Abstract
We analyzed changes in pneumococcal disease (PD) in Utah children <18 years of age from 1996 through 2009 using International Classification of Diseases, Ninth Revision ICD-9) coded hospital discharges. We observed a sustained decrease in the incidence of PD among children <5 years in 2001–2004 (−36%) and 2005–2009 (−34%) compared with 1996–2000 (pre-PCV7). Decreases were primarily in bacteremia, uncomplicated pneumonia and meningitis. In contrast, significant increases in complicated pneumonia/empyema were noted in children <5 years (+95% and +85%) and 5–17 years (+2% and +70%). Despite decreases in PD among Utah children, complicated pneumonia/empyema has increased during the PCV7 era.
Keywords: Streptococcus pneumoniae, pneumococcal disease, PCV7, PCV13
BACKGROUND
Invasive pneumococcal disease (IPD) remains a major cause of morbidity and mortality in children worldwide and a leading cause of community-acquired pneumonia, bacteremia and meningitis.(1, 2)
Widespread use of 7-valent pneumococcal conjugate vaccine (PCV7; Wyeth Lederle Vaccine) in the U.S. since 2000 has significantly changed the epidemiology of IPD.(3) The Centers for Disease Control and Prevention (CDC) Active Bacterial Core (ABC) surveillance sites report a decrease of >70% in the incidence of IPD in children <5 years from 95 cases/100,000 in 1999 to 23 cases/100,000 in 2004.(4) Some centers in the U.S. reported a less dramatic decrease in IPD and the early emergence of IPD due to non-PCV7 serotypes.(4–6) A 13-valent pneumococcal conjugate vaccine (PCV13; Wyeth Pharmaceuticals Inc.) was licensed in 2010, which includes PCV7 serotypes and emerging serotypes 1, 3, 5, 6A, 7F and 19A.
This study describes the epidemiology of pneumococcal disease (PD) from 1996 through 2009 using International Classification of Diseases, Ninth Revision ICD-9) coded hospital discharge data in Utah from the pre-vaccine period through the eve of the introduction of PCV13.
Methods
Human Subjects Protection
The institutional review boards of the University of Utah and Intermountain Healthcare (Intermountain) approved this study. Informed consent was waived.
Setting and Study Population
The study spanned 1996–2009. We defined 3 time periods for analysis based on levels of PCV7 coverage. We defined the pre-vaccine period as the 60-month period from 1996–2000, which preceded PCV7 licensure in the U.S. We defined the early post-vaccine period as the 48 months from 2001–2004, when PCV7 uptake (≥3 doses) was increasing but coverage was <80% for children <36 months. The late post-vaccine period included the 60 months from 2005–2009 when PCV7 coverage was >80% for children in Utah. Vaccine coverage rates for PCV7 in Utah were determined using the CDC’s National Immunization Survey and were similar to national rates.(3)
Intermountain is a large healthcare system that operates 20 hospitals in Utah. Over the study, 71–87% percent of all pediatric emergency encounters and hospital admissions in Utah occurred at an Intermountain facility (S. Lloyd, Intermountain, personal communication). The proportion of pediatric encounters at an Intermountain facility remained stable over this period.
Identification of ICD-9 coded Pneumococcal Disease
We defined a case of PD as a medically attended event occurring among children <18 years of age residing in Utah during the study period with an ICD-9 discharge diagnosis code specific for diseases caused by S. pneumoniae who received care at an Intermountain facility. We defined PD as previously described by Grijalva et al.(7) Briefly, patients were considered to have PD if they had a code specifying pneumococcal pneumonia, pneumococcal meningitis, pneumococcal septicemia or codes for a specific syndrome plus another code specifying pneumococcal infection.
Statistical Analyses
The age-specific incidence of PD among Utah children was calculated using Utah population estimates, which were adjusted for the proportion of children who received care at an IH facility (0.71–0.87) based upon annual market share data.(8) Mann-Whitney U tests were used for pairwise comparisons of age groups and Poisson regression was used to compare PD rates over the time periods.
Results
Incidence of PD in Utah
Between 1996–2009, 1463 children <18 years were treated at an Intermountain facility for an illness with an ICD-9 discharge code associated with PD. A total of 534 children were identified during 1996–2000, 354 during 2001–2004 and 575 during 2005–2009. Overall, the incidence of PD in Utah children <18 years of age decreased from 19.9/100,000 (1996–2000) to 14.4/100,000 during 2001–2004 (−28%; 95% CI −36%, −8%, p<0.001) and was 16.1/100,000 during 2005–2009 (−19%; 95% CI −28, −10, p<0.001). The increased incidence of PD from 2001–2009 did not reach statistical significance.
During the years 2001–2004, the incidence of PD among children <5 years, the PCV7 vaccine target group, decreased by 36% (p<0.001) compared with 1996–2000. During 2005–2009, the incidence of PD remained stable, but below 1996–2000 (−34%; p<0.001) (Table 1).
Table 1.
Changes in the incidence of PD per 100,000 Utah children younger than 5 years, 1996 to 2009.
| Year | Incidence rate/100,000 |
% Change (95 % CI) |
p value | |
|---|---|---|---|---|
| All IPD | 1996–2000 | 48.9 | - | - |
| 2001–2004 | 31.5 | −36% (−44%, −26%) |
<.0001 | |
| 2005–2009 | 32.0 | −34% (−42%, −25%) |
<.0001 | |
| Uncomplicated Pneumonia | 1996–2000 | 26.6 | - | - |
| 2001–2004 | 11.7 | −56% (−65%, −45%) |
<.0001 | |
| 2005–2009 | 13.4 | −50% (−58%, −39%) |
<.0001 | |
| Complicated pneumonia and empyema | 1996–2000 | 3.8 | - | - |
| 2001–2004 | 7.4 | 95% (+31%, +189%) |
0.0009 | |
| 2005–2009 | 7.0 | 85% (+27%, +170%) |
0.001 | |
| Meningitis | 1996–2000 | 4.9 | - | - |
| 2001–2004 | 2.6 | −47% (−67%, −14%) |
0.01 | |
| 2005–2009 | 1.9 | −62% (−76%, −38%) |
<.0001 | |
| IPD Others | 1996–2000 | 2.9 | - | - |
| 2001–2004 | 2.1 | −28% (−59%, +28%) |
0.27 | |
| 2005–2009 | 1.8 | −38% (−64%, +6%) |
0.08 | |
| Bacteremia without focus | 1996–2000 | 10.6 | - | - |
| 2001–2004 | 7.8 | −27% (−46%, −2%) |
0.04 | |
| 2005–2009 | 8.0 | −25% (−43%, −2%) |
0.04 |
There were sustained decreases in the incidence of PD during the 2001–2004 and 2005–2009 periods when compared to the pre-vaccine period among children <12 months (−52%, 95% CI −62, −39, p<0.0001 for 2001–2004 compared to the pre-vaccine period, and −41%, 95% CI −52, −38, p<0.0001 for 2005–2009 compared to the prevaccine period), 12–23 months (−27%, 95% CI −45, −4, p=0.03 and −35%, 95% CI −50, - 15, p<0.001) and 2-<5 years (−17%, 95% CI −35, 5, p=NS and −22%, 95% CI −37, −3, p=0.03). However, there was no change in the incidence of IPD among Utah children 5– 17 years (Table 1).
Clinical Syndromes of Pneumococcal Disease
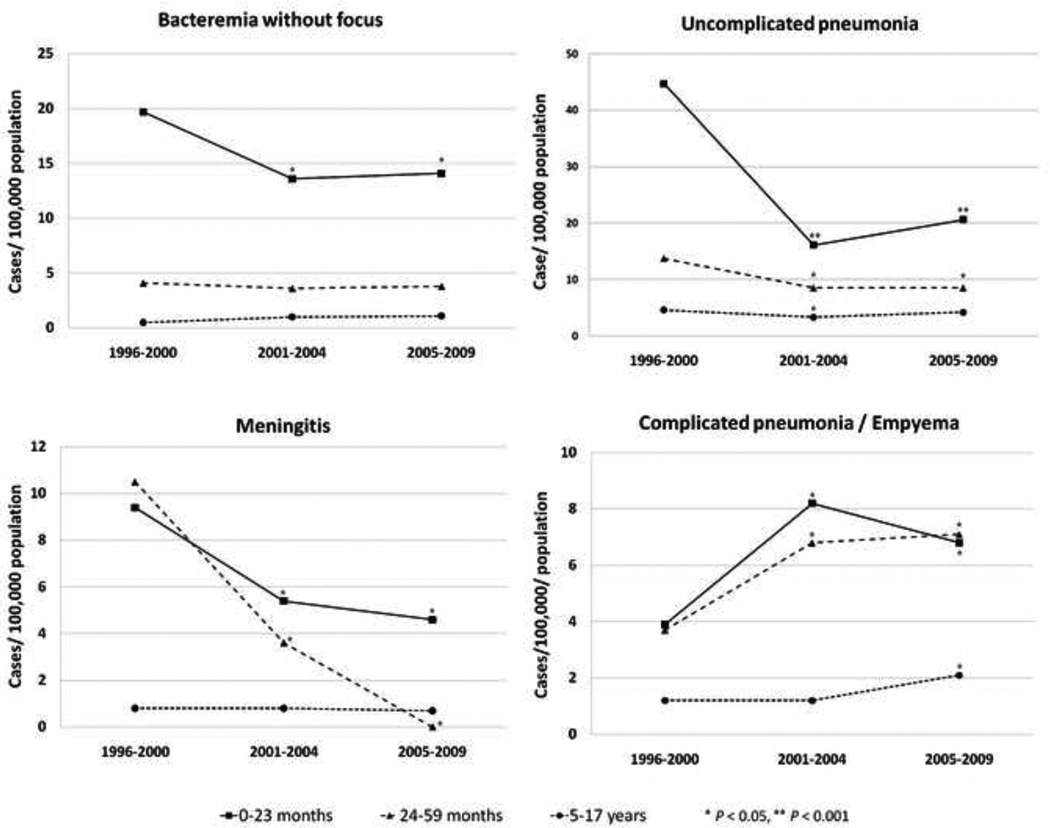
Of 1463 children with PD, 692 (47%) had uncomplicated pneumonia, 288 (20%) had bacteremia without focus, 257 (18%) had complicated pneumonia/empyema, 125 (9%) had meningitis, and 101 (7%) had other sterile site infection. Compared to the pre-vaccine period, there were sustained decreases in the incidence of uncomplicated pneumonia, and bacteremia without focus among children <5 years. In contrast, the incidence of complicated pneumonia/empyema increased significantly over the study period (Figure 1). Among children 5–17 years, there were significant increases of complicated pneumonia/empyema (2%, p=0.95 for 2001–2004 compared to the prevaccine period and 70%, p=0.02 for 2005–2009 compared to the pre-vaccine period) and bacteremia (92% and 102%).
Figure 1.
Incidence of pneumococcal disease syndromes by age before and after introduction of pneumococcal conjugate vaccine among Utah children, 1996–2009
DISCUSSION
In this study, we used hospital discharge data to study the incidence PD in Utah over 14 years, including 9 years of widespread use of PCV7. We observed a sustained modest decrease in the incidence of PD in children <18 years. The change was primarily due to decreasing PD in children <5 years. The introduction of PCV7 had a different impact on individual disease syndromes, with decreasing rates of uncomplicated pneumonia, bacteremia, and meningitis. In contrast, we observed a significant increase in the incidence of complicated pneumonia/empyema.
The widespread use of PCV7 has decreased PD in the U.S.(9, 10) The decrease in IPD due to PCV7 serotypes has been partially offset by increases in infection with non-PCV7 serotypes.(10, 11) PCV13 targets several common non-PCV7 serotypes that have emerged in the U.S. and is expected to further decrease pneumococcal disease burden, but changes in the epidemiology and serotype distribution seem likely.(12)
Shortly after the introduction of PCV7, several studies in the U.S. reported decreases of 65–70% in cases of IPD in children <5 years.(4, 6, 13) In comparison, our study found a modest 36% decrease in PD among children <5 years. Similarly, decreases in PD were observed among children <12 months and 12–23 months but lower than those reported at ABC sites.(10) Differences in methodology between active laboratory surveillance and hospital discharge-based surveillance may account for some of the difference. However, the differences could be partially due to differences in serotype distribution. PCV7 serotypes accounted for ~69% of IPD in Utah before the introduction of the vaccine, substantially lower than the 80–98% reported by other U.S. centers, but similar to the rate among Alaskan Native children. This difference in serotype distribution may have played a role in the overall impact of PCV7.(6)
Our findings of decreases in uncomplicated pneumonia, bacteremia and meningitis are similar to other U.S. reports.(4, 6, 10) In contrast, complicated pneumonia/empyema increased during the early- and late- post-vaccine periods (59% and 83%, respectively). The largest increase in complicated pneumonia/empyema was among children <5 years. The increase in complicated pneumonia/empyema in Utah is similar to findings reported by other investigators.(5–7) The cause(s) of increasing rates of empyema are unclear, but are likely related to the shift in circulating pneumococcal clones and possibly tissue-specific virulence factors.(14)
Other epidemiologic or host factors might explain the differences in epidemiology. In a case-control study of IPD in Utah, risk factors associated with IPD were similar to those reported from other geographic regions, although children ≥2 years were 2.2 times (95% CI: 1.3–3.7) more likely than younger children to have IPD caused by non–PCV7 serotypes.(15) Utah has the largest family sizes, the highest rates of household crowding and the greatest number of households with children in the U.S.(16) The high prevalence of invasive non-PCV7 serotypes in Utah before PCV7 introduction likely favored early serotype replacement.
This study has limitations. ICD-9 coding may be subject to misclassification, both potentially missing cases and incorrectly ascribing disease to S. pneumoniae. These problems are likely to be stable over time, allowing analysis of trends. Surveillance based on discharge diagnosis cannot be directly compared to active laboratory-based surveillance. However, our PD incidence rates are close to those derived from active laboratory-based surveillance.(10, 11) Our study is based in a single geographic area and pneumococcal epidemiology is known to display substantial regional variation.
In summary, we demonstrated a sustained modest decrease in PD of 34% among children <5 years and 27% among all children <18 years following the licensure of PVC7 in Utah. Pneumococcal bacteremia, uncomplicated pneumonia, and meningitis decreased substantially. Conversely, complicated pneumonia/empyema has increased among children <18 years. To optimize prevention strategies, continued surveillance with attention to regional variation and clinical syndromes will be essential to identify further evolution of the epidemiology and serotype distribution of invasive S. pneumoniae disease.
Acknowledgements
Intermountain Healthcare, Salt Lake City, Utah.
References
- 1.Holliman RE, Liddy H, Johnson JD, Adjei O. Epidemiology of invasive pneumococcal disease in Kumasi, Ghana. Trans R Soc Trop Med Hyg. 2007;101:405–413. doi: 10.1016/j.trstmh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–440. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Accessed May 13, 2009];Statistics and Surveillance: Immunization Coverage in the U.S. 2009 ( http://www.cdc.gov/vaccines/stats-surv/imzcoverage.htm#nis) [Google Scholar]
- 4.Hicks LA, Harrison LH, Flannery B, et al. Incidence of Pneumococcal Disease Due to Non-Pneumococcal Conjugate Vaccine (PCV7) Serotypes in the United States during the Era of Widespread PCV7 Vaccination, 1998–2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 5.Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups. Clin Infect Dis. 2005;41:21–29. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 6.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. Jama. 2007;297:1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 7.Grijalva CG, Nuorti JP, Zhu Y, Griffin MR. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis. 2010;50:805–813. doi: 10.1086/650573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed March 26th 2010];Population Estimates, Utah's Indicator-Based Information System for Public Health. 2010 ( http://ibis.health.utah.gov/)
- 9.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. Jama. 2006;295:1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 10.Pilishvili T, Lexau C, Farley MM, et al. Sustained Reductions in Invasive Pneumococcal Disease in the Era of Conjugate Vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 11.Hsu KK, Shea KM, Stevenson AE, Pelton SI. Changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001–2007. Pediatr Infect Dis J. 2010;29:289–293. doi: 10.1097/INF.0b013e3181c15471. [DOI] [PubMed] [Google Scholar]
- 12.Licensure of a 13-Valent Pneumococcal Conjugate Vaccine (PCV13) and Recommendations for Use Among Children --- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–261. [PubMed] [Google Scholar]
- 13.Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- 14.Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad MB, Porucznik CA, Joyce KE, et al. Risk factors for pediatric invasive pneumococcal disease in the Intermountain West, 1996–2002. Ann Epidemiol. 2008;18:139–146. doi: 10.1016/j.annepidem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed December 2nd, 2010];U.S. Census Bureau, 2006–2008 American Community Survey. ( http://factfinder.census.gov/servlet/ACSSAFFFacts?_event=Search&geo_id=01000US&_geoContext=01000US&_street=&_county=&_cityTown=&_state=04000US49&_zip=&_lang=en&_sse=on&ActiveGeoDiv=geoSelect&_useEV=&pctxt=fph&pgsl=010&_submenuId=factsheet_0&ds_name=ACS_2008_3YR_SAFF&_ci_nbr=null&qr_name=null®=null%3Anull&_keyword=&_industry=)