” with the following five answer options: i) I have never had one, ii) less than 3 years ago, iii) between 3 and 5 years ago, iv) between 5 and 10 years ago and v) more than 10 years ago (adapted from (21)).
Women eligible for the HPV immunisation programme (those born on or after 01/09/1990, up to 21 years by the end of the interview period, N=1094) were asked “Have you ever been vaccinated against cervical cancer (received HPV vaccination)?” with the following three answer options: i) Yes – I have completed three doses of the vaccine, ii) Yes – I have had one or two doses of the vaccine, but not all three doses and iii) No. Women who had not been vaccinated and those who had only received one or two doses were defined as not having completed the recommended 3-dose vaccination course. Women who reported not having been vaccinated were asked whether they had been offered the vaccination.
Urine collection and testing
Briefly, at the end of the interview a subsample of 16–44 year olds who reported at least one lifetime sexual partner were invited to provide a urine sample to be tested for STIs and 60% agreed (17). Written consent was provided for testing without return of results (22). Full details of the urine collection methods have been described previously (17,18).
Urine samples from 2569 women were tested for HPV (17). An in house Luminex®-based genotyping assay was used for the detection of HPV types (23). HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 were defined as HR-HPV according to the WHO International Agency for Research on Cancer definition (24).
Ethics
The Natsal-3 study was approved by the Oxfordshire Research Ethics Committee A [Ref: 10/H0604/27] (22).
Statistical analysis
Analyses were carried out using Stata (version 13) accounting for the stratification, clustering and weighting of the sample. To account for differences in the probability of selection for and response to providing a urine sample, an additional weight was applied to the urine data (17,18).
Logistic regression models were used to explore the factors associated with HR-HPV detection (N=2569), non-attendance for cervical screening in the past 5 years (N=5012) and non-completion of HPV catch-up vaccination (N=1050). Limited results have been presented previously (17) but are expanded here to present a more comprehensive picture of factors associated with HR-HPV and HPV catch-up vaccination in the general British female population.
Women under 26 or over 64 (60 in Scotland), women reporting having had a hysterectomy (N=365; who would not be invited for screening) and women reporting no lifetime sexual partners (N=39; who are advised that they might decline their screening invitation) were excluded from analyses of cervical screening. Factors associated with non-completion of HPV catch-up vaccination are presented for eligible women (born before 01/09/1995 (England and Wales) or 01/03/1995 (Scotland)).
We hypothesised that cervical screening non-attenders may have differing risk of HR-HPV and cervical cancer based on socio-demographics (e.g. ethnicity) and sexual behaviour (e.g. partner numbers). We explored the characteristics of women not attending for cervical screening, in order to examine how the prevalence of other cofactors for cervical cancer (25) differed by HR-HPV risk.
We explore the overlap between factors associated with HR-HPV and participation in cervical screening and HPV catch-up vaccination.
Results
HR-HPV prevalence
HR-HPV was detected in urine from 15.9% (95% confidence interval (CI) 14.4-17.5) of women aged 16-44 years reporting at least one lifetime partner. HR-HPV prevalence declined above age 24 and was associated with a number of socio-demographic characteristics (Table 1). Prevalence was higher in women not living with a partner, in women of lower socio-economic status, as measured by markers including area-level deprivation (age adjusted OR (AOR) 1.37, 1.05-1.80 for those living in the most deprived vs. least deprived two quintiles) and NS-SEC; and in those of mixed vs. white ethnicity (AOR 2.00, 1.09-3.67). Prevalence was lower in women of Asian/Asian-British ethnicity (AOR 0.40, 0.17-0.97). Prevalence did not vary significantly by sexual identity. Prevalence was higher in women who smoked (AOR 1.91, 1.49-2.43) or reported binge drinking regularly (AOR 1.80, 1.31-2.47).
Table 1.
Factors associated with high-risk HPV in urine in sexually-experienced women aged 16-44 years
| % | (95%CI) | OR | (95%CI) | Age adjusted OR | (95%CI) | Denom. (unwt, wt)a | |
|---|---|---|---|---|---|---|---|
| All | 15.9% | (14.4-17.5) | - | 2569, 2189 | |||
|
| |||||||
| Socio-demographic characteristics | |||||||
|
| |||||||
| Age (years) | p<0.0001 | ||||||
| 16-19 | 24.4% | (20.0-29.3) | 1 | - | 377, 203 | ||
| 20-24 | 26.6% | (22.8-30.8) | 1.13 | (0.82-1.56) | 580, 370 | ||
| 25-34 | 15.6% | (13.4-18.2) | 0.58 | (0.42-0.79) | 1108, 779 | ||
| 35-44 | 9.3% | (7.1-12.2) | 0.32 | (0.22-0.47) | 504, 837 | ||
| Relationship status at interview | p<0.0001 | p<0.0001 | |||||
| Living with a partner | 11.2% | (9.5-13.1) | 1 | - | 1 | - | 1256, 1357 |
| In a steady relationship (but not living with a partner) | 26.0% | (22.2-30.1) | 2.79 | (2.13-3.66) | 1.95 | (1.44-2.65) | 602, 360 |
| Previously in a live-in partnership | 19.9% | (15.3-25.4) | 1.97 | (1.37-2.84) | 1.92 | (1.34-2.76) | 353, 240 |
| Not in a steady relationship (never lived with partner) | 23.9% | (18.8-29.8) | 2.49 | (1.76-3.54) | 1.65 | (1.13-2.42) | 355, 229 |
| Index of Multiple Deprivation (quintiles) b | P=0.0238 | p=0.0578 | |||||
| 1-2 (least deprived) | 13.5% | (11.2-16.1) | 1 | - | 1 | - | 873, 778 |
| 3 | 15.0% | (11.8-18.7) | 1.13 | (0.80-1.58) | 1.12 | (0.80-1.57) | 502, 439 |
| 4-5 (most deprived) | 18.3% | (15.9-20.9) | 1.43 | (1.10-1.87) | 1.37 | (1.05-1.80) | 1194, 973 |
| Academic qualifications c | p=0.6717 | p=0.1250 | |||||
| No academic qualifications | 15.1% | (10.5-21.4) | 0.99 | (0.63-1.56) | 1.13 | (0.72-1.77) | 215, 191 |
| Academic qualifications typically gained at age 16 | 16.7% | (14.1-19.6) | 1.12 | (0.87-1.44) | 1.3 | (1.01-1.68) | 877, 748 |
| Studying for/attained further academic qualifications | 15.2% | (13.3-17.4) | 1 | - | 1 | - | 1348, 1157 |
| Housing tenure | p<0.0001 | p=0.0011 | |||||
| Own outright | 14.3% | (10.0-19.9) | 1.38 | (0.88-2.16) | 1.15 | (0.72-1.81) | 218, 201 |
| Buying with a mortgage or loand | 10.8% | (8.9-13.0) | 1 | - | 1 | - | 911, 912 |
| Rent it | 20.5% | (18.0-23.1) | 2.13 | (1.64-2.78) | 1.71 | (1.30-2.26) | 1325, 996 |
| Lives rent free | 24.1% | (16.6-33.8) | 2.64 | (1.59-4.38) | 1.53 | (0.91-2.56) | 106, 74 |
| Respondent’s National Statistics Socio-Economic Classification | p<0.0001 | p=0.0009 | |||||
| Managerial & prof occupations | 10.3% | (8.3-12.7) | 1 | - | 1 | - | 709, 714 |
| Intermediate occupations | 16.6% | (13.0-21.1) | 1.74 | (1.21-2.52) | 1.60 | (1.11-2.30) | 464, 423 |
| Semi-routine/routine occupations | 18.5% | (15.6-21.7) | 1.98 | (1.45-2.69) | 1.57 | (1.14-2.17) | 780, 617 |
| No job (10+ hrs/week) or not in last 10 years | 22.5% | (16.6-29.8) | 2.53 | (1.62-3.96) | 2.08 | (1.31-3.31) | 210, 173 |
| Student in full-time education | 19.8% | (15.8-24.6) | 2.16 | (1.50-3.11) | 1.01 | (0.66-1.55) | 398, 256 |
| Ethnic group e | p=0.0061 | p=0.0150 | |||||
| White | 16.2% | (14.6-18.0) | 1 | - | 1 | - | 2312, 1914 |
| Mixed | 29.7% | (19.4-42.7) | 2.18 | (1.24-3.85) | 2.00 | (1.09-3.67) | 74, 58 |
| Asian/Asian British | 7.0% | (2.9-15.5) | 0.39 | (0.16-0.95) | 0.40 | (0.17-0.97) | 82, 114 |
| Black/Black British | 12.6% | (6.8-22.0) | 0.74 | (0.38-1.46) | 0.69 | (0.36-1.32) | 77, 77 |
| Religion | p=0.0286 | p=0.2671 | |||||
| None | 17.7% | (15.7-20.0) | 1 | - | 1 | - | 1509, 1189 |
| Christian - Church of England/Anglican | 9.8% | (6.2-15.2) | 0.51 | (0.30-0.85) | 0.7 | (0.42-1.19) | 220, 235 |
| Christian - Roman Catholic | 14.2% | (10.3-19.3) | 0.77 | (0.52-1.14) | 0.84 | (0.57-1.25) | 261, 226 |
| Christian - other | 17.0% | (13.4-21.5) | 0.96 | (0.69-1.32) | 1.01 | (0.73-1.39) | 457, 396 |
| Non-Christian | 10.4% | (5.9-17.7) | 0.54 | (0.29-1.01) | 0.57 | (0.31-1.05) | 122, 142 |
| Sexual identity | p=0.2447 | p=0.1893 | |||||
| Heterosexual/straight | 16.1% | (14.5-17.8) | 1 | - | 1 | - | 2457, 2108 |
| Gay/lesbian/bisexual | 11.4% | (6.1-20.1) | 0.67 | (0.34-1.32) | 0.62 | (0.31-1.26) | 107, 79 |
|
| |||||||
| Health behaviours | |||||||
|
| |||||||
| Smoking status | p<0.0001 | p<0.0001 | |||||
| Non/Ex-smoker | 12.9% | (11.4-14.7) | 1 | - | 1 | - | 1702, 1568 |
| Current smoker | 23.4% | (20.1-26.9) | 2.05 | (1.61-2.60) | 1.91 | (1.49-2.43) | 867, 622 |
| Frequency of binge drinking f | p=0.0001 | p=0.0011 | |||||
| Never / less than monthly | 13.9% | (12.2-15.7) | 1 | - | 1 | - | 1730, 1573 |
| Monthly | 19.1% | (15.6-23.2) | 1.47 | (1.10-1.96) | 1.31 | (0.98-1.75) | 484, 355 |
| Weekly or more often | 23.8% | (19.1-29.3) | 1.94 | (1.42-2.66) | 1.80 | (1.31-2.47) | 355, 261 |
|
| |||||||
| Sexual behaviours | |||||||
|
| |||||||
| Age at first heterosexual sex (years) | p<0.0001 | p=0.0059 | |||||
| 18+ | 11.0% | (8.5-14.0) | 1 | - | 1 | - | 577, 642 |
| 17 | 12.9% | (9.8-16.7) | 1.20 | (0.79-1.82) | 1.18 | (0.78-1.80) | 432, 419 |
| 16 | 20.5% | (17.2-24.3) | 2.10 | (1.48-2.97) | 1.78 | (1.24-2.56) | 659, 517 |
| <16 | 20.2% | (17.4-23.4) | 2.06 | (1.47-2.89) | 1.65 | (1.17-2.34) | 859, 577 |
| Number of sexual partners, lifetime g | p<0.0001 | p<0.0001 | |||||
| 1 | 4.2% | (2.4-7.2) | 1 | - | 1 | - | 342, 361 |
| 2 | 11.3% | (7.5-16.5) | 2.89 | (1.40-5.96) | 2.74 | (1.32-5.69) | 234, 213 |
| 3-4 | 13.7% | (10.6-17.5) | 3.60 | (1.91-6.81) | 3.71 | (1.97-7.01) | 441, 388 |
| 5-9 | 17.2% | (14.3-20.6) | 4.74 | (2.55-8.79) | 5.67 | (3.07-10.46) | 709, 593 |
| 10+ | 24.0% | (20.9-27.4) | 7.19 | (3.94-13.10) | 9.35 | (5.14-17.02) | 822, 614 |
| No. of sexual partners, past 5 years g | p<0.0001 | p<0.0001 | |||||
| 0/1 | 7.1% | (5.7-8.9) | 1 | - | 1 | - | 1162, 1258 |
| 2 | 21.9% | (17.5-26.9) | 3.64 | (2.52-5.25) | 3.34 | (2.29-4.86) | 425, 316 |
| 3-4 | 23.0% | (19.0-27.4) | 3.88 | (2.77-5.42) | 3.43 | (2.45-4.79) | 424, 290 |
| 5+ | 37.5% | (32.7-42.7) | 7.82 | (5.63-10.86) | 6.62 | (4.68-9.38) | 544, 313 |
| Number of sexual partners without a condom, past year g | p<0.0001 | p<0.0001 | |||||
| 0 | 11.3% | (8.5-14.8) | 1 | - | 1 | - | 449, 405 |
| 1 | 14.0% | (12.3-15.9) | 1.28 | (0.91-1.80) | 1.35 | (0.96-1.90) | 1741, 1566 |
| 2+ | 40.1% | (33.9-46.5) | 5.27 | (3.49-7.95) | 4.35 | (2.87-6.60) | 347, 193 |
|
| |||||||
| Sexual health & services | |||||||
|
| |||||||
| Used hormonal contraceptionh, past year | p=0.0001 | p=0.1711 | |||||
| No | 13.1% | (11.1-15.5) | 1 | - | 1 | - | 1172, 1137 |
| Yes | 19.7% | (17.5-22.1) | 1.63 | (1.28-2.07) | 1.20 | (0.92-1.55) | 982, 1388 |
| Attended a sexual health (GUM) clinic, past 5 years | p<0.0001 | p<0.0001 | |||||
| No | 11.7% | (10.2-13.4) | 1 | - | 1 | - | 1779, 1686 |
| Yes | 30.4% | (26.8-34.2) | 3.29 | (2.62-4.14) | 2.54 | (2.00-3.23) | 765, 484 |
| STI diagnosisi, past 5 years | p<0.0001 | p<0.0001 | |||||
| No | 14.7% | (13.2-16.4) | 1 | - | 1 | - | 2316, 2038 |
| Yes | 35.3% | (29.2-41.9) | 3.16 | (2.33-4.28) | 2.36 | (1.76-3.16) | 237, 134 |
| Genital warts diagnosis, ever | p=0.2095 | p=0.0891 | |||||
| No | 15.8% | (14.3-17.5) | 1 | - | 1 | - | 2436, 2085 |
| Yes | 20.2% | (13.9-28.3) | 1.35 | (0.85-2.14) | 1.47 | (0.94-2.30) | 117, 86 |
Participants who reported at least one lifetime sexual partner, with urine test results (unweighted, weighted)
Index of Multiple Deprivation (IMD) is a multi-dimensional measure of area (neighbourhood)-level deprivation based on the participant’s postcode. IMD scores for England, Scotland and Wales were adjusted before being combined and assigned to quintiles, using a method by Payne and Abel (Payne and Abel, 2012).
Participants aged ≥17 years
Includes 29 women paying part mortgage & part rent (shared ownership)
Those of Chinese / Other ethnicity are excluded from the denominator due to small numbers
Binge drinking defined as having six units on one occasion
Includes both opposite-sex and same-sex partners
Defined as having used the oral contraceptive pill, hormonal IUD, injections, or implants
Defined as having been diagnosed with one of chlamydia, gonorrhoea, syphilis, genital herpes, genital warts, trichomonas, non-specific urethritis/non-gonococcal urethritis
HR-HPV was strongly associated with markers of more risky sexual behaviour including a younger age (≤16 years) at first heterosexual intercourse, increasing numbers of partners over the lifetime and in the past 5 years, as well as reporting two or more partners without a condom in the past year (AOR 4.31, 2.83-6.55). Prevalence was also higher in women who reported attending a sexual health (GUM) clinic (AOR 2.54, 2.00-3.23) or STI diagnosis/es (AOR 2.36, 1.76-3.16) in the past 5 years.
Cervical screening uptake
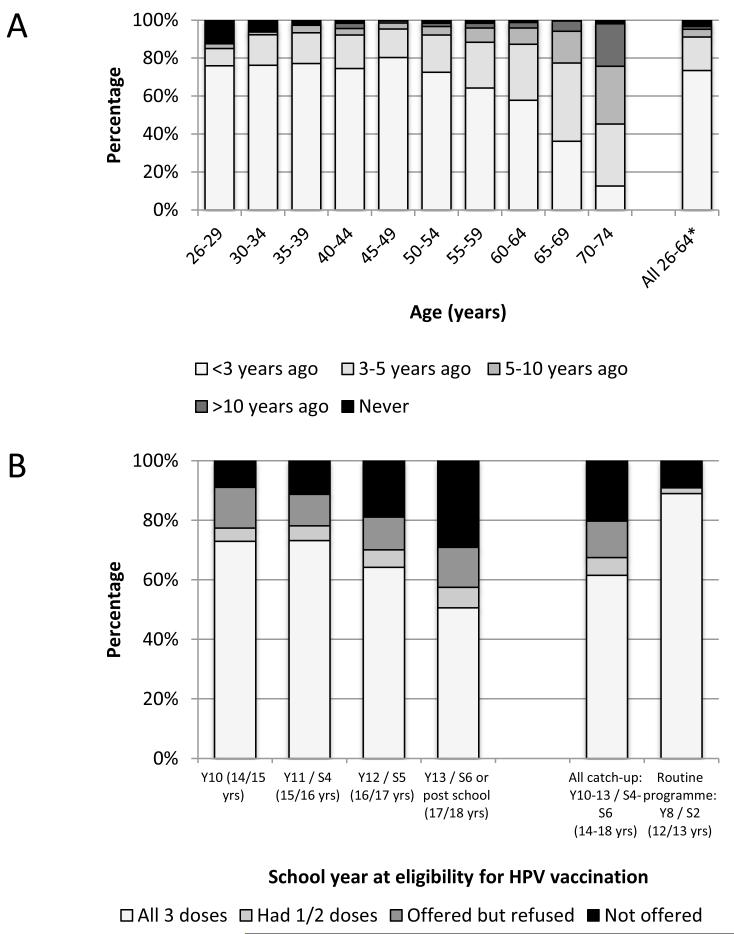
Figure 1A shows the time since last cervical screen in women aged 26-74 years. Overall, 96.8% of women aged 26-74 years reported ever having had a cervical screen. Over 70% of women aged 26-49 reported having attended screening within the last 3 years. Around 90% of women aged 50-64 years reported having attended for screening within the last 5 years. A notable proportion of 26-29 and 30-34 year olds reported never having had a cervical screen (12.1% and 5.9%, respectively),.
Figure 1.
Uptake of cervical cancer interventions. A) Time since last cervical smear test by age group among women aged 26-74 years; B) HPV vaccination uptake by school year at eligibility for vaccination in either the routine (Year 8/S2) or catch-up programmes
A) Women are eligible for cervical screening every 3-5 years depending on regional protocols (3 yearly to age 49 in England then 5 yearly to age 64; 3 yearly to age 64 in Wales and 3 yearly to age 60 in Scotland).
Denominators exclude women who report having had a hysterectomy & those with no lifetime sexual partners.
*All women in eligible age range for screening
Denominators (unwt., wt.) are: 26-29 (1121, 547), 30-34 (1025,648), 35-39 (580,664), 40-44 (571, 710), 45-49 (536, 694), 50-54 (427,553), 55-59 (399, 505), 60-64 (381,444), 65-69 (349, 387), 70-74 (225, 226), all eligible (5012, 4731)
Percentage screened in past 5 years when women reporting a hysterectomy are included in the denominator (N=5372, 5164) is 86.2%
B) Denominators (unwt., wt.) are: Y10 (153, 78), Y11 (244, 123), Y12 (238, 117), Y13 (415, 243), All catch-up (1050, 562), Routine (44, 21)
Table 2 shows factors associated with non-attendance for cervical screening in the past 5 years in women aged 26-64 (those eligible for screening), of which 8.9% (8.0-9.8) were non-attenders. Non-attendance was associated with a number of socio-demographic characteristics including younger (<30 years) or older (60+ years) age (OR 2.28, 1.72-3.00 and 2.01, 1.32-3.05, respectively, compared to those aged 30-39), lower socio-economic status, including area-level deprivation (AOR 1.91, 1.48-2.47 for most vs. least deprived two quintiles) and having no educational qualifications (AOR 1.95, 1.43-2.66), and being of Asian/Asian British ethnicity (AOR 1.96, 1.32-2.90). Women self-identifying as lesbian were more likely to be non-attenders (AOR 2.94, 1.36-6.38). Non-attendance was also strongly associated with being a current smoker (AOR 1.97, 1.57-2.47). The relationship with markers of risky sexual behaviour was not consistent. Overall, there was no association with age at first heterosexual intercourse or number of lifetime partners, although non-attendance was highest in those with one lifetime partner (11.4%). Women reporting no partners in the past 5 years (AOR 2.45, 1.67-3.61 vs. 1 partner), or no partners without a condom in the past year were more likely to be non-attenders. Non-attendance was lower in women who reported using hormonal contraceptives in the past year (AOR 0.53, 0.41-0.69) and in those who had ever attended a sexual health (GUM) clinic (AOR 0.53, 0.40-0.69) or had an STI diagnosis (AOR 0.49, 0.33-0.71). There was no difference in attendance by HR-HPV status overall (AOR 1.35, 0.79-2.31). Stratification of these analyses by age (<50 and 50+ years) and lifetime partners (1 and 2+) returned similar associations (data not shown).
Table 2.
Factors associated with non-attendance at cervical screening in the past 5 years in women aged 26-64 years
| Not in past 5 years | Not screened vs. screened in past 5 years | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| % | (95%CI) | OR | (95%CI) | Age adjusted OR | (95%CI) | Denom. (unwt, wt) a | |
| All ages | 8.9% | (8.0-9.8) | 5012, 4731 | ||||
|
| |||||||
| Socio-demographic characteristics | |||||||
|
| |||||||
| Age, years | p<0.0001 | ||||||
| 26-29 | 14.9% | (12.7-17.4) | 2.28 | (1.72-3.00) | 1121, 547 | ||
| 30-39 | 7.1% | (5.9-8.7) | 1 | - | 1605, 1312 | ||
| 40-49 | 6.2% | (4.9-8.0) | 0.86 | (0.61-1.21) | 1107, 1404 | ||
| 50-59 | 9.6% | (7.8-11.9) | 1.38 | (1.01-1.90) | 826, 1058 | ||
| 60-64 | 13.4% | (9.7-18.2) | 2.01 | (1.32-3.05) | 353, 411 | ||
| Relationship status at interview | p<0.0001 | p=0.0004 | |||||
| Living with a partner | 8.0% | (7.0-9.1) | 1 | - | 1 | - | 3151, 3476 |
| In a steady relationship (but not living with a partner) | 8.4% | (6.3-11.2) | 1.06 | (0.75-1.50) | 1.04 | (0.73 - 1.46) | 585, 373 |
| Previously in a live-in partnership | 11.5% | (9.4-14.0) | 1.50 | (1.14-1.96) | 1.43 | (1.09 - 1.87) | 1015, 717 |
| Not in a steady relationship (never lived with partner) | 18.5% | (13.1-25.4) | 2.61 | (1.71-3.99) | 2.31 | (1.49 - 3.57) | 234, 145 |
| Index of Multiple Deprivation (quintiles) b | p<0.0001 | p<0.0001 | |||||
| 1-2 (least deprived) | 6.3% | (5.2-7.6) | 1 | - | 1 | - | 1885, 1938 |
| 3 | 8.9% | (7.1-11.2) | 1.46 | (1.06-2.00) | 1.44 | (1.05 - 1.98) | 1003, 943 |
| 4-5 (most deprived) | 11.5% | (10.1-13.2) | 1.95 | (1.52-2.50) | 1.91 | (1.48 - 2.47) | 2124, 1850 |
| Academic qualifications | p<0.0001 | p=0.0001 | |||||
| No academic qualifications | 14.1% | (11.5-17.1) | 2.04 | (1.53-2.73) | 1.95 | (1.43 - 2.66) | 751, 764 |
| Academic qualifications typically gained at age 16 | 8.1% | (6.8-9.5) | 1.09 | (0.84-1.42) | 1.16 | (0.88 - 1.52) | 1828, 1730 |
| Studying for/attained further academic qualifications | 7.4% | (6.3-8.7) | 1 | - | 1 | - | 2278, 2102 |
| Housing tenure | p<0.0001 | p<0.0001 | |||||
| Own outright | 9.6% | (7.7-12.0) | 1.77 | (1.28-2.46) | 1.34 | (0.93 - 1.92) | 868, 1034 |
| Buying with a mortgage or loanc | 5.7% | (4.7-6.9) | 1 | - | 1 | - | 2092, 2118 |
| Rent it | 12.6% | (11.0-14.3) | 2.40 | (1.86-3.08) | 2.14 | (1.65 - 2.78) | 1967, 1505 |
| Lives rent free | 17.3% | (9.4-29.8) | 3.49 | (1.70-7.19) | 2.88 | (1.44 - 5.77) | 71, 59 |
| Respondent’s National Statistics Socio-Economic Classification | p<0.0001 | p<0.0001 | |||||
| Managerial & prof occupations | 6.4% | (5.3-7.7) | 1 | - | 1 | - | 1868, 1810 |
| Intermediate occupations | 7.0% | (5.5-8.8) | 1.10 | (0.80-1.53) | 1.07 | (0.77 - 1.49) | 1160, 1081 |
| Semi-routine/routine occupations | 11.8% | (10.0-13.9) | 1.96 | (1.49-2.59) | 1.88 | (1.42 - 2.49) | 1361, 1249 |
| No job (10+ hrs/week) or not in last 10 years | 14.8% | (11.4-18.9) | 2.54 | (1.77-3.65) | 2.40 | (1.66 - 3.47) | 475, 474 |
| Student in full-time education | 9.5% | (5.1-17.1) | 1.55 | (0.77-3.11) | 1.32 | (0.67 - 2.62) | 124, 95 |
| Ethnic group | p=0.0066 | p=0.0052 | |||||
| White | 8.3% | (7.4-9.2) | 1 | - | 1 | - | 4415, 4155 |
| Mixed | 11.7% | (5.7-22.4) | 1.46 | (0.68-3.17) | 1.49 | (0.68 - 3.25) | 89, 72 |
| Asian/Asian British | 15.1% | (10.9-20.6) | 1.97 | (1.32-2.93) | 1.96 | (1.32 - 2.90) | 254, 256 |
| Black/Black British | 11.8% | (6.8-19.6) | 1.48 | (0.81-2.71) | 1.62 | (0.88 - 2.97) | 174, 176 |
| Other | 12.5% | (6.5-22.7) | 1.58 | (0.78-3.24) | 1.52 | (0.73 - 3.16) | 69, 63 |
| Religion | p=0.0076 | p=0.0049 | |||||
| None | 9.4% | (8.2-10.9) | 1 | - | 1 | - | 2330, 2052 |
| Christian - Church of England/Anglican | 6.3% | (4.8-8.4) | 0.65 | (0.46-0.92) | 0.60 | (0.42 - 0.86) | 832, 906 |
| Christian - Roman Catholic | 7.9% | (5.9-10.6) | 0.83 | (0.58-1.19) | 0.80 | (0.55 - 1.16) | 582, 558 |
| Christian - other | 9.2% | (7.2-11.7) | 0.97 | (0.72-1.32) | 0.93 | (0.68 - 1.28) | 930, 903 |
| Muslim | 13.9% | (8.8-21.1) | 1.55 | (0.91-2.63) | 1.50 | (0.88 - 2.56) | 160, 152 |
| Hindu | 19.6% | (11.2-32.1) | 2.34 | (1.20-4.57) | 2.21 | (1.13 - 4.32) | 68, 57 |
| Other | 8.6% | (3.8-18.5) | 0.91 | (0.38-2.18) | 0.91 | (0.40 - 2.08) | 102, 94 |
| Sexual identity | p=0.0271 | p=0.0234 | |||||
| Heterosexual/straight | 8.7% | (7.9-9.7) | 1 | - | 1 | - | 4849, 4599 |
| Gay/lesbian | 20.9% | (11.1-35.7) | 2.76 | (1.31-5.78) | 2.94 | (1.36-6.38) | 63, 56 |
| Bisexual | 8.3% | (3.9-16.5) | 0.94 | (0.43-2.05) | 0.93 | (0.44-1.98) | 75, 53 |
|
| |||||||
| Health behaviours | |||||||
|
| |||||||
| Smoking status | p<0.0001 | p<0.0001 | |||||
| Non/Ex-smoker | 7.5% | (6.6-8.5) | 1 | - | 1 | - | 3700, 3646 |
| Current smoker | 13.5% | (11.5-15.7) | 1.92 | (1.54-2.40) | 1.97 | (1.57 - 2.47) | 1312, 1085 |
| Frequency of binge drinking d | p=0.0277 | p=0.0473 | |||||
| Never / less than monthly | 9.5% | (8.5-10.6) | 1 | - | 1 | - | 3769, 3636 |
| Monthly | 6.7% | (5.0-9.0) | 0.69 | (0.49-0.96) | 0.69 | (0.49 - 0.97) | 664, 568 |
| Weekly or more often | 7.1% | (5.3-9.4) | 0.73 | (0.52-1.02) | 0.77 | (0.55 - 1.09) | 578, 527 |
|
| |||||||
| Sexual behaviours | |||||||
|
| |||||||
| Age at first heterosexual sex (years) | p=0.3000 | p=0.5485 | |||||
| 18+ | 9.7% | (8.3-11.2) | 1 | - | 1 | - | 1971, 2033 |
| 16/17 | 8.2% | (6.9-9.7) | 0.83 | (0.65-1.06) | 0.89 | (0.69 - 1.14) | 1943, 1825 |
| <16 | 8.4% | (6.7-10.5) | 0.86 | (0.64-1.15) | 0.87 | (0.64 - 1.18) | 1040, 812 |
| No. of sexual partners, lifetime e | p=0.0612 | p=0.2391 | |||||
| 1 | 11.4% | (9.3-13.9) | 1 | - | 1 | - | 832, 923 |
| 2 | 9.4% | (7.0-12.4) | 0.80 | (0.54-1.19) | 0.81 | (0.55 - 1.21) | 468, 478 |
| 3-4 | 8.5% | (6.8-10.6) | 0.72 | (0.51-1.02) | 0.77 | (0.54 - 1.09) | 920, 890 |
| 5-9 | 8.0% | (6.5-9.9) | 0.68 | (0.49-0.94) | 0.74 | (0.54 - 1.03) | 1338, 1246 |
| 10+ | 7.5% | (6.0-9.3) | 0.63 | (0.45-0.87) | 0.68 | (0.48 - 0.96) | 1367, 1105 |
| No. of sexual partners, past 5 years e | p<0.0001 | p<0.0001 | |||||
| 0 | 19.3% | (15.0-24.5) | 2.72 | (1.94-3.82) | 2.45 | (1.67-3.61) | 358, 342 |
| 1 | 8.1% | (7.1-9.2) | 1 | - | 1 | - | 3133, 3311 |
| 2 | 8.2% | (6.1-10.9) | 1.02 | (0.72-1.45) | 0.94 | (0.66-1.34) | 625, 471 |
| 3-4 | 7.1% | (5.1-9.8) | 0.87 | (0.60-1.25) | 0.73 | (0.50-1.05) | 489, 328 |
| 5+ | 8.2% | (5.6-12.0) | 1.02 | (0.66-1.58) | 0.77 | (0.49-1.22) | 349, 216 |
| No. of sexual partners without a condom, past year e | p<0.0001 | p<0.0001 | |||||
| 0 | 14.1% | (12.0-16.6) | 1 | - | 1 | - | 1263, 1136 |
| 1 | 7.1% | (6.2-8.1) | 0.46 | (0.37-0.59) | 0.48 | (0.38 - 0.62) | 3420, 3358 |
| 2+ | 8.2% | (5.0-12.9) | 0.54 | (0.31-0.93) | 0.50 | (0.28 - 0.89) | 259, 163 |
|
| |||||||
| Health-related factors | |||||||
|
| |||||||
| Used hormonal contraceptionf, past year | p=0.0001 | p<0.0001 | |||||
| No | 9.8% | (8.7-10.9) | 1 | - | 1 | - | 3369, 3489 |
| Yes | 6.3% | (5.1-7.6) | 0.62 | (0.48-0.79) | 0.53 | (0.41 - 0.69) | 1573, 1168 |
| Ever attended a sexual health (GUM) clinic | p=0.0002 | p<0.0001 | |||||
| No | 9.7% | (8.7-10.8) | 1 | - | 1 | - | 3611, 3636 |
| Yes | 6.0% | (4.8-7.5) | 0.60 | (0.46-0.78) | 0.53 | (0.40 - 0.69) | 1353, 1041 |
| Ever diagnosed with a STI g | p=0.0004 | p=0.0002 | |||||
| No (or only thrush) | 9.5% | (8.6-10.6) | 1 | - | 1 | - | 4080, 3958 |
| Yes (excluding thrush) | 5.1% | (3.7-7.1) | 0.51 | (0.35-0.74) | 0.49 | (0.33 - 0.71) | 882, 717 |
| STI risk: to self | p=0.0377 | p=0.0200 | |||||
| Greatly at risk / Quite a lot | 5.5% | (2.9-10.0) | 1 | - | 1 | - | 130, 97 |
| Not very much | 7.1% | (5.6-9.1) | 1.33 | (0.65-2.71) | 1.34 | (0.67 - 2.67) | 903, 715 |
| Not at all at risk | 9.3% | (8.3-10.3) | 1.78 | (0.92-3.44) | 1.83 | (0.97 - 3.48) | 3958, 3900 |
|
| |||||||
| All women aged 26-44 who haven’t had a hysterectomy & who provided a urine sample | |||||||
|
| |||||||
| 1+ high-risk HPV type(s) | 10.6% | (8.7-12.8) | p=0.2062 | p=0.2775 | 1474, 1512 h | ||
| Negative | 10.1% | (8.1-12.5) | 1 | - | 1 | - | 1243, 1329 |
| Positive | 13.7% | (9.0-20.5) | 1.42 | (0.83-2.44) | 1.35 | (0.79 - 2.31) | 231, 184 |
Participants who haven’t had a hysterectomy & who reported at least 1 lifetime sexual partner (unweighted, weighted)
Index of Multiple Deprivation (IMD) is a multi-dimensional measure of area (neighbourhood)-level deprivation based on the participant’s postcode. IMD scores for England, Scotland and Wales were adjusted before being combined and assigned to quintiles, using a method by Payne and Abel (Payne and Abel, 2012).
Includes 46 women paying part mortgage & part rent (shared ownership)
Binge drinking defined as having six units on one occasion
Includes both opposite-sex and same-sex partners
Defined as having used the oral contraceptive pill, hormonal IUD, injections, or implants
Defined as having been diagnosed with one of chlamydia, gonorrhoea, syphilis, genital herpes, genital warts, trichomonas, non-specific urethritis/non-gonococcal urethritis
Participants aged 26-44 years who haven’t had a hysterectomy, who reported at least 1 lifetime sexual partner & who provided a urine sample
There were two distinct groups of non-attending women (Table 3). Overall, a quarter of non-attenders reported only 1 lifetime partner. A high proportion of these women were of Asian/Asian British ethnicity (25.5%, 17.2%-36.1%), few smoked (20.3%, 12.6%-31.1%), less than 1% reported first heterosexual intercourse before 16 years and 20.3% (12.6%-31.1%) reported no sexual partner in the past 5 years. Prevalence of HR-HPV in those providing a urine sample was 5.2% (1.4%-17.2%). In contrast, among the three-quarters of non-attenders reporting 2 or more lifetime partners, 89.6% (85.3%-92.7%) were of White ethnicity, 39.8% (34.4%-45.4%) were smokers and 21.7% (17.3%-26.8%) reported first heterosexual intercourse before 16 years. However, a similar proportion reported no partner in the past 5 years (14.5%, 10.6%-19.4%). Prevalence of HR-HPV in non-attenders providing a urine sample with 2 or more lifetime partners was 20.3% (12.9%-30.5%). This was non-significantly higher than the prevalence in attenders with 2 or more lifetime partners (13.3%, 11.3%-15.7%; p=0.079).
Table 3.
Key characteristics of women who have not attended for cervical screening in the past 5 years, by number of lifetime partners
| Not attended for screening in past 5 years | ||||||
|---|---|---|---|---|---|---|
| All not attended (100%) | 1 lifetime partnera (25%) | 2+ lifetime partnersa (75%) | ||||
| % | (95%CI) | % | (95%CI) | % | (95%CI) | |
| Denom. (unweighted, weighted) | 496, 420 | 111, 105 | 385, 314 | |||
| Age, years | ||||||
| 26-29 | 19.4% | (16.3-22.9) | 18.4% | (12.6-26.0) | 19.8% | (16.3-23.8) |
| 30-39 | 22.3% | (18.5-26.7) | 26.5% | (18.3-36.6) | 21.0% | (16.8-25.9) |
| 40-49 | 20.8% | (16.6-25.8) | 14.3% | (7.2-26.3) | 23.0% | (18.2-28.7) |
| 50-59 | 24.3% | (19.9-29.3) | 20.3% | (12.4-31.5) | 25.6% | (20.5-31.5) |
| 60-64 | 13.1% | (9.6-17.6) | 20.6% | (12.5-31.9) | 10.6% | (7.1-15.5) |
| Index of Multiple Deprivation (quintiles) c | p=0.4394b | |||||
| 1-2 (least deprived) | 29.1% | (24.4-34.2) | 28.8% | (20.3-39.2) | 29.1% | (23.8-35.1) |
| 3 | 20.0% | (16.0-24.7) | 15.7% | (10.0-23.9) | 21.5% | (16.7-27.2) |
| 4-5 (most deprived) | 50.9% | (45.7-56.1) | 55.5% | (44.9-65.6) | 49.4% | (43.4-55.4) |
| Academic qualifications | p=0.1289b | |||||
| No academic qualifications | 26.7% | (22.1-31.8) | 34.7% | (24.2-46.9) | 24.1% | (19.4-29.7) |
| Academic qualifications typically gained at age 16 | 34.6% | (29.6-39.9) | 26.4% | (17.4-38.0) | 37.2% | (31.5-43.2) |
| Studying for/attained further academic qualifications |
38.7% | (33.6-44.1) | 38.9% | (28.4-50.6) | 38.7% | (32.9-44.8) |
| Ethnic group | p<0.0001b | |||||
| White | 82.0% | (77.5-85.8) | 59.3% | (47.9-69.8) | 89.6% | (85.3-92.7) |
| Mixed | 2.0% | (1.0-4.1) | 1.4% | (0.3-5.8) | 2.2% | (1.0-4.9) |
| Asian/Asian British | 9.2% | (6.5-12.8) | 25.5% | (17.2-36.1) | 3.7% | (2.1-6.6) |
| Black/Black British | 4.9% | (2.8-8.5) | 9.4% | (3.8-21.2) | 3.5% | (1.7-6.9) |
| Other | 1.9% | (1.0-3.5) | 4.5% | (1.9-9.9) | 1.0% | (0.4-2.7) |
| Smoking status | p=0.0022b | |||||
| Non/Ex-smoker | 65.1% | (60.2-69.7) | 79.7% | (68.9-87.4) | 60.2% | (54.6-65.6) |
| Current smoker | 34.9% | (30.3-39.8) | 20.3% | (12.6-31.1) | 39.8% | (34.4-45.4) |
| Age at first heterosexual sex (years) | p<0.0001b | |||||
| 18+ | 47.4% | (42.4-52.6) | 81.6% | (71.3-88.8) | 36.1% | (30.6-41.9) |
| 16/17 | 36.1% | (31.2-41.2) | 17.5% | (10.4-27.9) | 42.3% | (36.5-48.3) |
| <16 | 16.5% | (13.1-20.5) | 0.9% | (0.3-2.9) | 21.7% | (17.3-26.8) |
| Sexual partner, past 5 years a | p=0.2239b | |||||
| No | 15.9% | (12.2-20.3) | 20.3% | (12.5-31.4) | 14.4% | (10.6-19.2) |
| Yes | 84.1% | (79.7-87.8) | 79.7% | (68.6-87.5) | 85.6% | (80.8-89.4) |
|
| ||||||
| Denom. (unwt, wt)d | 148, 160 | 31, 47 | 117, 112 | |||
| 1+ high-risk HPV type(s) | p=0.0216b | |||||
| Negative | 84.2% | (76.2-89.8) | 94.8% | (82.8-98.6) | 79.7% | (69.5-87.1) |
| Positive | 15.8% | (10.2-23.8) | 5.2% | (1.4-17.2) | 20.3% | (12.9-30.5) |
Includes both opposite-sex and same-sex partners
P-values for comparison between non-attenders with 1 and 2+ lifetime partners
Index of Multiple Deprivation (IMD) is a multi-dimensional measure of area (neighbourhood)-level deprivation based on the participant’s postcode. IMD scores for England, Scotland and Wales were adjusted before being combined and assigned to quintiles, using a method by Payne and Abel (Payne and Abel, 2012).
Non-attenders aged 26-44 years who provided a urine sample
We looked at the reported recent use of healthcare services among non-attenders. Overall, 6.1% (4.3%-8.5%) of non-attenders had been to a sexual health (GUM) clinic in the past 5 years, 14.3% (11.2%-18.0%) had attended an ante-natal clinic in the past 5 years and 19.2% (15.8%-23.1%) had obtained family planning from a clinical source in the past year. In total, 31.7% (27.1%-36.7%) of non-attending women had used one or more of these services. Use of healthcare services did not vary by lifetime partners.
HPV vaccine uptake
HPV catch-up vaccine uptake varied substantially by school year at eligibility (Figure 1B) with 72.9% of women eligible at 14 years reporting having received all 3 doses, compared with only 50.6% of women eligible at 17 years. In contrast, 89.0% of women in the routine programme reported having received all 3 doses (but denominators are small). Few women had received only one or two doses. The proportion of women who reported not having been offered the vaccine was higher in the older catch-up cohorts.
Of women eligible for the HPV catch-up immunisation programme, 38.5% reported not having completed the vaccination course. This was strongly associated with markers of lower socio-economic status (Table 4), non-white ethnicity (AOR 2.01, 1.29-3.13) and smoking (AOR 2.61, 1.93-3.55). Non-completion was also associated with reporting larger numbers of lifetime partners (AOR 1.70, 1.09-2.63 for 5+ vs. 1 lifetime partner). Among those with at least one lifetime partner, non-completion was higher in women reporting first heterosexual intercourse before 16 (AOR 1.68, 1.22-2.30) and unprotected sex with two or more partners in the past year (AOR 1.81, 1.15-2.84). Those using hormonal contraception were less likely to be non-completers (AOR 0.47, 0.34-0.67), while those attending sexual health (GUM) clinics (AOR 1.49, 1.10 2.02) and ever having been pregnant (AOR 2.94, 2.04-4.23) were more likely to report non-completion. Non-completion was higher in women who were HR-HPV positive (AOR 2.33, 1.45-3.74).
Table 4.
Factors associated with non-completion of HPV catch-up vaccination
| Not completed | Not completed vs. completed | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| % | (95%CI) | OR | (95%CI) | Age adjusted OR | (95%CI) | Denom. (unwt, wt) | ||
| All eligible for HPV catch-up vaccination programme | 38.5% | (35.3-41.9) | 1050, 562 | |||||
|
| ||||||||
| Socio-demographic factors | ||||||||
|
| ||||||||
| Age at interview (years) | p<0.0001 | |||||||
| 16-17 | 28.0% | (23.2-33.4) | 1 | - | 394, 195 | |||
| 18-19 | 41.7% | (36.7-47.0) | 1.84 | (1.33-2.56) | 449, 241 | |||
| 20-24 | 48.7% | (41.3-56.1) | 2.44 | (1.64-3.63) | 207, 125 | |||
| School year at eligibility for HPV vaccination programme | p<0.0001 | p=0.0060 | ||||||
| 14 (Y10/S3) | 27.1% | (20.1-35.3) | 1 | - | 1 | - | 153, 78 | |
| 15(Y11/S4) | 26.8% | (21.1-33.3) | 0.99 | (0.61-1.59) | 1.01 | (0.62-1.65) | 244, 123 | |
| 16(Y12/S5) | 35.8% | (29.5-42.6) | 1.50 | (0.94-2.39) | 1.57 | (0.90-2.74) | 238, 117 | |
| 17 (Y13/S6 or post school) | 49.4% | (44.1-54.8) | 2.64 | (1.69-4.10) | 2.87 | (1.39-5.95) | 415, 243 | |
| Grouped government office region | p<0.0001 | p<0.0001 | ||||||
| Rest of England | 36.4% | (32.7-40.2) | 1 | - | 1 | - | 803, 421 | |
| London | 62.4% | (52.3-71.5) | 2.90 | (1.87-4.50) | 2.76 | (1.77-4.30) | 100, 66 | |
| Scotland | 19.8% | (13.1-28.8) | 0.43 | (0.26-0.72) | 0.41 | (0.24-0.70) | 89, 46 | |
| Wales | 44.4% | (31.0-58.6) | 1.4 | (0.78-2.48) | 1.31 | (0.74-2.35) | 58, 29 | |
| Index of Multiple Deprivation (quintiles)a | p<0.0001 | p=0.0001 | ||||||
| 1-2 (least deprived) | 30.1% | (25.3-35.4) | 1 | - | 1 | - | 393, 210 | |
| 3 | 36.7% | (29.4-44.7) | 1.34 | (0.90-2.01) | 1.35 | (0.90-2.04) | 209, 116 | |
| 4-5 (most deprived) | 46.9% | (42.0-51.9) | 2.05 | (1.50-2.81) | 1.99 | (1.44-2.74) | 448, 236 | |
| Parents social class | p=0.0308 | p=0.0285 | ||||||
| I/II/III | 35.1% | (31.2-39.2) | 1 | - | 1 | - | 714, 385 | |
| IV/V | 44.9% | (37.0-53.1) | 1.51 | (1.04-2.19) | 1.52 | (1.05-2.21) | 196, 103 | |
| Academic qualifications b | p<0.0001 | p<0.0001 | ||||||
| No academic qualifications | 75.2% | (55.9-87.9) | 6.04 | (2.63-13.85) | 5.84 | (2.50-13.62) | 39, 18 | |
| Academic qualifications typically gained at age 16 | 57.2% | (49.3-64.7) | 2.66 | (1.85-3.83) | 2.52 | (1.75-3.65) | 188, 92 | |
| Studying for/attained further academic qualifications | 33.4% | (29.5-37.6) | 1 | - | 1 | - | 650, 361 | |
| Ethnic group | p=0.0015 | p=0.0001 | ||||||
| White | 36.3% | (32.9-39.7) | 1 | - | 1 | - | 937, 491 | |
| Non-white | 54.1% | (43.5-64.5) | 2.07 | (1.32-3.25) | 2.01 | (1.29-3.13) | 113, 71 | |
|
| ||||||||
| Health behaviours | ||||||||
|
| ||||||||
| Smoking status | p<0.0001 | p<0.0001 | ||||||
| Non/ex-smoker | 31.8% | (28.3-35.7) | 1 | - | 1 | - | 737, 400 | |
| Current smoker | 55.0% | (48.9-61.0) | 2.62 | (1.95-3.53) | 2.61 | (1.93-3.55) | 313, 162 | |
| Frequency of binge drinking c | p=0.0665 | p=0.1886 | ||||||
| Never / less than monthly | 36.8% | (32.9-41.0) | 1 | - | 1 | - | 712, 376 | |
| Monthly | 36.9% | (30.0-44.5) | 1.00 | (0.71-1.43) | 0.90 | (0.62-1.30) | 200, 107 | |
| Weekly or more often | 48.4% | (39.4-57.6) | 1.61 | (1.07-2.42) | 1.41 | (0.92-2.15) | 137, 78 | |
|
| ||||||||
| Sexual behaviours (all eligible for catch-up vaccination) | ||||||||
|
| ||||||||
| Number of sexual partners, lifetime d | p<0.0001 | p=0.0107 | ||||||
| 0 | 24.5% | (18.4-31.7) | 0.62 | (0.38-1.01) | 0.72 | (0.43-1.18) | 205, 109 | |
| 1 | 34.4% | (27.1-42.5) | 1 | - | 1 | - | 203, 113 | |
| 2 | 37.6% | (29.1-46.8) | 1.15 | (0.69-1.89) | 1.12 | (0.68-1.84) | 147, 77 | |
| 3-4 | 39.6% | (31.6-48.2) | 1.25 | (0.77-2.03) | 1.22 | (0.75-2.00) | 171, 93 | |
| 5+ | 49.9% | (43.6-56.1) | 1.89 | (1.23-2.91) | 1.70 | (1.09-2.63) | 317, 167 | |
| All eligible for HPV catch-up vaccination programme with 1+ lifetime partner d | 41.9% | (38.3-45.6) | 843, 451 | |||||
|
| ||||||||
| Sexual behaviours (those with 1+ lifetime partner) | ||||||||
|
| ||||||||
| Had heterosexual sex before 16 | p=0.0088 | p=0.0014 | ||||||
| No | 37.5% | (32.7-42.6) | 1 | - | 1 | - | 456, 252 | |
| Yes | 47.7% | (42.0-53.4) | 1.52 | (1.11-2.07) | 1.68 | (1.22-2.30) | 355, 181 | |
| Number of sexual partners, past year d | p=0.3294 | p=0.2689 | ||||||
| 0/1 | 39.7% | (35.0-44.6) | 1 | - | 1 | - | 475, 260 | |
| 2 | 44.8% | (36.2-53.6) | 1.23 | (0.82-1.84) | 1.28 | (0.85-1.93) | 156, 78 | |
| 3+ | 45.7% | (38.2-53.3) | 1.28 | (0.89-1.84) | 1.3 | (0.90-1.88) | 203, 108 | |
| Number of sexual partners without a condom, past year d | p=0.0092 | p=0.0065 | ||||||
| 0 | 38.8% | (31.0-47.2) | 1 | - | 1 | - | 196, 106 | |
| 1 | 39.6% | (34.7-44.7) | 1.03 | (0.68-1.56) | 1.03 | (0.69-1.55) | 443, 238 | |
| 2+ | 53.1% | (45.1-60.9) | 1.79 | (1.13-2.83) | 1.83 | (1.16-2.88) | 185, 98 | |
|
| ||||||||
| Health-related factors | ||||||||
|
| ||||||||
| Used hormonal contraception, past year e | p<0.0001 | p<0.0001 | ||||||
| No | 54.3% | (47.1-61.4) | 1 | - | 1 | - | 235, 131 | |
| Yes | 36.4% | (32.4-40.7) | 0.48 | (0.34-0.68) | 0.47 | (0.34-0.67) | 570, 299 | |
| Ever attended a sexual health (GUM) clinic | p=0.0044 | p=0.0100 | ||||||
| No | 37.2% | (32.6-42.1) | 1 | - | 1 | - | 462, 251 | |
| Yes | 47.8% | (42.3-53.3) | 1.54 | (1.15-2.08) | 1.49 | (1.10-2.02) | 377, 199 | |
| Ever diagnosed with an STI (excluding thrush) f | p=0.1735 | p=0.4147 | ||||||
| No (or only thrush) | 41.0% | (37.1-45.0) | 1 | - | 1 | - | 730, 395 | |
| Yes | 48.5% | (38.3-58.8) | 1.36 | (0.87-2.10) | 1.2 | (0.77-1.88) | 109, 55 | |
| Ever been pregnant | p<0.0001 | p<0.0001 | ||||||
| No | 35.4% | (31.4-39.8) | 1 | - | 1 | - | 633, 346 | |
| Yes | 63.4% | (55.9-70.2) | 3.15 | (2.21-4.49) | 2.94 | (2.04-4.23) | 210, 105 | |
| All eligible for HPV catch-up vaccination programme with 1+ lifetime partnerd who provided a urine sample | 41.0% | (36.1-46.1) | 481, 273 | |||||
|
| ||||||||
| HPV markers in urine | ||||||||
|
| ||||||||
| HPV positive | p=0.0302 | p=0.0383 | ||||||
| HPV negative | 36.0% | (29.3-43.2) | 1 | - | 1 | - | 253, 152 | |
| HPV positive | 47.2% | (40.0-54.6) | 1.6 | (1.04-2.44) | 1.57 | (1.02-2.40) | 228, 121 | |
| 1+ high-risk HPV type(s) | p=0.0003 | p=0.0005 | ||||||
| Negative | 35.3% | (29.6-41.4) | 1 | - | 1 | - | 347, 200 | |
| Positive | 56.6% | (46.8-65.9) | 2.39 | (1.49-3.83) | 2.33 | (1.45-3.74) | 134, 73 | |
Index of Multiple Deprivation (IMD) is a multi-dimensional measure of area (neighbourhood)-level deprivation based on the participant’s postcode. IMD scores for England, Scotland and Wales were adjusted before being combined and assigned to quintiles, using a method by Payne and Abel (Payne and Abel, 2012).
Participants aged >17 years
Binge drinking defined as having six units on one occasion
Includes both opposite-sex and same-sex partners
Defined as having used the oral contacepive pill, hormonal IUD, injections, or implants
Defined as having been diagnosed with one of chlamydia, gonorrhoea, syphilis, genital herpes, genital warts, trichomonas, non-specific urethritis/non-gonococcal urethritis
Associations with having had no doses of the vaccine were similar (data not shown), although a stronger association was seen with area-level deprivation and slightly weaker associations with sexual behaviours, GUM clinic attendance and ever having been pregnant.
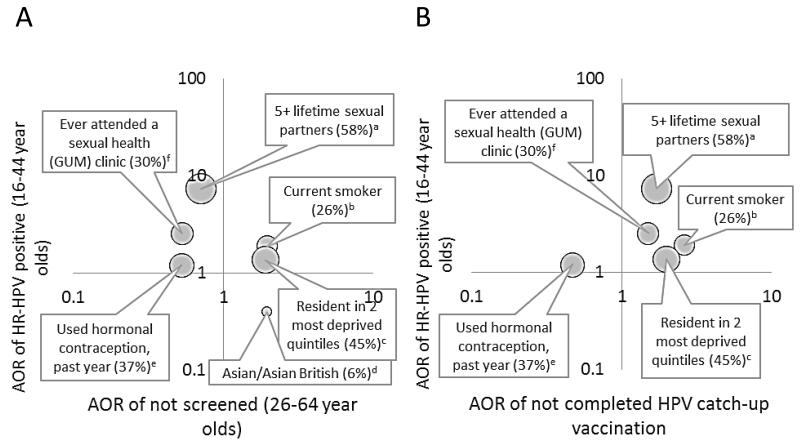
Overlap between factors associated with HR-HPV infection and uptake of cervical screening & HPV vaccination
Figure 2 shows factors associated with HR-HPV infection (vertical axes) plotted against factors associated with non-attendance for cervical screening (Figure 2A) and non-completion of HPV vaccination (Figure 2B). The top right hand quadrant for each figure indicates increased risk of HR-HPV infection and lower uptake of the cervical cancer prevention programme. The area of the bubble represents the size of the group as a proportion of those eligible for screening. There was evidence of overlap of HR-HPV infection risk and cervical screening uptake for some factors (Figure 2A). Living in more deprived areas and smoking were associated with both HR-HPV infection and non-attendance for cervical screening. These factors were also associated with non-completion of HPV vaccination (Figure 2B). Associations between smoking and HR-HPV infection, and uptake of cervical screening and HPV vaccination persisted after adjustment for area-level deprivation (data not shown). In contrast, HR-HPV prevalence was lower in women of Asian/Asian British ethnicity, another group less likely to attend for screening (Figure 2A). Women with 5 or more lifetime partners and those who reported attending a sexual health (GUM) clinic, had a higher prevalence of HR-HPV infection, and were more likely to have attended for cervical screening but less likely to have completed HPV vaccination.
Figure 2.
Relationship between risk factors for HR-HPV and uptake of cervical cancer programmes: (A) cervical screening and (B) HPV catch-up vaccination
All ORs adjusted for age. 95%CIs for AORs exclude 1 with the exception of the association between hormonal contraception use and HR-HPV (see Tables 1, 2 and 4).
Top right quadrant for each graph indicates increased risk of HR-HPV and lower uptake of cervical cancer prevention programme. The area of the bubble represents the size of the group as a proportion of those eligible for screening. Letters indicate reference groups: a) 1 lifetime sexual partner; b) non/ex-smoker; c) resident in 2 least deprived quintiles; d) White/White British; e) Not used hormonal contraception, past year; f) Never attended a sexual health (GUM) clinic
Discussion
In this cross-sectional probability-sample survey of the British general population we found markers of lower socio-economic status and smoking to be common risk factors for HR-HPV infection and non-uptake of both cervical screening and HPV catch-up vaccination. Overall, cervical screening attendance was not lower in women reporting more risky sexual behaviours and there was no difference in attendance by HR-HPV status. However, our analysis suggests that there are two distinct groups of non-attenders, one of which would be considered at higher risk of developing cervical cancer due to high prevalence of other lifestyle risk factors such as smoking and early age at first sex, whose non-attendance might augment their overall risk of cervical cancer, and one of which would be considered lower risk, whose non-attendance might negate their lower lifestyle risk.
The major strength of this study is that it is a population-based survey with individual-level data from a nationally representative sample. We were able to link behavioural and biological data and look at risk factors for different outcomes in the same survey. One limitation is the accuracy of self-reporting, especially of cervical screening (26,27). Our estimates of cervical screening uptake are higher than official figures, which estimate 5-year coverage in 2011-12 as 78.6% (6), and one other study (28), which asked for year and month of last cervical screen. We believe that social desirability bias is unlikely to have had a substantial effect since this question was asked in the self-completion part of the questionnaire. However, ‘telescoping’, where an event is remembered as occurring more recently than it did, is a strong possibility both for us and other studies (27,29). Any variation in such a bias by the socio-demographic or behavioural variables that we report could mean that we have over- or under-estimated associations, for example, if telescoping errors were greater amongst more educated women, the association between attendance and education would be over-estimated. Women may also not be able to accurately report their vaccination status (30) and accurate reporting may vary by other variables. Uptake estimates may be affected by biases in the women who agreed to participate in Natsal-3. The Natsal-3 response rate was 57.7%, which is comparable with other population-based surveys completed around the same time (31,32). After weighting our data to match the British population for age, gender and geographic region, the sample was comparable with the 2011 census data on other key demographic characteristics (18). However, women who do not attend for screening may be less likely to participate in research studies or engage more generally (33).
Another limitation is that urine is a suboptimum specimen for HPV detection (34) with recent estimates of 77% sensitivity of cervical HR-HPV (35) and therefore a likely underestimate of HR-HPV prevalence, although this would weaken, not bias, our identification of risk factors. Finally, due to the years the Natsal-3 fieldwork was carried out, our study could only focus on the catch-up programme, and the factors we describe as associated with vaccination uptake in the catch-up cohorts may not be generalizable to routine vaccination at 12 years of age.
To our knowledge, no population-based studies have examined the associations between cervical screening and sexual behaviour or HR-HPV infection. We found lower screening uptake among women with lower levels of education and of non-White ethnicity as in other British population studies (21,28). Other studies have shown lower uptake of HPV catch-up vaccination in women of Black/Black British and Asian/Asian British ethnicity (36,37). Our sample of women of these ethnic minorities was too small to examine associations between vaccination and each ethnic group but completion of catch-up vaccination was lower in women of non-White ethnicity.
It is a reasonable expectation that herd immunity should lead to a reduction in cervical cancer incidence among unvaccinated women in the catch-up vaccination cohorts (38). However, the effect of multiple risks in some groups of women has the potential to widen inequalities in cervical cancer incidence. Women who live in more deprived areas and who smoke were less likely to complete catch-up vaccination. These women were also at higher risk of HR-HPV and their cervical cancer risk is compounded by smoking, which is itself a cofactor in cervical cancer development (39). Additionally, these women were less likely to attend for cervical screening, thereby losing the opportunity for early detection and treatment of cancer abnormalities. Special efforts may be warranted to ensure women who missed vaccination are engaged by the cervical screening programme, especially since girls with low intentions to attend for cervical screening may be less likely to be fully-vaccinated (40). Good linkage between vaccination and screening records will be important in order to target those not vaccinated.
As some non-attenders for cervical screening seem to be at low risk for HR-HPV, tailored approaches may be appropriate to increase screening among higher risk women. On the other hand there is evidence of lower uptake of cervical screening among women who may be considered at lower risk for cervical cancer or may perceive themselves to be. For example, as in other studies, we found lower uptake in women self-identifying as lesbian (41,42). Previous studies have also found that women who are not sexually active are less likely to attend for screening (33). Cervical screening prevents approximately 75% of cervical cancers by detecting and treating cervical abnormalities in women who attend regularly (5,43). The odds of cervical cancer are approximately six times higher in women with no adequate screens at age 50-64 compared to those with adequate negative screening (44) so despite being at lower relative risk for cervical cancer, by missing the prevention opportunity offered by cervical screening these women may end up at increased risk. Although they have a lower incidence of cervical cancer overall, Asian/Asian British women aged 65 and over have a higher incidence than do women of White ethnicity (45). Since these women are unlikely to access sexual health services, engaging them in screening through general practice (family doctor) is important. The cervical screening programme also needs to counter this risk-based tendency for non-participation. This will be particularly important in the era of vaccination, where careful messaging will be needed to promote uptake of screening among those who may perceive themselves at less risk.
Overall those at increased risk of HR-HPV were no more or less likely to attend for screening. We found markers of engagement with healthcare, such as sexual health (GUM) clinic attendance and using hormonal contraception, were associated with higher cervical screening attendance. In 2011-12, 17% of women having a cervical screen in England had a test which was outside the invitation system of the cervical screening programme, i.e. opportunistic tests which were initiated by the person taking the sample or by the woman (46). This underlines the importance of maintaining integrated sexual health services to ensure that screening levels remain high in those at highest risk. However, around 30% of women who had not attended cervical screening in the past 5 years reported attending ante-natal or sexual health (GUM) clinics in the past 5 years or obtaining contraceptives from clinical sources in the past year, suggesting missed opportunities to engage these women with cervical screening.
Changes to the cervical screening programme are likely in coming years, due both to HPV immunisation effects on HPV epidemiology and the use of HPV testing in screening algorithms. HPV testing has already been introduced to help manage women with borderline and mildly abnormal cytology results. A pilot of HPV testing as the primary screening test (in place of cytology) is currently underway (46). It is unclear how changes will impact cervical screening uptake.
To date, there are few data relating to HPV vaccination uptake in the routine cohorts by the variables we have explored. It will be important to study factors associated with routine HPV vaccination uptake in the same way. Uptake of cervical screening among women who have not received HPV vaccination should be studied as these women reach screening age.
As some non-attenders for cervical screening seem to be at low risk for HR-HPV, tailored approaches may be appropriate to increase screening among higher-risk women. Socio-economic markers and smoking were associated with HR-HPV positivity, non-completion of catch-up HPV vaccination and non-attendance for cervical screening. This highlights the importance of general practice considering all aspects of the cervical cancer prevention pathway: vaccination, healthy lifestyle advice and cervical screening. To avoid a potential widening of cervical cancer disparities in the catch-up age cohorts, special efforts may be warranted to ensure that those who missed catch-up HPV vaccination are engaged by the cervical screening programme.
Acknowledgements
Natsal-3 is a collaboration between University College London (London, UK), the London School of Hygiene and Tropical Medicine (London, UK), NatCen Social Research, Public Health England (formerly the Health Protection Agency), and the University of Manchester (Manchester, UK). We thank the study participants, the team of interviewers from NatCen Social Research, and operations and computing staff from NatCen Social Research; Chinelo Obi, Rebecca Howell-Jones, David Mesher, Heather Northend, Krishna Gupta, and Tracey Cairns (Department of HIV and Sexually Transmitted Infections, Public Health England) for data linkage, anonymisation, and data entry; laboratory staff for their contributions to development of protocols and testing: Natasha de Silva, and Mohammed-Abbas Fazal (Virus Reference Department, Public Health England); and Laura Marlow (Research Department of Epidemiology and Public Health, University College London) for help designing questions on cervical screening and HPV vaccination.
Financial support: The study was supported by grants (to A.M. Johnson) from the Medical Research Council (G0701757) and the Wellcome Trust (084840), with contributions from the Economic and Social Research Council and Department of Health. N. Field is supported by a National Institute for Health Research Academic Clinical Lectureship.
Footnotes
Conflict of interest declaration: AMJ has been a Governor of the Wellcome Trust since 2011. All other authors declare that they have no conflicts of interest.
Role of the sponsor The sponsors of the study had no role in study design and the collection, analysis and interpretation of data, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Bosch FX, de Sanjosé S. Human papillomavirus in cervical cancer. Curr Oncol Rep. 2002;4:175–83. doi: 10.1007/s11912-002-0079-y. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK . Cervical Cancer Incidence Statistics [Internet] Cancer Research UK; London: 2011. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/cervix/incidence/ [Google Scholar]
- 3.Cancer Research UK . Cervical Cancer Key Stats [Internet] Cancer Research UK; London: 2011. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/cervical-cancer/uk-cervical-cancer-statistics. [Google Scholar]
- 4.International Agency for Research on Cancer . Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 [Internet] International Agency for Research on Cancer; Lyon: 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- 5.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. The Lancet. 2004;364:249–56. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 6.Health and Social Care, Information Centre. Screening and Immunisations team . Cervical Screening Programme, England 2011-12 [Internet] The Health and Social Care Information Centre; 2012. Available from: http://www.cancerscreening.nhs.uk/cervical/cervical-statistics-bulletin-2011-12.pdf. [Google Scholar]
- 7.Shack L, Jordan C, Thomson CS, Mak V, Møller H, UK Association of Cancer Registries Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer. 2008;8:271. doi: 10.1186/1471-2407-8-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currin LG, Jack RH, Linklater KM, Mak V, Møller H, Davies EA. Inequalities in the incidence of cervical cancer in South East England 2001-2005: an investigation of population risk factors. BMC Public Health. 2009;9:62. doi: 10.1186/1471-2458-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Health . Cancer Reform Strategy. DH; London: 2007. [Google Scholar]
- 10.Department of Health . Improving Outcomes: A Strategy for Cancer. London: 2011. [Google Scholar]
- 11.Foley G, Alston R, Geraci M, Brabin L, Kitchener H, Birch J. Increasing rates of cervical cancer in young women in England: an analysis of national data 1982-2006. Br J Cancer. 2011;105:177–84. doi: 10.1038/bjc.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancuck L, Patnick J, Vessey M. A cohort effect in cervical screening coverage? J Med Screen. 2008;15:27–9. doi: 10.1258/jms.2008.007068. [DOI] [PubMed] [Google Scholar]
- 13.Department of Health Annual HPV vaccine uptake in England: 2010/11 [Internet] 2012 Available from: http://media.dh.gov.uk/network/211/files/2012/03/120319_HPV_UptakeReport2010-11-revised_acc.pdf.
- 14.Hughes A, Mesher D, White J, Soldan K. Coverage of the English national human papillomavirus (HPV) immunisation programme among 12 to 17 year-old females by area-level deprivation score, England, 2008 to 2011. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014;19 doi: 10.2807/1560-7917.es2014.19.2.20677. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell S, Roberts R, Thomas D. Factors affecting uptake of HPV vaccination in Wales. University of Warick; 2012. [Google Scholar]
- 16.Sinka K, Kavanagh K, Gordon R, Love J, Potts A, Donaghy M, et al. Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health. 2014;68:57–63. doi: 10.1136/jech-2013-202620. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382:1795–806. doi: 10.1016/S0140-6736(13)61947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erens B, Phelps A, Clifton S, Mercer CH, Tanton C, Hussey D, et al. Methodology of the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3) Sex Transm Infect. 2014;90:84–9. doi: 10.1136/sextrans-2013-051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal) Lancet. 2013;382:1781–94. doi: 10.1016/S0140-6736(13)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne RA, Abel GA. UK indices of multiple deprivation - a way to make comparisons across constituent countries easier. Health Stat Q Off Natl Stat. 2012:22–37. [Google Scholar]
- 21.Sutton S, Rutherford C. Sociodemographic and attitudinal correlates of cervical screening uptake in a national sample of women in Britain. Soc Sci Med. 2005;61:2460–5. doi: 10.1016/j.socscimed.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Field N, Tanton C, Mercer CH, Nicholson S, Soldan K, Beddows S, et al. Testing for sexually transmitted infections in a population-based sexual health survey: development of an acceptable ethical approach. J Med Ethics. 2012;38:380–2. doi: 10.1136/medethics-2011-100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissett SL, Howell-Jones R, Swift C, De Silva N, Biscornet L, Parry JV, et al. Human papillomavirus genotype detection and viral load in paired genital and urine samples from both females and males. J Med Virol. 2011;83:1744–51. doi: 10.1002/jmv.22167. [DOI] [PubMed] [Google Scholar]
- 24.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 25.International Collaboration of Epidemiological Studies of Cervical Cancer Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119:1108–24. doi: 10.1002/ijc.21953. [DOI] [PubMed] [Google Scholar]
- 26.Bowman JA, Redman S, Dickinson JA, Gibberd R, Sanson-Fisher RW. The accuracy of Pap smear utilization self-report: a methodological consideration in cervical screening research. Health Serv Res. 1991;26:97–107. [PMC free article] [PubMed] [Google Scholar]
- 27.Caplan LS, McQueen DV, Qualters JR, Leff M, Garrett C, Calonge N. Validity of Women’s Self-Reports of Cancer Screening Test Utilization in a Managed Care Population. Cancer Epidemiol Biomarkers Prev. 2003;12:1182–7. [PubMed] [Google Scholar]
- 28.Moser K, Patnick J, Beral V. Inequalities in reported use of breast and cervical screening in Great Britain: analysis of cross sectional survey data. BMJ. 2009;338:b2025. doi: 10.1136/bmj.b2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klungsøyr O, Nygård M, Skare G, Eriksen T, Nygård JF. Validity of self-reported Pap smear history in Norwegian women. J Med Screen. 2009;16:91–7. doi: 10.1258/jms.2009.008087. [DOI] [PubMed] [Google Scholar]
- 30.Stupiansky NW, Zimet GD, Cummings T, Fortenberry JD, Shew M. Accuracy of self-reported human papillomavirus vaccine receipt among adolescent girls and their mothers. J Adolesc Health. 2012;50:103–5. doi: 10.1016/j.jadohealth.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig R, Mindell J. Health Survey for England 2010 - volume 1: respiratory health. The NHS Information Centre; Leeds: 2011. [Google Scholar]
- 32.British Social Attitudes: the 28th report. NatCen Social Research; London: 2012. [Google Scholar]
- 33.Waller J, Bartoszek M, Marlow L, Wardle J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16:199–204. doi: 10.1258/jms.2009.009073. [DOI] [PubMed] [Google Scholar]
- 34.Enerly E, Olofsson C, Nygård M. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol. 2013;5:67–79. doi: 10.2147/CLEP.S39799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. 2014;349:g5264–g5264. doi: 10.1136/bmj.g5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher H, Audrey S, Mytton JA, Hickman M, Trotter C. Examining inequalities in the uptake of the school-based HPV vaccination programme in England: a retrospective cohort study. J Public Health Oxf Engl. 2014;36:36–45. doi: 10.1093/pubmed/fdt042. [DOI] [PubMed] [Google Scholar]
- 37.Roberts SA, Brabin L, Stretch R, Baxter D, Elton P, Kitchener H, et al. Human papillomavirus vaccination and social inequality: results from a prospective cohort study. Epidemiol Infect. 2011;139:400–5. doi: 10.1017/S095026881000066X. [DOI] [PubMed] [Google Scholar]
- 38.Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de González A, Colin D, Franceschi S, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118:1481–95. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 40.Bowyer HL, Dodd RH, Marlow LAV, Waller J. Association between human papillomavirus vaccine status and other cervical cancer risk factors. Vaccine. 2014;32:4310–6. doi: 10.1016/j.vaccine.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fish J, Anthony D. UK national Lesbians and Health Care Survey. Women Health. 2005;41:27–45. doi: 10.1300/J013v41n03_02. [DOI] [PubMed] [Google Scholar]
- 42.Bailey JV, Kavanagh J, Owen C, McLean KA, Skinner CJ. Lesbians and cervical screening. Br J Gen Pract. 2000;50:481–2. [PMC free article] [PubMed] [Google Scholar]
- 43.Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer. 2003;89:88–93. doi: 10.1038/sj.bjc.6600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castañón A, Landy R, Cuzick J, Sasieni P. Cervical Screening at Age 50-64 Years and the Risk of Cervical Cancer at Age 65 Years and Older: Population-Based Case Control Study. PLoS Med. 2014;11:e1001585. doi: 10.1371/journal.pmed.1001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Cancer Intelligence Network Cancer Incidence and Survival By Major Ethnic Group, England, 2002 - 2006 [Internet] 2006 Available from: http://www.ncin.org.uk/view?rid=75.
- 46.NHS Cervical Screening Programme . NHS Cervical Screening Programme: Annual Review 2012 [Internet] Sheffield: 2012. Available from: http://www.cancerscreening.nhs.uk/cervical/publications/cervical-annual-review-2012.pdf. [Google Scholar]