Abstract
Muscarinic acetylcholine receptors (mAChRs) are involved in the control of nociception in the spinal cord. The M2, M3, and M4 mAChR subtypes are present in the spinal dorsal horn. However, the role of the individual subtypes in the antinociceptive effect produced by mAChR agonists is uncertain. Here we determined the contribution of M2, M3, and M4 subtypes to spinal muscarinic analgesia by using small-interference RNA (siRNA) targeting specific mAChR subtypes in rats. The neuronal uptake and distribution of a chitosan-siRNA conjugated fluorescent dye in the spinal cord and dorsal root ganglion were confirmed after intrathecal injection. The control and gene-specific siRNA-chitosan complexes were injected intrathecally for 3 consecutive days. Quantitative RT-PCR analysis showed that treatment with siRNA targeting M2, M3, or M4 subtype produced a large reduction in the corresponding mRNA levels in the dorsal root ganglion and dorsal spinal cord. Also, the protein levels of the mAChR subtypes in the spinal cord were significantly downregulated by siRNA treatment, as determined by the immunoprecipitation and receptor binding assay. Treatment with the M2-siRNA caused a large reduction in the inhibitory effect of muscarine on the nociceptive withdrawal threshold. Furthermore, M4 knockdown at the spinal level significantly reduced the antinociceptive effect of muscarine. However, the antinociceptive effect of muscarine was not significantly changed by the M3-specific siRNA. Our study suggests that chitosan nanoparticles can be used for efficient delivery of siRNA into the neuronal tissues in vivo. Our findings also provide important functional evidence that M2 and M4, but not M3, contribute to nociceptive regulation by mAChRs at the spinal level.
Introduction
Muscarinic acetylcholine receptors (mAChRs) play an important role in the regulation of nociception at the spinal level in both animals and humans (Iwamoto and Marion 1993; Hood et al. 1997; Duttaroy et al. 2002). To date, five mAChR subtypes (M1-M5) have been identified and characterized. The M1, M3, and M5 subtypes are coupled to the Gq/G11 family of G proteins, and the M2 and M4 subtypes preferentially interact with the Gi/Go family (Wess 1996; Caulfield and Birdsall 1998). It has been shown that M2, M3, and M4 are present in the spinal cord dorsal horn (Hoglund and Baghdoyan 1997; Chen et al. 2005). However, because of the lack of highly selective mAChR subtype agonists and antagonists, many pharmacologic studies have yielded contradictory results about how the different mAChR subtypes are involved in the analgesic effect of mAChR agonists (Iwamoto and Marion 1993; Naguib and Yaksh 1997; Ellis et al. 1999; Honda et al. 2000; Mulugeta et al. 2003). For instance, one report has suggested a role for M3 in spinal muscarinic analgesia when 4-diphenylacetoxy-N-methylpiperidine (4-DAMP) was used as an M3-specific antagonist (Honda et al. 2000). Other studies have shown that M1, M2, and M4 in the spinal cord are involved in the regulation of nociception in rodents (Iwamoto and Marion 1993; Ellis et al. 1999; Mulugeta et al. 2003). Notably, 4-DAMP can block other mAChR subtypes with almost equal potencies (Ehlert 1996), and the highly M1-selective agonist xanomeline does not produce any analgesic effect (Sheardown et al. 1997). Thus, the roles of M2, M3, and M4 subtypes in the nociceptive regulation should be defined by using specific approaches independent of pharmacological agents.
Studies using single or double mAChR subtype knockout mice have shown that the M2 and, to a lesser extent, M4 are essential for mAChR agonist-induced anaglesia (Gomeza et al. 1999a; Duttaroy et al. 2002; Wess et al. 2003). However, we recently found significant differences between mice and rats in the function of individual mAChR subtypes in the spinal cord. For example, although activation of M2 and M4 inhibits synaptic GABA and glycine release in the mouse spinal cord (Zhang et al. 2006; Zhang et al. 2007b), stimulation of these two subtypes in the rat spinal cord potentiates synaptic GABA and glycine release (Zhang et al. 2005; Wang et al. 2006). Also, knockout of one mAChR subtype in mice may cause developmental compensatory changes in other mAChR subtypes or altered activity of signaling molecules associated with mAChRs (Nishiyama et al. 2007). Therefore, it is important to determine the contribution of individual mAChR subtypes to the muscarinic control of nociception at the spinal level using other state-of-the-art techniques.
Small-interference RNA (siRNA)-mediated gene-specific silencing has been used to downregulate target genes both in vitro and in vivo (Wall and Shi 2003; Leung and Whittaker 2005). However, an effective in vivo siRNA delivery system remains a major obstacle. We have selected chitosan as a candidate delivery vehicle, as chitosan has excellent biodegradability, biocompatibility, and high positive charges that can easily form complexes with negatively charged double-stranded siRNA (Howard et al. 2006; Katas and Alpar 2006; Liu et al. 2007). In one of our recent studies, we successfully downregulated M3 in the spinal cord using chitosan nanoparticles to deliver siRNA (Zhang et al. 2009). Therefore, in the present study, we used chitosan-conjugated siRNA to specifically knockdown the M2, M3, and M4 mAChR subtypes to determine each subtype's role in controlling nociception at the spinal level.
Materials and Methods
Animals
We used male Sprague-Dawley rats (Harlan, Indianapolis, IN) that initially weighed 225–250 g were for this study. To surgically implant the intrathecal catheters, animals were anesthetized with 2% isoflurane. The catheter was inserted through an incision in the cisternal membrane and advanced about 8 cm caudal so that the tip of each catheter was positioned at the lumbar spinal level. The rats next received an intrathecal siRNA injection 5 days after recovery from cannulation. The surgical preparation and experimental protocols were approved by the Animal Care and Use Committee at the University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical care and use of animals.
Formulation of chitosan-siRNA nanoparticles and intrathecal treatment
All siRNA sequences targeting the three mAChR subtypes were carefully selected using a web-based design software. We subjected the siRNA sequences to BLAST analysis to minimize the potential off-target effects of siRNA. All chemically synthesized siRNA duplexes with more than 90% purity were purchased from Qiagen (Valencia, CA), except the M4-siRNA (Dharmacon Inc., Lafayette, Co). To confirm the specificity of the siRNA target sequences, one mismatch sequence was selected for each mAChR subtype as the control siRNA. The sequences for the mAChR subtype-specific and control siRNAs used in this study are listed in Table 1.
Table 1.
List of siRNA sequences used to target the mAChR subtypes
| Gene | Target Sequences (position of coding sequence) |
|---|---|
| Rat Chrm2 (NM_031016) | M2-specific siRNA (1): 5'-AAT TCC GCG ACA GGT TTA AAT-3' (399–419) |
| M2-specific siRNA (2): 5'-AAC TGC AGA TCT AAG GAA AAA-3' (2095–2115) | |
| M2 control siRNA: 5'-AAT TCT CCG AAC GTG TCA CGT-3' | |
| Rat Chrm3 (NM_012527) | M3-specific siRNA (1): 5'-AAG GAG AGG CAT ACC GCT AAA-3'(2005–2025) |
| M3-specific siRNA (2): 5'-AAC GGC GAT CGC TGC CTT TTA-3' (739–759) | |
| M3 control siRNA: 5'-AAG GCG AGG CTT ACC GGT AAC-3' | |
| Rat Chrm4 (NM_345403) | M4-specific siRNA: 5'-GCG CAA AGT GAC TCG GAC ATT-3' (1656–1674) |
| M4 control siRNA: 5'-AAT TCT CCG AAC GTG TCA CGT-3' |
Chitosan-siRNA nanoparticles were prepared as described in a previous study (Katas and Alpar 2006). Briefly, chitosan (Cat. # 448869, Sigma-Aldrich, St. Louis, MO) was dissolved in 1% acetate buffer and was then adjusted to a pH level of 4.6 with 10 N NaOH. A sodium tripolyphosphate (TPP) solution (0.25%, w/v) containing siRNA (70 µg/ml) was then drop-wise added to a 5 mg/ml chitosan solution with rapid stirring. After 30 min, the mixture was centrifuged at 9,000 × g for 30 min at 4°C. The pellet was diluted with sterile RNase-free water to obtain a final chitosan-siRNA nanoparticle solution concentration of 1 µg/µl. The chitosan-siRNA solution was intrathecally injected in the rats for 3 consecutive days at a dosage of 5 µg siRNA per injection.
Neuronal uptake of chitosan-siRNA in the spinal cord and dorsal root ganglion (DRG)
To validate effective neuronal uptake of chitosan-siRNA, the rats were intrathecally injected with 5 µl (1 µg/µl) of Alexa Fluor 488-labeled chitosan-siRNA nanoparticles. After 24 hr, animals were anesthetized with 60 mg/kg (i.p.) pentobarbital and fixed by perfusion with 4% paraformaldehyde. To determine the neuronal uptake of the chitosan-siRNA nanoparticles in the rat dorsal root ganglion (DRG) and spinal cord, we performed double labeling of anti-Alexa Fluor-488 antibody and Nissl, a neuronal marker, in rats treated with Alexa Fluor-488-labeled chitosan-siRNA complexes and Alexa Fluor 488-labeled naked siRNA (i.e., without chitosan). The spinal cord sections were cut into 25-µm sections, and the DRG sections were cut into 30-µm sections and collected free floating in 0.1 M PBS. Sections were blocked with a 5% blocking reagent (PerkinElmer Life Sciences, Boston, MA) in 0.1 M Tris-HCl (TNB) for 1 hr at room temperature. The sections were then incubated with the primary antibody (rabbit anti-Alexa Fluor-488, dilution 1:400; Molecular Probes, Eugene, OR) diluted in TNB for 2 hr at room temperature and then overnight at 4°C. Sections were subsequently rinsed in TBS and incubated with the secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, dilution: 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 2 hr at room temperature. The sections were then rinsed and incubated with Cy3-tyramide (dilution: 1:100; PerkinElmer Life Sciences, Waltham, MA) for 10 min. Finally, the sections were rinsed and incubated with green NeuroTrace Nissl stains (dilution 1:200; Molecular Probes) for 20 min. Sections were rinsed and mounted on slides, dried and coverslipped. The sections were examined on a confocal microscope (Carl Zeiss, Jena, Germany) and the areas of interest were photographed.
Nociceptive behavioral test
Intrathecal injection of muscarine, a specific muscarinic agonist that activates all 5 mAChR subtypes, was used to determine the antinociceptive effect produced by mAChR stimulation at the spinal level. We measured the thermal sensitivity of the rat hindpaw to a noxious heat stimulus by placing each rat in an individual Plexiglas enclosure on the transparent glass surface, which was maintained at a constant temperature of 30°C. After a 30-min acclimation period, the heat-emitting projector lamp of a thermal testing apparatus (IITC Life Sciences Inc., Woodland Hills, CA) was activated, and the beam was focused directly onto the plantar surface of the hindpaw of each rat. A built-in digital timer was used to record the paw withdrawal latency. Muscarine (10 µg in 5 µl) was injected intrathecally after the baseline withdrawal threshold was measured. This dose of muscarine was chosen because it produces a potent analgesic effect that can be readily detected in rats (Chen and Pan 2003b). The paw withdrawal latency was measured every 15 min for 60 min after drug injection. The mean value of the withdrawal latency on 2 or 3 consecutive trials was calculated. A cutoff of 30 s was used to avoid potential tissue damage.
RNA isolation and quantitative RT-PCR analysis
Three days after the last siRNA injection, animals were killed by inhalation of 5% isoflurane. The total RNA was extracted from the lumbar dorsal spinal cord and DRG using the Purelink total RNA purification system (Invitrogen) with on-column DNase I digestion according to the manufacturer’s instructions. For each sample, 2 µg of total RNA was reverse transcribed for 60 min at 37°C with random primers and M-MLV reverse transcriptase. cDNA was prepared using the Superscript III first strand synthesis kit (Invitrogen).
Real-time PCR was performed using the ABI Prism 7000 Sequence Detection System with SYBR green reagents (Applied BioSystems, Foster City, CA). All samples were run in duplicate using an annealing temperature of 60°C, and the experiment was repeated at least once for each sample. The PCR primers are listed in Table 2 and were purchased from Invitrogen. To calculate the relative transcript levels of the mAChR subtypes, we analyzed the mRNA level of each mAChR subtype using the comparative threshold (Ct) cycle method, as described previously (Pfaffl 2001). Standard curves were generated by a 2-fold dilution of the cDNA sample of the spinal cord or DRG as the PCR templates. For each mAChR subtype, the transcript level in each sample was normalized to the β-actin mRNA (an endogenous reference gene used as the internal control) within that sample by subtracting the Ct of β-actin from the Ct of the mAChR subtype to obtain the ΔCt. Relative mRNA levels of M2, M3, and M4 subtypes in the DRG and spinal cord tissues were obtained from the derived Ct values (2-ΔCt) for comparisons between the control and specific siRNA-treated groups.
Table 2.
List of primers used for real-time PCR
| Gene | Primers |
|---|---|
| Rat Chrm2 (NM_031016) | Fwd: 5'-TCC CGG GCA AGC AAG AGT AGA ATA AAG A-3' |
| Rev: 5'-CCA GGC CGC CAT CAC CAG-3' | |
| Rat Chrm3 (NM_012527) | Fwd: 5'-ACC ACG GCT ACT CTA CCT CTG TCC TTC A-3' |
| Rev: 5'-AGC GTC TGG GCG GCC TTC TC-3' | |
| Rat Chrm4 (NM_345403) | Fwd: 5'-GCC CCT GGG TGC CGT GGT CTG TGA-3' |
| Rev: 5'-GGC GGG CGG GAT AGG TGA GGG GTT TG-3' | |
| Rat β-actin (NM_031144) | Fwd: 5'-TGA ACC CTA AGG CCA ACC GTG AAA AGA T-3' |
| Rev: 5'-GAC CAG AGG CAT ACA GGG ACA CAG C-3' |
Quantification of mAChR subtypes using immunoprecipitation and receptor binding assay
We used the radioactive ligand-binding assay combined with immunoprecipitation to measure the protein level of the mAChR subtypes in the dorsal spinal cord of siRNA-treated animals (Chen et al. 2005). The M2-, M3-, and M4-specific rabbit polyclonal antisera (provided by Dr. Jürgen Wess from the National Institute of Diabetes Digestive and Kidney Diseases) were raised against non-conserved regions of the third cytoplasmic loops of the mouse mAChR proteins (Gomeza et al. 1999a; Gomeza et al. 1999b). The specificity of M2, M3, and M4 antibodies (for immunoprecipitation) used in our study has been extensively validated using mAChR subtype-knockout mice (Yamada et al. 2001; Duttaroy et al. 2002; Chen et al. 2005; Gautam et al. 2005). In brief, lumbar dorsal spinal tissues were homogenized and further disrupted by sonication in ice-cold 0.32 M sucrose in 5 mM Tris-HCl buffer containing 1 mM phenylomethanesulfonyl fluoride (PMSF) to avoid protein degradation. The homogenates were centrifuged at 500 g for 10 min at 4°C. The pellets were discarded, and the supernatants were centrifuged again at 48,000 × g for 20 min at 4°C. The pellets were then resuspended in assay buffer (25 mM phosphate buffer containing 5 mM MgCl2 and 1 mM PMSF, pH 7.4) and centrifuged as described above. The membrane protein samples were incubated for 1 h with 2 nM of the non-selective mAChR antagonist, [3H]quinuclidinyl benzliate ([3H]QNB, 42 Ci/mmol; PerkinElmer Life Sciences), washed thoroughly, and solubilized with 1% digitonin, followed by immunoprecipitation of solubilized [3H]QNB-labeled receptors with receptor subtype-selective antisera, as we described previously (Chen et al. 2005).
Statistical analysis
All data are expressed as means ± S.E.M. Differences in the mRNA and protein levels of the mAChR subtypes between the subtype-specific siRNA-treated and control siRNA-treated animals were analyzed using the Student's t-test or one-way analysis of variance followed by Dunnett’s post hoc test, as appropriate. To compare the different analgesic effects between rats receiving the control siRNA and those receiving subtype-specific siRNA, we calculated the percent analgesic effect produced by intrathecal injection of muscarine using the following formula: [(postdrug effect!baseline)/baseline] × 100. Statistical differences in the analgesic effects of muscarine between the control and subtype-specific siRNA-treated groups were analyzed using one-way analysis of variance followed by Dunnett’s post hoc test. A P value less than 0.05 was considered statistically significant.
Results
Relative mRNA levels of M2, M3, and M4 in the dorsal spinal cord and DRG
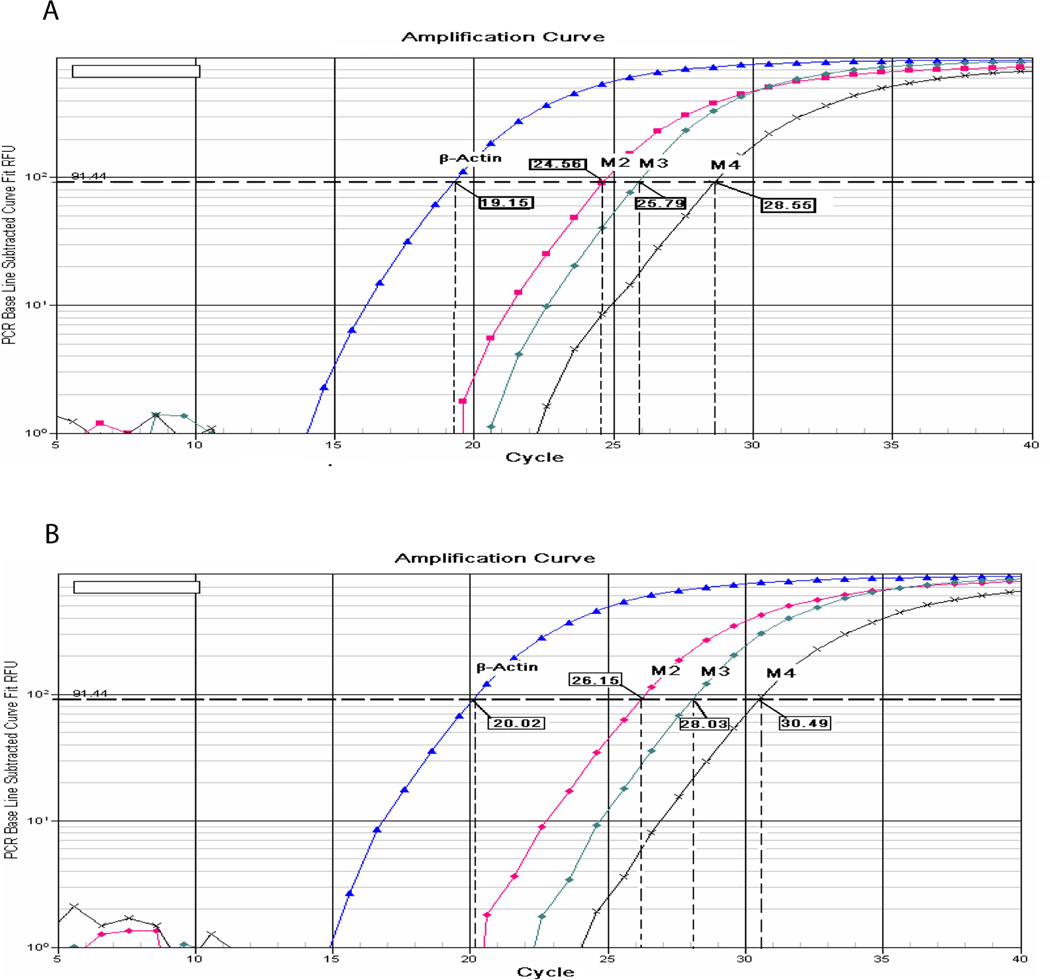
We used quantitative RT-PCR to determine the relative mRNA expression levels of M2, M3, and M4 in the rat dorsal spinal cord and DRG. All three mAChR subtypes were present in the DRG and spinal cord (Fig. 1). In the dorsal spinal cord, the cycle numbers (cycle threshold, Ct) of the same cDNA sample for M2, M3, and M4 were 24.56, 25.79, and 28.55, respectively. Thus, M2 was the most abundant mAChR subtype present in the spinal cord (Fig. 1A). When the mRNA level of M2 was considered to be 100% (the quantitative PCR efficiency was close to 100%), the relative mRNA levels of M3 and M4 subtypes were 42.6% and 6.3%, respectively, of M2 in the dorsal spinal cord tissue.
Figure 1.
Quantitative PCR analysis of the relative expression level of M2, M3, and M4 mRNA in the (A) dorsal spinal cord and (B) DRG. Four real-time amplification plots are shown for β-actin, M2, M3, and M4 (background at the bottom of the graph). The cycle numbers (cycle threshold, Ct) necessary to achieve the given level of fluorescence of 2 (horizontal dashed lines) lies in the log-linear phase of the PCR reactions (the PCR efficiency is close to 100%). The Ct values were 24.56, 25.79, and 28.55 for M2, M3, and M4, respectively, in the dorsal spinal cord (vertical dashed lines). The Ct values were 26.15, 28.03, and 30.49 for M2, M3, and M4, respectively, in the DRG (vertical dashed lines).
In the lumbar DRG, the Ct values of the same cDNA sample for M2, M3, and M4 were 26.15, 28.03, and 30.49, respectively (Fig. 1B). When we considered the mRNA level of M2 to be100%, the mRNA levels of M3 and M4 subtypes were 27.2% and 4.94%, respectively, of M2 in the DRG tissue.
Efficient delivery of chitosan-siRNA nanoparticles in the DRG and spinal cord
Twenty-four hr after intrathecal injection of 5 µg of fluorescence-tagged siRNA encapsulated in chitosan, fluorescent particles were present throughout the DRG and spinal cord tissue sections. Confocal images revealed that the fluorescence-labeled siRNAs were particularly concentrated in the cytoplasm of all Nissl-positive neurons, suggesting an efficient neuronal uptake of siRNA (Fig. 2). When the fluorescence-tagged siRNAs were injected without chitosan, fluorescence-labeled siRNA uptake in the DRG and spinal cord was negligible. Therefore, chitosan is an effective vector that can be used to deliver the synthetic siRNA duplex into the neural tissues in vivo.
Figure 2.
Confocal images showing the neuronal uptake of the fluorescence-labeled siRNA-chitosan (red fluorescent particles in the cytoplasm) in the lumbar DRG and spinal dorsal horn 24 hr after intrathecal injection in a rat. Nissl counter-staining (green) was used to outline the boundaries of the DRG and spinal dorsal horn neurons in the tissue sections.
Role of M2 in intrathecal muscarine-produced antinociception
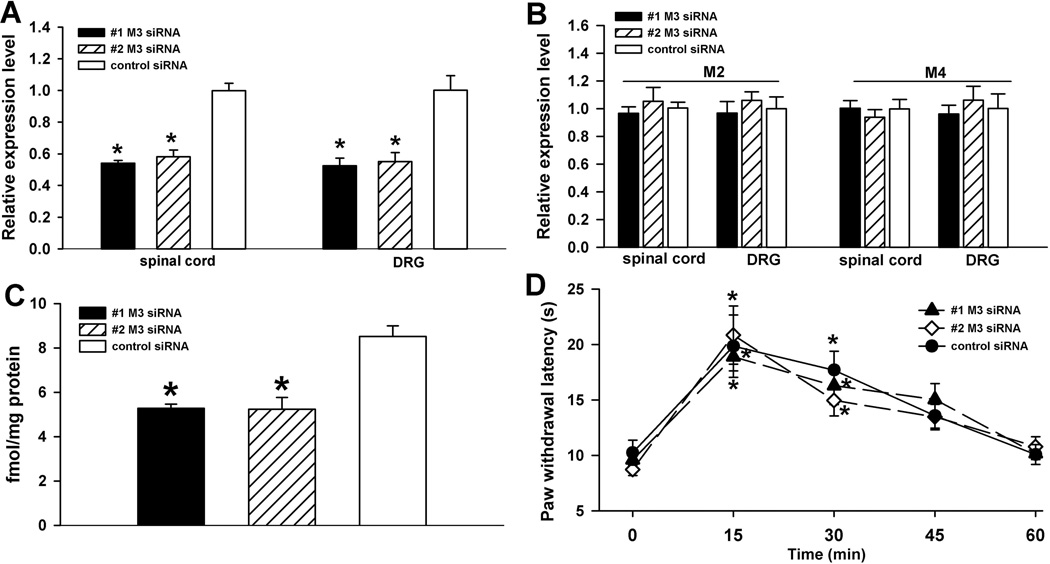
Because the M2 is the most predominant mAChR subtype in the DRG and spinal cord in rats, we first studied its role in the antinociceptive effect produced by intrathecal injection of muscarine. We examined the effects of intrathecal administration of 2 specific siRNAs targeting M2 and M2 control siRNA for 3 days on the mRNA and protein levels of M2. In animals treated with either of the M2-specific siRNAs, but not the control siRNA, there was a large reduction in the mRNA level of M2 (~ 60%) in the lumbar DRG and spinal cord (Fig. 3A). To ensure the specificity of the M2 siRNA, we also examined the mRNA levels of M3 and M4 in rats treated with one of the M2-specific siRNAs. Treatment with the M2-specific siRNAs had no significant effect on the mRNA level of M3 and M4 subtypes in both the DRG and dorsal spinal cord (Fig. 3B). Furthermore, treatment with either of the M2-specific siRNAs significantly reduced the M2 membrane protein level (~ 30%) in the dorsal spinal cord (Fig. 3C). However, the M2-specific siRNA had no significant effects on the membrane protein level of M3 and M4 in the spinal cord (Fig. 3D).
Figure 3.
Effects of intrathecal treatment with M2-specific siRNA on the mRNA and protein levels of M2 and muscarine-produced analgesia in rats. A and B, effects of intrathecal treatment with M2 control siRNA and the two M2-specific siRNAs on the mRNA levels of M2, M3, and M4 in the DRG and dorsal spinal cord. The expression level of the mAChR subtypes in the M2 control siRNA-treated group was considered to be 1. C, the membrane protein level of M2 in the dorsal spinal cord of rats treated with M2 control siRNA and the two M2-specific siRNAs. * P < 0.05 compared with the value in control siRNA-treated rats. D, the membrane protein level of M3 and M4 in the dorsal spinal cord of rats treated with M2 control siRNA and the #1 M2-specific siRNA (n = 4 rats per group). E, effects of intrathecal injection of 10 µg muscarine on the paw withdrawal latency in rats treated with M2 control siRNA and the two M2-specific siRNAs (n = 8 or 9 rats per group). * P < 0.05 compared to the baseline control. # P < 0.05 compared to the M2 control siRNA-treated animals.
Three days after the last intrathecal injection of siRNA, intrathecal administration of 10 µg muscarine significantly increased the paw withdrawal latency in rats treated with the control siRNA. In contrast, intrathecal injection of muscarine failed to significantly change the paw withdrawal latency in rats treated with one of the M2-specific siRNAs (Fig. 3E).
Role of M3 in intrathecal muscarine-produced antinoception
Because M3 is also expressed in the dorsal spinal cord and DRG and because some studies using pharmacological agents suggest that M3 may mediate mAChR agonist-induced antinociception (Naguib and Yaksh 1997; Honda et al. 2000), we next evaluated the role of M3 in mAChR agonist-induced spinal analgesia using the siRNA approach. Intrathecal treatment with either of the two M3-specific siRNAs caused a profound decrease in the mRNA level of the M3 subtype in the lumbar DRG and dorsal spinal cord (Fig. 4A). However, treatment with the M3-specific siRNAs had no significant effect on the mRNA levels of M2 and M4 in the DRG and dorsal spinal cord (Fig. 4B). Furthermore, the M3 protein level was significantly reduced (~35%) in the dorsal spinal cord of rats treated with the M3-siRNAs (Fig. 4C).
Figure 4.
Effects of intrathecal treatment with M3-specific siRNA on the mRNA and protein levels of M3 and muscarine-produced analgesia in rats. A and B, effects of intrathecal treatment with the M3 control siRNA and the two M3-specific siRNAs on the mRNA levels of M2, M3, and M4 subtypes in the DRG and dorsal spinal cord. C, the membrane protein level of M3 in the dorsal spinal cord of rats treated with the M3 control siRNA and the two M3-specific siRNAs. * P < 0.05 compared to the value in M3 control siRNA-treated rats. D, effects of intrathecal injection of 10 µg muscarine on the paw withdrawal latency in rats treated with M3 control siRNA and the two M3-specific siRNAs (n = 7 or 8 rats per group). * P < 0.05 compared to the baseline control.
In animals treated with the M3 control siRNA, intrathecal administration of 10 µg muscarine significantly increased the paw withdrawal latencies. However, pretreatment with the M3-specific siRNAs did not significantly change the antinociceptive effect produced by intrathecal injection of muscarine when compared to rats treated with the M3 control siRNA (Fig. 4D).
Role of M4 in intrathecal muscarine-produced antinociception
The M4 subtype has low expression levels in the rat spinal cord and DRG, as shown in our quantitative PCR data and in previous studies (Hoglund and Baghdoyan 1997; Tata et al. 2000; Chen et al. 2005). Nevertheless, functional evidence obtained from using M4 and M2/M4 double-knockout mice suggests that M4 also contribute to the muscarinic analgesic effect (Duttaroy et al. 2002). Here, we determined the role of M4 in spinal muscarinic analgesia using the specific siRNA targeting the M4 subtype. Intrathecal treatment with the M4-specific siRNA significantly reduced the mRNA level of M4 (~ 50%) in the dorsal spinal cord and DRG (Fig. 5A). However, the M4-specific siRNA had no significant effect on the mRNA levels of M2 and M3 in the DRG and dorsal spinal cord (Fig. 5B). Furthermore, intrathecal treatment with the M4-specific siRNA significantly decreased the M4 protein levels (~30%) in the dorsal spinal cord when compared to treatment with the M4 control siRNA (Fig. 5C). However, the M4-specific siRNA had no significant effects on the membrane protein level of M2 and M3 in the spinal cord (Fig. 5D). A second siRNA sequence [5'-CCC GAA AGT TTG CCA GCA T-3' (1592–1610)] originally designed to target M4 failed to significantly change the mRNA and protein levels of M4 in the spinal cord and DRG (data not shown), and thus this siRNA was not used further in this study.
Figure 5.
Effects of intrathecal treatment with M4-specific siRNA on the mRNA and protein levels of M4 and muscarine-produced analgesia in rats. A and B, effects of intrathecal treatment with M4 control siRNA and M4-specific siRNA on the mRNA levels of M2, M3, and M4 in the DRG and dorsal spinal cord. C, the membrane protein level of the M4 subtype in the dorsal spinal cord of rats treated with M4 control siRNA and M4-specific siRNA. * P < 0.05 compared to the value in M4 control siRNA-treated rats. D, the membrane protein level of M2 and M3 in the dorsal spinal cord of rats treated with M4 control siRNA and the M4-specific siRNA (n = 4 rats per group). E, effects of intrathecal injection of 10 µg muscarine on the paw withdrawal latency in rats treated with M4 control siRNA and M4-specific siRNA (n = 7 or 8 rats per group). * P < 0.05 compared to the baseline control. # P < 0.05 compared to M4 control siRNA-treated animals.
In the M4 control siRNA-treated rats, intrathecal injection of 10 µg of muscarine caused a large increase in the paw withdrawal latencies, and this effect was comparable to that observed in rats treated with the M2 or M3 control siRNAs. Intrathecal administration of muscarine produced a significant increase in the paw withdrawal latencies in rats treated with the M4-specific siRNA. However, pretreatment with the M4-specific siRNA significantly reduced the effect of muscarine on the nociceptive threshold when compared to treatment with the M4 control siRNA (Fig. 5E).
Discussion
The major objective of our study was to use specific siRNAs to define the contribution of the M2-M4 mAChR subtypes to the control of nociception at the spinal level in rats. We found that the M2 mRNA level was the highest among the three mAChR subtypes in the both DRG and spinal cord in rats. Furthermore, the specific siRNAs targeting M2-M4 caused a profound and specific downregulation of the mRNA and protein levels of M2-M4 in the DRG and spinal cord. Importantly, selective knockdown of the M2 or M4 subtypes significantly decreased the antinociceptive effect of muscarine administered intrathecally. However, treatment with the M3-specific siRNA had no effect on muscarine-induced antinociception in rats. Therefore, the chitosan nanoparticle system is a valuable tool for in vivo studies of gene function in the nervous system. Our findings clearly suggest that the M2 and M4 subtypes are essential for the muscarinic regulation of nociception at the spinal level.
Compared with the antisense technique, which requires potentially toxic concentrations to achieve gene-specific suppression, the efficient and reproducible silencing effects of double-stranded siRNA make RNA interference highly advantageous (Elbashir et al. 2002). However, efficient delivery of siRNA into the neural tissues in vivo has proven to be a major difficulty in the application of this novel technique. Chitosan is an attractive vector for siRNA delivery, as its protonated amine groups allow transport across cellular membranes and subsequent endocytosis into the neurons. In addition, chitosan is biodegradable and biocompatible (Howard et al. 2006; Katas and Alpar 2006; Liu et al. 2007). Furthermore, the positively charged amines (under slightly acidic conditions) permit electrostatic interaction with phosphate-bearing nucleic acids to form polyelectrolyte complexes. Using intrathecal injection of the fluorescence-labeled siRNA, we demonstrated an efficient delivery of siRNA to the spinal cord and DRG neurons by chitosan. Also, the effective downregulation of M2, M3, and M4 in the DRG and spinal cord was documented using the quantitative PCR analysis and immunoprecipitation and receptor-binding assay. In general, intrathecal administration of mAChR subtype-specific chitosan-siRNA resulted in a 50–60% reduction in the mRNA levels of the targeted mAChR subtypes. We also demonstrated that two different sequences of siRNAs targeting the same subtype produced a similar degree of reduction in the mRNA and protein levels of the targeted mAChR subtypes. Furthermore, the siRNA targeting a particular mAChR subtype had no significant effect on the mRNA and protein levels of other subtypes present in the spinal cord. Hence, these data strongly suggest that the siRNAs used in our study were highly specific. Our findings clearly show that intrathecal delivery of siRNA using chitosan can effectively and specifically knock down mAChR subtypes in the DRG and spinal cord, and chitosan-siRNA could be used to study the function of other genes and molecular targets in the DRG and spinal cord.
By M2-specific siRNA, our study provides strong evidence that the M2 subtype in the spinal cord plays a major role in the cholinergic regulation of nociceptive transmission at the spinal level. Data from our real-time PCR and membrane protein analyses suggest that M2 is the most abundant mAChR subtype in the rat spinal cord and DRG. It has been reported that there is a strong expression of M2 in the rat DRG, while the mRNA signal of M3 and M4 is weak (Tata et al. 2000). Also, M2-M4 are preferentially distributed in small- to medium-sized DRG neurons (Tata et al. 1999; Tata et al. 2000). In the rat spinal cord, radioligand-binding studies suggest that M2, M3, and M4, but not M1, are present in the superficial dorsal horn (Hoglund and Baghdoyan 1997). Strong M2 immunoreactivity is primarily located in laminae I-III of the spinal cord in rats and mice (Yung and Lo 1997; Duttaroy et al. 2002; Li et al. 2002). We found that treatment with either of two siRNAs targeting the M2 subtype caused a large reduction in the analgesic effect produced by intrathecal injection of muscarine. Our finding is consistent with the results seen in other studies of M2 knockout mice (Gomeza et al. 1999a; Duttaroy et al. 2002), suggesting a critical role of the M2 subtype in the muscarinic control of nociception. We have shown that stimulation of the M2 subtype inhibits glutamate release from primary afferent terminals in rats (Zhang et al. 2007a). Furthermore, activation of the M2 subtype increases synaptic glycine and GABA release to rat spinal dorsal horn neurons (Zhang et al. 2005; Wang et al. 2006), leading to attenuation of the glutamatergic input through activation of GABAA and GABAB receptors (Chen and Pan 2003a; Liu et al. 2007; Yuan et al. 2009). These mechanisms of actions could explain how the M2 subtype is involved in the control of nociceptive transmission at the spinal level.
Our findings also suggest that although the M4 subtype is expressed at a low level in the rat DRG and spinal cord, it contributes importantly to the antinociceptive effect produced by mAChR activation. In this regard, downregulation of M4 by the M4-specific siRNA significantly decreased the spinal analgesic effect of muscarine. Our results are consistent with previous studies about the functional significance of M4 in muscarinic analgesia in rats and mice (Ellis et al. 1999; Duttaroy et al. 2002; Mulugeta et al. 2003). It has been shown that M4 is mainly distributed on small- and medium-sized DRG neurons and superficial spinal dorsal horn in rats (Hoglund and Baghdoyan 1997; Bernardini et al. 1999; Tata et al. 2000). We have shown that the functional activity of M4 is dependent on the presence of M2, and some M2/M4 mAChRs may coexist as oligomers in the spinal cord (Chen et al. 2005). Our electrophysiological studies have suggested that M4 activation can inhibit nociceptive transmission probably by attenuation of glutamatergic synaptic transmission and potentiation of GABAergic and glycinergic inputs to the rat spinal dorsal horn neurons (Zhang et al. 2005; Wang et al. 2006; Zhang et al. 2007a). Because M4 has a limited distribution in the nociceptive pathway, it is a potential therapeutic target to treat acute and chronic pain conditions.
The contribution of the M3 subtype in the spinal cord to the muscarinic control of nociception has not been specifically defined owing to a lack of M3-specific mAChR agonists and antagonists. In the rat spinal cord, M3 is present in interneurons and is involved in stimulation of GABA and glycine release and inhibition of glutamatergic synaptic transmission (Zhang et al. 2005; Wang et al. 2006; Zhang et al. 2007a). In the present study, we found that selective downregulation of M3 with M3-specific siRNA had no significant effect on muscarine-produced analgesia at the spinal level. Therefore, our data suggest that M3 in the spinal cord does not have a major role in the cholinergic control of nociception. However, because a low level of M3 was still present in the spinal cord of siRNA-treated rats, we cannot completely exclude the possibility that M3 may participate, to a small extent, in the spinal muscarinic analgesic action.
Our findings suggest that a 30–40% reduction in the membrane protein of mAChRs can result in a significant loss of function. The dose of muscarine was selected from our previous dose-response study (Chen and Pan 2003b), and intrathecal injection of 10 µg muscarine produces a near maximal analgesic effect without evident side effects in rats. The siRNA we used reduced the expression of mAChR subtypes in both the dorsal root ganglion and spinal cord, which may explain a profound loss of the muscarinic analgesic effect. Also, the mRNA and protein levels were measured from the entire tissues and do not reflect their amount at the cellular level. It is possible that the siRNA may produce a more substantial reduction in the subtype expression at the primary afferent terminals than the interneurons at the spinal level. As we have characterized in the electrophysiological studies (Zhang et al. 2005; Wang et al. 2006; Zhang et al. 2007a), attenuation of neurotransmitter release from the primary afferent terminals by activation of M2 and M4 subtypes may be more important for the muscarinic analgesic effect than their effects on interneurons in the spinal dorsal horn. Thus, the full function of the receptor in the control of nociception may be best reflected by the reduced receptor expression at a critical cellular location rather than the total amount of the receptor protein reduced by siRNA at the spinal level. Although both M2 and M4 subtypes are important for the control of nociceptive transmission, they are separately involved in the control of synaptic transmission at the spinal level (Zhang et al. 2005; Wang et al. 2006; Zhang et al. 2007a). Therefore, their role in the control of spinal nociceptive transmission likely is not redundant, and the loss of function of one of the subtypes after acute knockdown with the specific siRNA is not compensated for by the other subtype.
In summary, we demonstrated that intrathecal treatment with chitosan-siRNA can effectively and specifically downregulate M2, M3, or M4 in the DRG and spinal cord in vivo. Our results provide further evidence that both M2 and M4 subtypes are important for the muscarinic regulation of nociception at the spinal level. Upregulation of the mAChRs in the spinal cord has been shown in rats with diabetic neuropathic pain (Chen and Pan 2003b). Therefore, defining the functional significance of individual mAChR subtypes in the spinal cord in different pain conditions could lead to new and more effective treatments for patients with chronic pain.
Acknowledgments
This study was supported by the National Institutes of Health grants GM64830 and NS45602 and the Hawkins Endowment to H.L.P. We thank Dr. Jürgen Wess for providing the subtype-selective mAChR antibodies used in this study.
List of abbreviations
- GABA
λ-aminobutyric acid
- DRG
dorsal root ganglion
- mAChRs
muscarinic acetylcholine receptors
- RT-PCR
reverse-transcription polymerase chain reaction
- siRNA
small-interference RNA
Contributor Information
You-Qing Cai, Department of Anesthesiology and Perioperative Medicine, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030.
Shao-Rui Chen, Department of Anesthesiology and Perioperative Medicine, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030.
Hee-Dong Han, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030.
Anil K. Sood, Departments of Gynecologic Oncology and Cancer Biology, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030
Gabriel Lopez-Berestein, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030.
Hui-Lin Pan, Department of Anesthesiology and Perioperative Medicine, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030; Program in Neuroscience, The University of Texas Graduate School of Biomedical Sciences, Houston, TX 77225.
References
- Bernardini N, Levey AI, Augusti-Tocco G. Rat dorsal root ganglia express m1-m4 muscarinic receptor proteins. J Peripher Nerv Syst. 1999;4:222–232. [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res. 2003a;965:67–74. doi: 10.1016/s0006-8993(02)04123-9. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Up-regulation of spinal muscarinic receptors and increased antinociceptive effect of intrathecal muscarine in diabetic rats. J Pharmacol Exp Ther. 2003b;307:676–681. doi: 10.1124/jpet.103.055905. [DOI] [PubMed] [Google Scholar]
- Chen SR, Wess J, Pan HL. Functional activity of the M2 and M4 receptor subtypes in the spinal cord studied with muscarinic acetylcholine receptor knockout mice. J Pharmacol Exp Ther. 2005;313:765–770. doi: 10.1124/jpet.104.082537. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, Smith PL, Felder CC, Levey AI, Wess J. Evaluation of muscarinic agonist-induced analgesia in muscarinic acetylcholine receptor knockout mice. Mol Pharmacol. 2002;62:1084–1093. doi: 10.1124/mol.62.5.1084. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. The interaction of 4-DAMP mustard with subtypes of the muscarinic receptor. Life Sci. 1996;58:1971–1978. doi: 10.1016/0024-3205(96)00187-7. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Harman D, Gonzalez J, Spera ML, Liu R, Shen TY, Wypij DM, Zuo F. Development of muscarinic analgesics derived from epibatidine: role of the M4 receptor subtype. J Pharmacol Exp Ther. 1999;288:1143–1150. [PubMed] [Google Scholar]
- Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999a;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999b;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglund AU, Baghdoyan HA. M2, M3 and M4, but not M1, muscarinic receptor subtypes are present in rat spinal cord. J Pharmacol Exp Ther. 1997;281:470–477. [PubMed] [Google Scholar]
- Honda K, Harada A, Takano Y, Kamiya H. Involvement of M3 muscarinic receptors of the spinal cord in formalin-induced nociception in mice. Brain Res. 2000;859:38–44. doi: 10.1016/s0006-8993(99)02456-7. [DOI] [PubMed] [Google Scholar]
- Hood DD, Mallak KA, James RL, Tuttle R, Eisenach JC. Enhancement of analgesia from systemic opioid in humans by spinal cholinesterase inhibition. J Pharmacol Exp Ther. 1997;282:86–92. [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Characterization of the antinociception produced by intrathecally administered muscarinic agonists in rats. J Pharmacol Exp Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115:216–225. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Howard KA, Dong M, Andersen MO, Rahbek UL, Johnsen MG, Hansen OC, Besenbacher F, Kjems J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–1288. doi: 10.1016/j.biomaterials.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Mulugeta E, El-Bakri N, Karlsson E, Elhassan A, Adem A. Loss of muscarinic M4 receptors in spinal cord of arthritic rats: implications for a role of M4 receptors in pain response. Brain Res. 2003;982:284–287. doi: 10.1016/s0006-8993(03)03025-7. [DOI] [PubMed] [Google Scholar]
- Naguib M, Yaksh TL. Characterization of muscarinic receptor subtypes that mediate antinociception in the rat spinal cord. Anesth Analg. 1997;85:847–853. doi: 10.1097/00000539-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Nakamura T, Obara K, Inoue H, Mishima K, Matsumoto N, Matsui M, Manabe T, Mikoshiba K, Saito I. Up-regulated PAR-2-mediated salivary secretion in mice deficient in muscarinic acetylcholine receptor subtypes. J Pharmacol Exp Ther. 2007;320:516–524. doi: 10.1124/jpet.106.113092. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown MJ, Shannon HE, Swedberg MD, Suzdak PD, Bymaster FP, Olesen PH, Mitch CH, Ward JS, Sauerberg P. M1 receptor agonist activity is not a requirement for muscarinic antinociception. J Pharmacol Exp Ther. 1997;281:868–875. [PubMed] [Google Scholar]
- Tata AM, Vilaro MT, Mengod G. Muscarinic receptor subtypes expression in rat and chick dorsal root ganglia. Brain Res Mol Brain Res. 2000;82:1–10. doi: 10.1016/s0169-328x(00)00165-0. [DOI] [PubMed] [Google Scholar]
- Tata AM, Vilaro MT, Agrati C, Biagioni S, Mengod G, Augusti-Tocco G. Expression of muscarinic m2 receptor mRNA in dorsal root ganglia of neonatal rat. Brain Res. 1999;824:63–70. doi: 10.1016/s0006-8993(99)01109-9. [DOI] [PubMed] [Google Scholar]
- Wall NR, Shi Y. Small RNA: can RNA interference be exploited for therapy? Lancet. 2003;362:1401–1403. doi: 10.1016/S0140-6736(03)14637-5. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Li DP, Chen SR, Pan HL. Dynamic regulation of glycinergic input to spinal dorsal horn neurones by muscarinic receptor subtypes in rats. J Physiol. 2006;571:403–413. doi: 10.1113/jphysiol.2005.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Gomeza J, Zhang W, Yamada M, Felder CC, Bernardini N, Reeh PW. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: a review. Life Sci. 2003;72:2047–2054. doi: 10.1016/s0024-3205(03)00082-1. [DOI] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- Yuan WX, Chen SR, Chen H, Pan HL. Stimulation of alpha(1)-adrenoceptors reduces glutamatergic synaptic input from primary afferents through GABA(A) receptors and T-type Ca(2+) channels. Neuroscience. 2009;158:1616–1624. doi: 10.1016/j.neuroscience.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Lo YL. Immunocytochemical localization of muscarinic m2 receptor in the rat spinal cord. Neurosci Lett. 1997;229:81–84. doi: 10.1016/s0304-3940(97)00426-6. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Regulation of glutamate release from primary afferents and interneurons in the spinal cord by muscarinic receptor subtypes. J Neurophysiol. 2007a;97:102–109. doi: 10.1152/jn.00586.2006. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Li DP, Chen SR, Pan HL. M2, M3, and M4 receptor subtypes contribute to muscarinic potentiation of GABAergic inputs to spinal dorsal horn neurons. J Pharmacol Exp Ther. 2005;313:697–704. doi: 10.1124/jpet.104.079939. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Matsui M, Gautam D, Wess J, Pan HL. Opposing functions of spinal M2, M3, and M4 receptor subtypes in regulation of GABAergic inputs to dorsal horn neurons revealed by muscarinic receptor knockout mice. Mol Pharmacol. 2006;69:1048–1055. doi: 10.1124/mol.105.018069. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Zhou HY, Chen SR, Gautam D, Wess J, Pan HL. Control of glycinergic input to spinal dorsal horn neurons by distinct muscarinic receptor subtypes revealed using knockout mice. J Pharmacol Exp Ther. 2007b;323:963–971. doi: 10.1124/jpet.107.127795. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Cai YQ, Richardson TE, Driver LC, Lopez-Berestein G, Pan HL. Signaling mechanisms mediating muscarinic enhancement of GABAergic synaptic transmission in the spinal cord. Neuroscience. 2009;158:1577–1588. doi: 10.1016/j.neuroscience.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]