Abstract
Objective
To prospectively evaluate for changes in objective cognitive performance (attention, memory, and executive function) and psychiatric symptom severity (depression, anxiety, fatigue, and pain) in patients before, during and after interferon-alpha based therapy (IFN) for chronic hepatitis C virus infection (HCV).
Methods
33 HCV+ adults were evaluated two months before IFN initiation (baseline), three months into IFN, and six months following IFN termination (IFN+ Group). 31 HCV+ adults who did not undergo IFN therapy were evaluated at baseline and six months later (IFN− Group). At each evaluation, participants completed the Neuropsychological Assessment Battery (NAB) Attention, Memory and Executive Functions Modules, the Beck Depression Inventory, Second Edition (BDI), Generalized Anxiety Disorder Inventory (GADI), Fatigue Severity Scale (FSS), and Brief Pain Inventory (BPI).
Results
Compared with the IFN−Group, the IFN+ Group experienced significantly (p < 0.050) increased symptoms of depression, anxiety, fatigue and pain during IFN therapy relative to baseline. In the IFN+ Group, psychiatric symptoms generally returned to baseline levels following IFN termination. Sustained viral response was associated with significantly lower depression and fatigue. No significant changes in cognitive performance were observed.
Conclusions
During IFN, patients with HCV evidence significantly increased psychiatric symptoms, including symptoms of depression, anxiety, fatigue and pain. These psychiatric symptoms are generally short-term and remit following IFN termination, with increased benefit if viral clearance is achieved. However, IFN is not associated with significant declines in objective cognitive performance during or following IFN.
Keywords: Anxiety, Cognition, Depression, Fatigue, Interferon, Hepatitis C, Pain
Introduction
Approximately 2.2% of adults world-wide are chronically infected with the hepatitis C virus (HCV) [1], and approximately 10–15% of these cases progress to advanced liver disease resulting in decompensated liver cirrhosis, hepatocellular carcinoma, liver transplantation, or death [2]. Until recently, standard of care for HCV was combination therapy including both PEGylated interferon-alpha and ribavirin. For HCV genotype 1, combination therapy is typically for 48 weeks, while for genotype 2/3, treatment is typically for 24 weeks. In 2011, the Food and Drug Administration (FDA) approved two protease inhibitors, telaprevir and boceprevir, for the treatment of HCV [3]. Thus, current antiviral therapy for HCV can either entail combination therapy or triple drug therapy with pegylated interferon-alpha, ribavirin, and a protease inhibitor. Following combination therapy, sustained viral response (SVR) (i.e., viral clearance for at least six months following treatment termination) is achieved in approximately 40–50% of those with HCV genotype 1, and 75–80% of those with genotype 2/3 [4]. Recent clinical trials suggest that triple drug therapy significantly increases SVR rates among individuals with HCV genotype 1 to above 65% [5–7].
Interferon-alpha is an endogenous cytokine that can also be administered exogenously for the treatment of malignancies such as malignant melanoma as well as chronic viral diseases including hepatitis B and HCV. Interferon-alpha based antiviral therapy for HCV (IFN) is associated with significant side effects, the most commonly reported ones including flu-like symptoms (e.g., fever, chills, myalgia, nausea, fatigue), psychiatric symptoms (e.g., depressed mood, anxiety, irritability, emotional lability, agitation, apathy, anhedonia, anorexia, psychomotor retardation, sleep disturbance, sexual dysfunction) and cognitive complaints [8].
Although cognitive complaints are frequently reported during IFN, relatively few studies have attempted to longitudinally characterize neuropsychological function before, during and after IFN using objective neuropsychological tests. Objective neuropsychological testing is important because people do not typically assess their cognitive skills accurately, and subjective cognitive complaints poorly correlate with objective neuropsychological performance [9]. Perhaps due in part to widely varying methodology, results from available cognitive studies are mixed, showing no clear pattern of whether objective cognitive performance declines during IFN, whether impairments abate following treatment termination, nor which cognitive domains are most sensitive to IFN effects [10–24]. In terms of psychiatric side effects, there is a relatively large literature documenting high rates of psychiatric symptoms during IFN administration but significantly less information regarding the possible persistence of these side effects following IFN termination. A recent community survey of 200 patients treated with IFN for HCV found that 84.5% reported psychiatric side effects during IFN, and 42.5% reported psychiatric side effects that persisted up to six months following IFN termination [25]. IFN induced depression is the most prevalent and well-studied IFN induced psychiatric side effect. The most recent meta-analysis on this topic [26] evaluated 26 prospective observational studies that reported on the incidence of IFN induced major depressive disorder in patients treated for HCV; overall cumulative incidence of depression was 25% following 24 weeks of IFN, and 28% following 48 weeks of IFN. Although the depressive symptoms associated with IFN are generally considered to be transient and remit following termination of therapy [8], a handful of case reports have described worsening depression, and at times increased suicidality, following IFN termination [27–30]. In a case series of five patients treated with IFN, suicide was attempted in four cases after IFN termination and was responsible for two deaths [30]. In another report, two attempted suicides and one successful suicide during or shortly after IFN were described [28]. The rates and time course of other IFN induced psychiatric symptoms have been less rigorously studied. However, an expert panel convened by the European Liver Patient's Organization (ELPA) recently published a consensus statement regarding treatment recommendations for the management of mental health problems among HCV infected patients [31]. Based on their review of the available literature on HCV, IFN, and mental health, this consensus statement reports prevalence rates of IFN induced psychiatric side effects ranging from 30 to 70% for depression, 39–80% for fatigue, 18–45% for sleep disturbances, 16–50% for irritability, 11–45% for anxiety, 0–3.2% for mania, 0–0.6% for psychosis, 3.5–10% for suicidal ideation, and 0–0.2% for suicidal attempts.
In light of the inconsistent findings within the cognitive literature, additional well-designed longitudinal studies are warranted to better characterize the trajectory of potential IFN induced cognitive effects both during IFN and following IFN termination. The present study, therefore, utilizes a comprehensive battery of widely used, well-validated, and adequately normed neuropsychological assessment instruments to prospectively evaluate neuropsychological functioning in patients before, during, and after IFN, and also includes a demographically similar (i.e., age, race/ethnicity, gender, education, baseline estimated IQ) control group of untreated HCV patients to control for possible confounding factors such as practice effects. This study additionally adds to the literature on the psychiatric side effects of IFN by simultaneously including well-validated symptom questionnaires to evaluate the severity and persistence of symptoms of IFN induced depression, anxiety, fatigue and pain.
Methods
Participants
A total of 64 adults were recruited from the Portland, Oregon area and assigned to one of two groups: 1) adults with chronic HCV (>5 years) who were about to initiate IFN (IFN+, n=33), 2) a control group of adults with chronic HCV (>5 years) who were not planning to initiate IFN (IFN−, n = 31). Participants were recruited from Portland area hepatology clinics through referral by the hepatologists, announcements at hepatology clinic HCV education classes, mailings to patients who had previously participated in HCV research, or study advertisements posted in hepatology clinics and hospitals. Inclusion Criteria: 1) Able to provide informed consent, 2) HCV status confirmed by the treating hepatologist, medical record verification, and a detectable HCV viral load based on polymerase chain reaction (PCR) test at the time of study enrollment. Exclusion Criteria: 1) History of antiviral therapy or chemotherapy for any purpose. 2) Visual or auditory impairments that would prevent valid neuropsychological test administration. 3) History of a major medical or psychiatric condition, or currently unstable medical or psychiatric condition, that was likely to be associated with severe neurological, cognitive, or immune dysfunction at the time of enrollment or would preclude informed consent or valid testing [e.g., stroke, seizures, brain tumors, Parkinson's disease, neurodegenerative dementia, mental retardation, hepatic encephalopathy, human immunodeficiency virus (HIV), traumatic brain injury with loss of consciousness ≥30 min, schizophrenia, bipolar I disorder]. 3) Within twenty-four hours of testing, use of alcohol, illicit substances, or medications with acute cognitive effects such as sedation or intoxication (e.g., benzodiazepines, opiates, muscle relaxants, psychostimulants, steroids, anticholinergics). 4) Alcohol or drug dependence within the past three months (except nicotine or caffeine), based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria [32], confirmed with the Mini-International Neuropsychiatric Interview (MINI) [33].
Procedures
All research was conducted with permission from the Portland Veterans Affairs Medical Center (PVAMC)'s Institutional Review Board and in accordance with the Helsinki Declaration as revised in 1989. All patients were paid $75 per study visit to complete the following study procedures: clinical interview, comprehensive medical record review, a battery of cognitive assessment measures, a battery of psychiatric questionnaires to assess severity of depression, anxiety, fatigue, and pain, and blood sample collection for standard medical laboratory tests including a liver panel (serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), ammonia, bilirubin, and albumin levels), human immunodeficiency virus (HIV) antibody screening, and HCV testing (HCV antibody, followed by HCV recombinant immunoblot assay, HCV PCR Qualitative, and HCV PCR Quantitative if HCV antibody positive). Blood samples were collected by certified phlebotomists in the PVAMC medical laboratory. All other study procedures were administered by one of eight study personnel (VW, SJ, CE, MK, DK, JA, KB, HO) who were trained and supervised by a clinical neuropsychologist (MH). To ensure accuracy, all cognitive and psychiatric measures were scored and then re-scored by separate study personnel. All study data were entered into a database initially and then double-checked by separate study personnel prior to analyses.
Clinical interviews were conducted using a structured case report form, developed specifically for this study, including prompts to screen patients based on each inclusion criteria, gather relevant demographic data, assess for a full range of current and past Axis I psychiatric and substance use disorders using DSM-IV [32] criteria and the MINI [33], evaluate for history of head injuries, and record a comprehensive list of current and previous medical conditions and medications. Study personnel additionally reviewed each participant's complete electronic medical record if treated at PVAMC, or the medical records forwarded by a treating hepatologist or primary care provider if treated elsewhere to cross-validate the psychiatric, substance use, and medical history gathered in the clinical interview.
The IFN+ Group was followed longitudinally before, during, and after IFN and repeated all study visit procedures according to the following study visit schedule: 1) Visit 1 — (baseline) eligible to initiate IFN within approximately two months, 2) Visit 2 — approximately three months into IFN, 3) Visit 3—approximately 6 months post IFN termination. The IFN− Group was followed longitudinally according to an equivalent schedule even though this group did not initiate IFN, with Visit 2 occurring approximately five to six months following baseline; however, the IFN−Group was not assessed at Visit 3.
Cognitive measures
Wechsler Test of Adult Reading (WTAR). [34]
A widely used word recognition reading test, validated for use in estimating baseline cognitive ability prior to injury or disease. Standard Index Scores are derived from age corrected norms.
Neuropsychological Assessment Battery (NAB) [35]
A well-validated, comprehensive battery of subtests assessing a range of cognitive domains. The Attention, Memory, and Executive Functions Modules were administered, each consisting of several subtests relevant to that domain. Based on demographically corrected norms (i.e., age, gender, education), standard T scores are derived for each subtest, and standard Index Scores are derived as summary measures of performance across subtests for each Module.
Psychiatric questionnaires
Depression. Beck Depression Inventory, Second Edition (BDI) [36]
A well-validated 21-item measure of depression severity. As previously described [37], we conducted a factor analysis of BDI data from a large sample of 671 HCV+ patients which yielded a two-factor model and showed that HCV+ adults scored significantly higher on the Somatic Factor (i.e., loss of energy, changes in sleeping pattern, irritability, changes in appetite, concentration difficulty, tiredness or fatigue, loss of interest in sex) than the Cognitive Affective Factor (i.e., sadness, pessimism, past failure, guilty feelings, punishment feelings, self-dislike, self-criticalness, suicidal thoughts, crying, agitation, worthlessness). Thus, for the present study, the total BDI scores (Depression—Total) as well as the two BDI factor scores [Depression—Cognitive Affective Factor and Depression—Somatic Factor, derived according to the previously published methods [37]] are reported and analyzed.
Anxiety. Generalized Anxiety Disorder Inventory (GADI) [38]
A well-validated 18-item measure of anxiety severity.
Fatigue. Fatigue Severity Scale (FSS) [39–41]
A 9-item fatigue severity scale, previously validated for use with patients with HCV, multiple sclerosis, and other chronic illnesses.
Pain. Brief Pain Inventory, Short Form (BPI) [42–44]
A well-validated 12-item inventory assessing both the intensity of recent pain [BPI Pain Severity (BPI-PS)] as well as the level at which it interferes with daily activities [BPI Pain Interference (BPI-PI)].
Statistical analyses
Analyses were conducted using Stata v12. Results with p values < 0.05 were considered significant, unless multiple models were run and then a Bonferroni correction was employed. Primary outcome measures were the summary cognitive (NAB Attention Index, NAB Memory Index, and NAB Executive Functions Index) and psychiatric (BDI Total, BDI-Cognitive–Affective Factor, BDI-Somatic Factor, GADI, FSS, BPI-PI, BPI-PS) scores.
For Table 1, between group comparisons of baseline demographic and clinical characteristics were conducted using F tests for continuous variables; chi-square tested differences between categorical variables.
Table 1.
Between group comparisons of baseline demographic data, clinical characteristics and hepatitis C and liver biomarkers by study group.a
| IFN+ | IFN− | p value | |
|---|---|---|---|
| N | 33 | 31 | |
| Demographics | |||
| Age, mean years (SD) | 52 (10) | 52 (8) | 0.984 |
| Male gender | 64% | 61% | 0.846 |
| Caucasian | 94% | 86% | 0.323 |
| Veteran status | 33% | 35% | 0.891 |
| Years of education | 13 (2) | 13 (2) | 0.724 |
| Estimated cognitive reserve (WTAR), mean standard score (SD) | 101 (14) | 105 (14) | 0.309 |
| Clinical characteristics | |||
| Body mass index, mean (SD) | 30 (5) | 29 (6) | 0.251 |
| Lifetime alcohol use disorderb | 53% | 58% | 0.543 |
| Lifetime other drug use disorderb | 64% | 88% | 0.022* |
| Lifetime medical diagnoses (any) | 55% | 61% | 0.627 |
| Diabetes | 9% | 10% | 0.936 |
| Hyperlipidemia | 18% | 10% | 0.328 |
| Hypertension | 39% | 29% | 0.383 |
| Asthma/pulmonary | 9% | 16% | 0.395 |
| Lifetime psychiatric diagnoses (any)b | 41% | 55% | 0.618 |
| Major depressive disorderb | 31% | 48% | 0.861 |
| Posttraumatic stress disorderb | 16% | 23% | 0.482 |
| Other anxiety disorderb | 13% | 23% | 0.264 |
| Hepatitis C and liver biomarkers | |||
| HCV RNA (log10 IU/ml), mean (SD) | 5.9 (0.2) | 6.1 (0.2) | 0.673 |
| AST (IU/L), mean (SD) | 71.0 (36.2) | 46.1 (32.4) | 0.754 |
| ALT (IU/L), mean (SD) | 100.9 (56.7) | 63.0 (47.0) | 0.915 |
| Ammonia (µg/dL), mean (SD) | 41.2 (20.0) | 45.0 (29.4) | 0.572 |
| Bilirubin (mg/dL), mean (SD) | 0.6 (0.3) | 2.6 (11.1) | 0.273 |
| Albumin (g/dL), mean (SD) | 4.2 (0.3) | 4.4 (0.5) | 0.019* |
Data expressed as n, with (%) in terms of n over total N unless otherwise stated. p values reflect comparisons between the IFN+ group versus the IFN− control group. For categorical variables, chi square analysis was used. F tests were used for continuous variables.
Substance use and psychiatric diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria verified using the Mini-International Neuropsychiatric Interview.
ALT = Serum alanine aminotransferase levels. AST = Serum aspartate aminotransferase levels. HCV = Hepatitis C virus. IFN+ = Adults with chronic hepatitis C virus infection who were planning to initiate antiviral treatment. IFN− = Adults with chronic hepatitis C virus infection who were not planning to initiate antiviral treatment. IU = International units. RNA = Ribonucleic acid. SD = Standard deviation. WTAR = Wechsler Test of Adult Reading.
p ≤ 0.050.
Due to the structure of the data, two sets of analyses were necessary for adequate modeling. To determine whether IFN was associated with significantly increased cognitive and psychiatric symptoms during treatment, the first analysis included all data from the first two visits (i.e., Visit 1 pre-treatment, and Visit 2 during treatment) for both the IFN+ and IFN− groups. For these data, a 2 (Visit 1, Visit 2) × 2 (IFN+ Group, IFN− Group) mixed-effects regression model was conducted for each of the primary outcome measures. The models assumed two levels, Participants (Level 1) nested within Visit (Level 2), in order to adjust standard errors for the longitudinal effects. GADI, BDI Total Score, FSS and both scales of the BPI used Poisson mixed-effects modeling to account for non-normal distributions. The NAB Indices and BDI Factors were analyzed with normal mixed-effects regression methods. To control for multiple comparisons, a Bonferroni correction was employed, and only omnibus tests with p values ≤ 0.005 (0.050/10 omnibus tests) were deemed significant and interpreted.
To determine whether any IFN associated cognitive or psychiatric symptoms remitted upon treatment termination, data from the IFN+ group only was analyzed across all three study visits (i.e., Visit 1: pre-treatment, Visit: 2 during treatment, and Visit 3: post-treatment). For these data, normal (NAB scores, BDI factors) or Poisson (GADI, BDI Total Score, FSS, BPI scales) regression models with clustered standard errors by participant identified differences in scores across visits for each of the primary cognitive and psychiatric outcome measures. Two models evaluated differences between two intervals: Visit 1 and Visit 2, and between Visit 2 and Visit 3. Two separate models were specified in order to avoid statistical suppression between two dummy codes. To control for multiple comparisons, a Bonferroni correction was employed, and only omnibus tests with p values ≤ 0.0025 (0.05/20 omnibus tests) were deemed significant and interpreted.
Results
Baseline characteristics
As summarized in Table 1, groups were similar in terms of age, gender, ethnicity, Veteran status, education, estimated baseline cognitive ability (i.e., WTAR scores), and body mass index, as well as rates of lifetime psychiatric disorders and medical conditions. Although rates of past alcohol use disorders were similar across groups, the IFN− group had significantly higher rates of past drug use disorders. There were no significant differences across groups in terms of HCV viral load [i.e., HCV ribonucleic acid (RNA) levels], AST, ALT, ammonia, or bilirubin levels; the IFN− had significantly higher albumin levels. Within the total sample, 70% reported contracting HCV through injection drug use, 3% through blood transfusions, 6% through tattoos, 5% through accidental work exposure, and 15% through unknown or otherwise unspecified causes.
IFN Effects during treatment and following treatment termination
Means and standard errors for each of the primary outcome measures are reported by group (IFN+, IFN−) and visit (Visit 1, Visit 2, Visit 3) in Table 2. Results from the 2 (Visit 1, Visit 2) × 2 (IFN+ Group, IFN− Group) mixed effects regression models for each of the primary outcome measures are summarized in Table 3, including Wald X2, df, b, robust standard errors, z and p values. Results from the mixed effects regression models assessing change across visits within the IFN+ group are summarized in Table 4, including df, z and p values.
Table 2.
Means and standard errors on primary cognitive and psychiatric outcome measures by study group and visit.a
| IFN+ (n = 33) | IFN− (n = 31) | ||||
|---|---|---|---|---|---|
| Outcome Measure | Visit 1 | Visit 2 | Visit 3 | Visit 1 | Visit 2 |
| NAB Attention | 94.85 (2.51) | 98.00 (2.59) | 100.96 (3.03) | 97.87 (2.59) | 101.87 (2.63) |
| NAB Memory | 97.55 (2.59) | 96.36 (2.59) | 102.37 (3.09) | 93.00 (2.67) | 96.42 (2.67) |
| NAB Executive Functions | 103.31 (2.80) | 104.09 (2.76) | 107.48 (3.14) | 99.68 (2.84) | 105.74 (2.84) |
| BDI Total (Depression) | 7.64 (1.29) | 14.34 (1.31) | 9.85 (1.66) | 7.26 (1.33) | 7.35 (0.33) |
| Somatic Factor | 0.60 (0.08) | 1.16 (0.08) | 0.67 (0.10) | 0.53 (0.08) | 0.49 (0.09) |
| Cognitive Affective Factor | 0.21 (0.06) | 0.39 (0.06) | 0.34 (0.08) | 0.23 (0.06) | 0.20 (0.06) |
| GADI (Anxiety) | 11.73 (1.87) | 21.50 (1.89) | 15.85 (2.25) | 11.94 (1.93) | 12.81 (1.93) |
| FSS (Fatigue) | 3.36 (0.86) | 7.31 (0.88) | 3.60 (1.14) | 3.67 (0.89) | 3.41 (0.89) |
| BPI-PI (Pain Interference) | 1.80 (0.43) | 3.54 (0.44) | 2.35 (0.49) | 2.09 (0.45) | 2.78 (0.44) |
| BPI-PS (Pain Severity) | 2.17 (0.40) | 2.69 (0.40) | 2.18 (0.46) | 2.26 (0.41) | 2.86 (0.41) |
Data expressed as mean (standard error). NAB Attention, Memory and Executive Functions Module scores are reported as demographically corrected standard index scores. Psychiatric symptom severity scores are reported as total scale scores (BDI Total, GADI, FSS, BPI-PI, BPI-PS) or factor scores (BDI Somatic Factor, BDI Cognitive Affective Factor).
BDI = Beck Depression Inventory, Second Edition. BPI-PI = Brief Pain Inventory-Pain Interference. BPI-PS = Brief Pain Inventory-Pain Severity. FSS = Fatigue Severity Scale. GADI = Generalized Anxiety Disorder Inventory. IFN+ = Adults with chronic hepatitis C virus infection who were planning to initiate interferon-alpha based antiviral treatment. IFN− = Adults with chronic hepatitis C virus infection who were not planning to initiate interferon-alpha based antiviral treatment. NAB = Neuropsychological Assessment Battery.
Table 3.
Results from a 2 (Visit 1, Visit 2) × 2 (IFN+ Group, IFN− Group) mixed effects model to evaluate the effect of interferon-alpha based antiviral therapy (IFN) for hepatitis C on primary cognitive and psychiatric outcome measures during treatment.a
| Neuropsychological Assessment Battery — Attention (Normal) Wald X2 = 13.86, df = 3, p = 0.0031* | |||
| Effect | b (robust SE) | z | p |
| Intercept | 97.87 (2.27) | 43.02 | <0.000 |
| Visit | 4.56 (1.51) | 3.02 | 0.002 |
| Group | −3.02 (3.49) | −0.87 | 0.386 |
| Visit × group | −1.70 (2.06) | −0.83 | 0.409 |
| Neuropsychological Assessment Battery — Memory (Normal) Wald X2 = 5.45, df = 3, p = 0.1415 | |||
| Effect | b (robust SE) | z | p |
| Intercept | 93.00 (2.35) | 39.58 | <0.000 |
| Visit | 3.42 (1.58) | 2.16 | 0.031 |
| Group | 4.54 (3.78) | 1.20 | 0.230 |
| Visit × group | −4.60 (2.33) | −1.97 | 0.048 |
| Neuropsychological Assessment Battery — Executive Functions (Normal) Wald X2 = 11.38, df = 3, p = 0.0098 | |||
| Effect | b (robust SE) | z | p |
| Intercept | 99.67 (2.98) | 33.46 | <0.000 |
| Visit | 6.06 (1.85) | 3.29 | 0.001 |
| Group | 3.28 (4.16) | 0.79 | 0.430 |
| Visit × group | −4.93 (2.51) | −1.96 | 0.050 |
| Beck Depression Inventory — Total Score (Poisson) Wald X2 = 40.37, df = 3, p < 0.0000* | |||
| Effect | b (robust SE) | z | p |
| Intercept | 1.62 (.17) | 9.65 | <0.000 |
| Visit | −0.0007 (0.14) | −0.01 | 0.996 |
| Group | 0.14 (0.23) | 0.61 | 0.542 |
| Visit × group | 0.72 (.19) | 3.85 | <0.000 |
| Beck Depression Inventory — Somatic Factor (Normal) Wald X2 = 54.99, df = 3, p < 0.0000* | |||
| Effect | b (robust SE) | z | p |
| Intercept | 0.52 (0.073) | 7.14 | <0.000 |
| Visit | −0.019 (0.082) | −0.23 | 0.816 |
| Group | 0.072 (0.11) | 0.69 | 0.493 |
| Visit × group | 0.57 (0.11) | 5.00 | <0.000 |
| Beck Depression Inventory — Cognitive Affective Factor (Normal) Wald X2 = 11.84, df = 3, p = 0.0079 | |||
| Effect | b (robust SE) | z | P |
| Intercept | 0.23 (.061) | 3.69 | <0.000 |
| Visit | −0.011 (.035) | −0.33 | 0.745 |
| Group | −0.014 (0.081) | −0.17 | 0.865 |
| Visit × group | 0.18 (0.061) | 2.95 | 0.003 |
| Generalized Anxiety Disorder Inventory (Poisson) Wald X2 = 45.28, df = 3, p < 0.0000* | |||
| Effect | b (robust SE) | z | P |
| Intercept | 2.12 (0.17) | 12.77 | <0.000 |
| Visit | 0.037 (0.12) | 0.30 | 0.761 |
| Group | 0.027 (0.23) | 0.12 | 0.908 |
| Visit × group | 0.69 (0.17) | 4.15 | <0.000 |
| Fatigue Severity Scale (Poisson) Wald X2 = 24.39, df = 3, p < 0.0000* | |||
| Effect | b (robust SE) | z | p |
| Intercept | 1.23 (0.12) | 9.95 | <0.000 |
| Visit | −0.075 (0.17) | −0.45 | 0.653 |
| Group | −0.089 (0.17) | −0.51 | 0.608 |
| Visit × group | 0.71 (0.23) | 3.17 | 0.002 |
| Brief Pain Inventory-Pain Interference (Poisson) Wald X2 = 18.48, df = 3, p = 0.0003* | |||
| Effect | b (robust SE) | z | P |
| Intercept | 0.39 (0.22) | 1.76 | <0.078 |
| Visit | 0.26 (0.17) | 1.56 | 0.119 |
| Group | −0.15 (0.30) | −0.50 | 0.614 |
| Visit × group | 0.38 (0.23) | 1.65 | 0.099 |
| Brief Pain Inventory-Pain Severity (Poisson) Wald X2 = 8.78, df = 3, p = 0.0323 | |||
| Effect | b (robust SE) | z | p |
| Intercept | 0.52 (0.20) | 2.55 | <0.011 |
| Visit | 0.24 (0.16) | 1.48 | 0.138 |
| Group | −0.063 (0.28) | −0.23 | 0.821 |
| Visit × group | −0.010 (0.23) | −0.05 | 0.963 |
Data reflect results from a 2 within (Visit 1, Visit 2) × 2 between (IFN+ Group, IFN− Group) groups design to assess the effects of IFN on patients with HCV+ during treatment. Models are classified as either normal or Poisson mixed-effect regression models depending on the shape of the distribution of the outcome variable.
IFN+ = Adults with chronic hepatitis C virus infection who were planning to initiate interferon-alpha based antiviral treatment. IFN− = Adults with chronic hepatitis C virus infection who were not planning to initiate interferon-alpha based antiviral treatment. Visit 1 = Baseline (two months before treatment.) Visit 2 = Three months into IFN treatment, or approximately six months following baseline for the IFN− group.
p ≤ 0.005, indicates the overall model remained significant after a Bonferroni correction for multiple comparisons (p ≤ 0.050/10 tests).
Table 4.
Results from mixed-effects regression models evaluating change across visits on primary cognitive and psychiatric measures in patients with hepatitis C two months before (Visit 1), three months into (Visit 2), and six months after (Visit 3) interferon-alpha based antiviral treatment, n = 33.a
| Test between visits | 1 and 2 | 2 and 3 |
|---|---|---|
| Degrees of freedom = 2 | z (p) | z (p) |
| NAB Attention | 1.81 (0.071) | 2.21 (0.027) |
| NAB Memory | −0.65 (0.515) | 3.25 (0.001)* |
| NAB Executive Functions | 0.48 (0.631) | 1.98 (0.047) |
| BDI Total (depression) | 7.93 (<0.000)* | −5.22 (<0.000)* |
| Somatic Factor | 2.35 (0.019) | −0.07 (<0.000)* |
| Cognitive Affective Factor | 5.79 (<0.000)* | −4.42 (<0.000)* |
| GADI (Anxiety) | 9.55 (<0.000)* | −5.26 (<0.000)* |
| FSS (Fatigue) | 7.04 (<0.000)* | −6.74 (<0.000)* |
| BPI-PI (Pain Interference) | 4.15 (<0.000)* | −2.83 (0.005) |
| BPI-PS (Pain Severity) | 1.58 (0.113) | −1.61 (0.107) |
Data reflect results from a mixed-effects regression model (with adjusted standard errors for participant) based on the distribution of the outcome variable (Poisson or Normal). Two models are displayed: one for the difference between Visit 1 and Visit 2, and another independent model for the difference between Visit 2 and Visit 3 to evaluate change on measures of cognitive and psychiatric function across visits in patients with hepatitis C. All models had 2 degrees of freedom.
BDI = Beck Depression Inventory, Second Edition. BPI-PI = Brief Pain Inventory-Pain Interference. BPI-PS = Brief Pain Inventory-Pain Severity. FSS = Fatigue Severity Scale. GADI = Generalized Anxiety Disorder Inventory. NAB = Neuropsychological Assessment Battery.
p ≤ 0.005, indicates the overall model remained significant after a Bonferroni correction for multiple comparisons (p ≤ 0.0025/20 tests).
Regarding IFN effects on cognition, following a Bonferroni correction for multiple comparisons, the 2 (Visit 1, Visit 2) × 2 (IFN+ Group, IFN− Group) mixed effects regression model (Table 3) remained significant and was therefore interpreted for NAB Attention (but not for NAB Memory nor NAB Executive Function). Although there was a significant Visit effect for NAB Attention, the IFN and interaction effects were non-significant. Results from the mixed-effects regression model evaluating changes across visits before, during and after IFN in the IFN+ group (Table 4) were largely consistent with the results in Table 3. Following a Bonferroni correction for multiple comparisons, NAB Memory showed a significant increase between treatment and post-treatment. However, given the small magnitude of this increase (and the non-significant effects in Table 3), this change is most consistent with a modest practice effect rather than a robust IFN effect. No other effects were significant in these models. Overall, these results indicate there was no statistically robust or clinically significant effect of IFN on objective cognitive performance during treatment or following treatment termination.
Regarding IFN effects on psychiatric symptoms, following a Bonferroni correction for multiple comparisons, the 2 (Visit 1, Visit 2) × 2(IFN+ Group, IFN− Group)mixed effects regression models (Table 3) remained significant and were therefore interpreted for BDI Total Score, BDI Somatic Factor, GADI, FSS, and BPI-PI (but not for BDI Cognitive Affective Factor or BPI-PS). Significant interaction effects were observed for the BDI Total Score and the BDI Somatic Factor, suggesting that IFN is associated with significantly increased somatic but not cognitive–affective symptoms of depression during treatment. Significant interaction effects were also observed on the GADI and FSS (but not on the BPI-PI), suggesting that IFN is also associated with increased anxiety and fatigue during treatment. Results from the mixed effects regression model evaluating changes across visits before, during and after IFN in the IFN+ group (Table 4) were largely consistent with the results in Table 3. Following Bonferroni corrections for multiple comparisons, BDI Total Score, BDI Cognitive Affective Factor, GADI, FSS and BPI-PI showed significant increases between baseline and mid-treatment. BDI Total Score, BDI Somatic Factor, BDI Cognitive Affective Factor, GADI, and FSS showed significant decreases between treatment and post-treatment (see means across visits in Table 2). Psychiatric rating scales with scores that had significant between visit effects are also shown in Fig. 1. Overall, these results indicate that IFN is associated with significant increases in psychiatric symptoms (depression, anxiety, fatigue, pain) during treatment which decrease or remit following treatment termination.
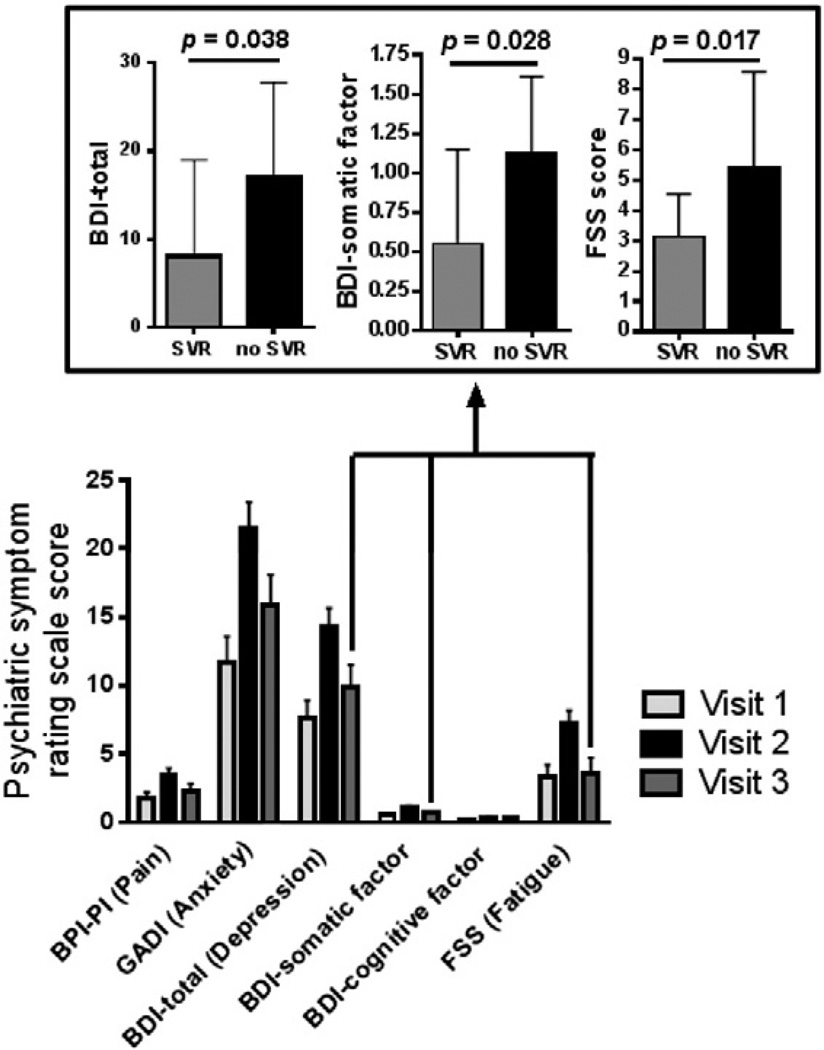
Fig. 1.
Psychiatric symptom rating scale scores before, during and after interferon alpha-based antiviral therapy (IFN) for hepatitis C virus (HCV) infection (IFN+ Group, n = 33). Significant (p < 0.05) changes across visits were observed on the Beck Depression Inventory, Second Edition (BDI) Total, BDI Somatic Factor, BDI Cognitive Affective Factor, Generalized Anxiety Disorder Inventory (GADI), Fatigue Severity Scale (FSS), and Brief Pain Inventory-Pain Interference (BPI-PI); specifically, psychiatric symptoms increased at Visit 2 during IFN and then returned to near baseline levels at Visit 3 following IFN termination. Inset box illustrates that for individuals undergoing IFN for HCV (IFN+ Group), there were significant effects of sustained viral response (SVR) on fatigue (FSS), overall depression (BDI Total), and somatic depression (BDI Somatic Factor) scores. Participants who achieved an SVR (n= 19) reported less fatigue (p= 0.017), less overall depression (p = 0.038), and fewer somatic depressive symptoms (p = 0.028), as compared with those who did not achieve an SVR (n= 6).
Post-hoc analyses
Post-hoc exploratory regression models were constructed to determine whether HCV disease variables [AST, ALT, ammonia, bilirubin, and albumin levels] significantly mediated changes in cognitive or psychiatric outcomes across visits. This was accomplished by a series of mixed effects regression analyses (with adjusted standard errors for participant) with appropriate distributional assumptions, with the primary outcome measures entered as the dependent variables, visit number entered as an independent variable, and the HCV disease variables entered as simultaneous covariates. When ALT and AST were included in the FSS regression, the increase in FSS from Visit 1 to Visit 2 became non-significant (but the decrease from Visit 2 to Visit 3 remained significant) showing that ALT and AST in part mediated the effect between FSS and IFN. None of the other HCV disease variables were significant in the FSS model. Otherwise, results were consistent with those shown in Table 4, with none of the other outcomes being mediated by any of the HCV diseases variables.
Additional post-hoc exploratory analyses were conducted to determine whether SVR contributed to cognitive and psychiatric outcomes within the IFN+ group. Normal and Poisson regressions compared the psychiatric and cognitive outcomes as dependent variables and SVR at Visit 3 as an independent variable. SVR was significantly associated with the BDI Total Score (z = −2.07, p = 0.038), indicating that those with a SVR were less depressed (M= 8.05, SD= 10.92) than those who did not achieve a SVR (M = 17.0, SD = 10.8) (Fig. 1). SVR was significantly associated with the BDI Somatic factor (z = −2.19, p = 0.028), similarly showing lower scores for those attaining a SVR (M = 0.55, SD = 0.60) than those who did not (M = 1.12, SD = 0.49) (Fig. 1). FSS scores were also significantly lower for those who attained a SVR (z = −2.38, p = 0.017) (M = 3.14, SD = 1.39) than those who did not (M = 5.44, SD = 3.15) (Fig. 1). SVR was not significant for the NAB indices, BDI Cognitive Affective Factor, GADI or the BPI scales.
Lastly, rates of clinically significant depressive episodes, defined as a BDI score >18, were explored post-hoc across groups, and based on SVR in the IFN+ group using chi square analysis. At Visit 1, rates of clinically significant depressive episodes were comparable across groups (IFN− group= 10%, IFN+ = 9%). At Visit 2, rates were significantly higher in the IFN+ group (IFN− = 6%, IFN+ = 24%). At Visit 3, individuals in the IFN+ group who achieved a SVR had a much lower rate of clinically significant depressive episodes (15%) compared with those who did not achieve a SVR (50%), but this was a non-significant trend (p = 0.087).
Discussion
Overall, our results indicate that adults undergoing IFN treatment for HCV report significantly increased psychiatric symptoms, including depression, anxiety, fatigue and pain that interferes with daily life during treatment, but that these symptoms remit or are markedly reduced six months following treatment termination. Although many studies have documented increased rates of depression during IFN therapy [8, 26], our data reveals that while the somatic symptoms of depression, including loss of energy, fatigue, changes in sleep patterns, changes in appetite, loss of interest in sex, irritability, and concentration difficulties, are significantly increased during treatment, the changes are not as robust for many other classic symptoms of depression, namely cognitive and affective symptoms including sadness, pessimism, feelings of failure, feeling guilty, feeling punished, self-dislike, self-criticism, suicidal ideation, crying, agitation, or worthlessness. These findings extend our previous work demonstrating that somatic, but not cognitive and affective, symptoms of depression are increased in untreated HCV+ adults [37,45].
Moreover, we found evidence that individuals who achieved a SVR following IFN termination reported significantly less depression and fatigue following IFN than those who did not achieve a SVR. This latter finding is consistent with our recent work demonstrating that peripheral immune activation, particularly as indicated by altered levels of immunoregulatory factors such as inflammatory cytokines, is significantly associated with increased psychiatric symptoms in individuals with and without HCV [46]. Similarly, an expanding literature demonstrates that elevations of pro-inflammatory cytokines and chemokines are evidenced in patients diagnosed with a range of chronic psychiatric disorders including depression [47,48], anxiety [49,50], chronic fatigue syndrome [51], and pain disorders [52–55]. Although the present study design prevents definitive confirmation of mechanism, results support the hypothesis that, upon HCV viral clearance in individuals treated with IFN, cytokines and other immune factors return to normal homeostatic levels, reversing a pattern of extended peripheral immune activation and inflammation, and contributing toward improved mood and psychiatric function.
In contrast, we found no evidence of significant decline in terms of objective cognitive performance on neuropsychological tests during or following IFN treatment for HCV. Nor did we find evidence that SVR or other HCV disease variables were associated with improved cognitive performance following IFN termination. As the results of previous longitudinal studies evaluating change on objective neuropsychological measures across IFN for HCV have been largely mixed, our results can neither be deemed consistent nor inconsistent with the available literature. Table 5 provides a summary of results from selected published studies prospectively following the neuropsychological performance of adults with HCV across IFN. Of the published studies that measured neuropsychological performance both at baseline (i.e., prior to treatment) as well as during IFN, 9/14 reported a significant IFN associated decline in at least one cognitive domain [11,15–19,21,22,24], while 5/14 did not [10,12–14,23]. Across the nine studies that detected cognitive decline, there was little consistency with regard to which domains were significantly affected. The discrepant study results are likely due to large variations in study design including selection of neuropsychological measures and sample characteristics. For example, only 6/14 of these studies included a non-treated control group [12,13,15,19,21,22] (4/6 detecting significant decline), six primarily utilized computerized batteries [17–19,22–24] (5/6 detecting significant decline), three were designed primarily as neuroimaging studies [one was an electroencephalogram (EEG) study [10], one a magnetic resonance spectroscopy (MRS) study [12], and another a positron emission tomography (PET) study [16]] (1/3 detecting decline), three report only on one global cognitive score (1/3 finding a decline), two report on only one cognitive domain [15,22] (both finding decline), and two include only previous non-responders to IFN undergoing repeat IFN rather than treatment naïve adults [13,14] (neither finding a decline).
Table 5.
Summary of results from published studies prospectively evaluating objective cognitive performance across interferon-alpha based treatment (IFN) in adults with chronic hepatitis C virus (HCV) infection.
| Study | Sample. Evaluation time points across IFN. |
Cognitive domains tested during IFN (Was there a significant IFN related decline in this domain during IFN relative to baseline?) |
Cognitive domains tested following IFN termination (Was there a significant IFN related change in this domain post IFN relative to baseline?) |
|---|---|---|---|
| Amodio et al., 2005 [10] | HCV+ adults: 1) IFN+ (n = 20). Evaluated at baseline; during IFN (2 m, 6 m) | Auditory Attention/Working Memory (N); Speeded Visual Processing (N); Visuoconstruction (N); Verbal Learning (N); Verbal Fluency (N); Executive-Inhibition/Switching (N) | None |
| Baranyi et al., 2013 [11] | HCV+ adults: 1) IFN+ (n = 41). Evaluated at baseline; during IFN (1 m, 3 m, 6 m, 9 m); post IFN | Global Score of Memory and Attention (Y) | Global Score of Memory and Attention (N) |
| Byrnes et al., 2012 [12] | Non cirrhotic HCV+ adults: 1) IFN+ (n = 15), 2) IFN− (n = 7). Evaluated at baseline; during IFN (12w); post IFN (12w) | Verbal Learning (N); Verbal Recall (N); Visual–Spatial Learning (N); Visual–Spatial Recall (N); Visuoconstruction (N); Speeded Visual Processing (N); Auditory Attention/Working Memory (N); Verbal Fluency (N); Motor Speed (N); Executive-Inhibition/Switching (N) | Individuals with an SVR, but not those without an SVR, significantly improved on the following domains: Verbal Learning (Y); Verbal Recall (T); Visual–Spatial Learning (Y); Visual–Spatial Recall (N); Visuoconstruction (N); Speeded Visual Processing (T); Auditory Attention/Working Memory (N); Verbal Fluency (N); Motor Speed (N); Executive-Inhibition/Switching (N) |
| Fontana et al., 2007 [14] | HCV+ adults with advanced fibrosis who were previous non-responders to IFN: 1) retreated with IFN for 24w (n = 177), 2) had an SVR at 24w and continued IFN for 48w (n = 57). Evaluated at baseline; during IFN (24w, 48w); post IFN (24w) | Visual–Spatial Learning (N); Auditory Attention/Working Memory (N); Speeded Visual Processing (N); Motor Speed (N); Executive-Problem Solving/Mental Flexibility (N); Verbal Fluency (N); Reaction Time (N) | Adults in Group 2 showed significant improvements at 24w post IFN on the following: Visual-Spatial Learning (Y); Auditory Attention/Working Memory (N); Speeded Visual Processing (Y); Motor Speed (N); Executive-Problem Solving/Mental Flexibility (Y); Verbal Fluency (Y); Reaction Time (N) |
| Fontana et al., 2010 [13] | HCV+ adults with advanced fibrosis who were previous non-responders to IFN, randomized to: 1) IFN+ for 3.5 years (n = 66), 2) IFN− (n = 63). Evaluated at baseline; during IFN (12 m, 24 m, 36 m, and 48 m) | Global Deficit Score (N) | None |
| Hilsabeck et al., 2005 [15] | HCV+ adults: 1) IFN+ (n = 11), 2) IFN− (n = 19). Evaluated at baseline; during IFN (6 m). | Speeded Visual Processing (Y) | None |
| Juengling et al., 2000 [16] | HCV+ adults: 1) IFN+ (n = 11). Evaluated at baseline; during IFN (12w) | Verbal Learning (Y); Verbal Fluency (N); Speeded Visual Processing (N) | None |
| Kraus et al., 2005 [17] | HCV+ adults: 1) IFN+ (n = 70). Evaluated at baseline; during IFN (4w, 3–4 m, 6–8 m); post IFN (6–8 m) | Reaction Time/Motor Speed (Y); Divided Attention (N); Sustained Visual Attention (N); Visual Attention/Working Memory (Y) | Reaction Time/Motor Speed (N); Divided Attention (N); Sustained Visual Attention (N); Visual Attention/Working Memory (N) |
| Lieb et al., 2006 [18] | 33 HCV+, 4 HBV+, and 1 HBV+/HCV+ adults: 1) IFN+ (n = 38). Evaluated at baseline; during IFN (12w) | Verbal Learning (Y); Speeded Visual Processing (N); Verbal Fluency (Y) | None |
| Majer et al., 2008 [19] | HCV+ adults: 1) IFN+ (n = 20); 2) IFN− (n = 12). Evaluated at baseline; during IFN (12w) | Reaction Time/Motor Speed (Y); Speeded Visual Processing (N); Executive-Inhibition/Switching (N); Executive-Problem Solving/Mental Flexibility (N) | None |
| Pattullo et al., 2011 [20] | HCV+ adults with no other cognitive risk factors: 1) IFN+ with SVR (n = 31), 2) IFN+ without SVR (n = 9), 3) IFN− (n = 39). Evaluated at baseline; post IFN (6 m) | None | Verbal Learning (N); Visual Learning (N); Speeded Visual Processing (Y — Group 2 only declined); Auditory Attention/Working Memory (N); Motor Speed (N); Executive-Inhibition/Switching (N); Executive-Problem Solving/Mental Flexibility (N) |
| Pawelczyk et al., 2008 [21] | HCV+ adults: 1) IFN+ (n = 26), 2) IFN− (n = 21). Evaluated at baseline; during IFN (12w) | Executive-Inhibition/Switching (Y); Speeded Visual Processing (Y) | None |
| Raison et al., 2010 [22] | HCV+ adults: 1) IFN+ (n = 19), 2) IFN− (n = 12). Evaluated at baseline; during IFN (12w) | Reaction Time/Motor Speed (Y) | None |
| Thein et al., 2007 [23] | IFN+ (n = 34): 1) HCV+ Monoinfected (n = 19), 2) HIV+/HCV+ Coinfected (n = 15). Evaluated at baseline; during IFN (18w, 42w); post IFN (24w) | Global Cognitive Performance (Subtests measured Speeded Visual Processing and Reaction Times/Motor Speed) (N) | At 24w post IFN, Group 2 only significantly improved in terms of Global Cognitive Performance related to Speeded Visual Processing. In the Total Sample, those who achieved an SVR, but not those who did not, performed significantly better following IFN termination on tests of Reaction Time/Motor Speed. |
| Wobrock et al., 2009 [24] | HCV+ adults: 1) IFN +(n = 17). Evaluated at baseline; during IFN (12w) | Attention (N); Speeded Visual Processing (Y); Motor Speed (N); Reaction Time/Motor Speed (N) | None |
HCV = Hepatitis C virus. HCV+ = Infected with the hepatitis C virus. HIV+ = Infected with the human immunodeficiency virus. IFN = Interferon-alpha based antiviral therapy. IFN+ = Undergoing interferon-alpha based antiviral therapy. m = Month. N = No, there were no significant effects in this cognitive domain. SVR = Sustained viral response. T = There was a non-significant trend suggesting a possible effect in this cognitive domain. w = Week. Y = Yes, there was a significant effect in this cognitive domain.
The results of the six published studies that compared neuropsychological performance at baseline with performance several months following IFN termination are also mixed (Table 5). These types of studies are particularly important because patients and clinicians want to know whether potential IFN associated cognitive effects are permanent (i.e., persist long-term) versus temporary (i.e., subside once IFN is terminated). One study found significant decreases in cognitive performance during IFN, which appeared to abate following treatment termination regardless of depression [11]. Another study [17] found no significant differences in cognitive performance between baseline and 6–8 months post IFN termination, regardless of antiviral response. Since these two studies had found significant declines during IFN, these results might suggest that IFN induced cognitive effects are temporary and return to baseline following IFN termination. However, another study [20] measured cognitive performance at baseline and 6 months post IFN termination and found no differences across time points except that a subgroup of individuals who did not achieve an SVR significantly declined between baseline and IFN termination in Speeded Visual Processing but no other cognitive domains; this study did not measure performance during IFN. In contrast, the remaining 3/5 studies [12,14,23] found that the subgroup of IFN treated individuals who maintained an SVR following IFN termination (but not those who did not achieve an SVR) improved in at least one cognitive domain compared with baseline. The cognitive domains that improved were not consistent across these latter three studies, and none of these three studies detected significant cognitive decline during IFN in their samples.
In light of the inconsistencies across available neuropsychological studies, it is worth highlighting important strengths that increase the validity of our study demonstrating non-significant effects of IFN on cognition. We evaluated participants before, during, and after IFN therapy, and our IFN treated group was compared with an untreated control group that was similar in terms of key demographic, psychiatric and medical characteristics (Table 1). This design allowed us to rule out confounds such as practice effects or medical factors that might otherwise explain improvement or decline across time in a study without a control group. The neuropsychological assessment measures we selected, namely the NAB Attention, Memory, and Executive Functions Modules, are from a widely-used, well-validated, and clinically relevant instrument yielding demographically corrected standard summary index scores based on a very large normative sample; the structure of the NAB allowed us to focus our primary analyses on a small number of robust index scores that were each derived from multiple individual subtests within a cognitive domain. We also used a mixed effects model that accounted for repeated measures and individual baseline scores, and we controlled for multiple comparisons. This analysis plan minimized our risk of over-interpreting weak effects of limited clinical significance.
Despite strengths, several limitations should be discussed. Our control group was assessed at two rather than three time points, requiring us to conduct two separate analyses to effectively assess for IFN effects during and following treatment. Our sample size was adequate for the present analyses, but not large, potentially increasing the risk of sample specific findings. For example, our sample was 63% male and 91% Caucasian, potentially limiting its generalizability to more diverse populations.
It is worth noting that in the very near future there will likely be new and improved treatments for HCV that do not use IFN, and, fortunately, these new treatment options are not anticipated to cause cognitive or psychiatric side effects [56–58]. Until these new treatments are available, healthcare providers should continue to screen for and treat depression and other psychiatric symptoms in patients who are considering IFN for HCV [59–62]. Further, it is well-documented that the development of depression during IFN therapy can be effectively treated using pharmacotherapeutic interventions (e.g., selective serotonin reuptake inhibitor antidepressants) or even prevented (in subsets of patients) by the use of prophylactic antidepressant treatment [60,63–66].
In summary, our study adds to the literature by using both well-validated objective neuropsychological measures as well as psychiatric symptom questionnaires to longitudinally characterize symptom course in patients with HCV who were either initiating or not initiating IFN for HCV. Our results indicate that patients report significant increases in psychiatric symptoms, including symptoms of depression, anxiety, fatigue and pain, during IFN treatment, which remit following treatment termination, with added benefit if viral clearance is achieved. However, IFN treatment for HCV was not associated with significant declines in objective cognitive performance on tests of attention, memory, or executive functions during IFN treatment or following IFN treatment termination.
Acknowledgments
This work was supported by career development awards to M.H. (Staff Psychologist and Neuropsychologist) and J.M.L. (Research Scientist) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development. This material is the result of work supported with resources and the use of facilities at the Portland Veterans Affairs Medical Center, Portland, Oregon. The authors thank the study participants and staff at each of the recruitment sites, especially Betsy Zucker and Janice Voukidis. The authors also acknowledge Arthur Vandenbark, Peter Hauser, William Hoffman, Diane Howieson, Daniel Storzbach, and Alexander Stevens for consultation regarding study design and implementation. All authors read and approved the final contents of the manuscript.
Footnotes
Conflict of interest statement
The authors have no competing interests to declare.
References
- 1.Global Burden Of Hepatitis CWG. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. doi: 10.1177/0091270003258669. http://dx.doi.org/10.1177/0091270003258669 [PubMed PMID: 14681338]. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7:261–287. doi: 10.1016/s1089-3261(02)00078-8. [PubMed PMID: 12691470]. [DOI] [PubMed] [Google Scholar]
- 3.Yee HS, Chang MF, Pocha C, Lim J, Ross D, Morgan TR, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107:669–689. doi: 10.1038/ajg.2012.48. http://dx.doi.org/10.1038/ajg.2012.48 [quiz 90, PubMed PMID: 22525303]. [DOI] [PubMed] [Google Scholar]
- 4.Hauser P, Morasco BJ, Linke A, Bjornson D, Ruimy S, Matthews A, et al. Antiviral completion rates and sustained viral response in hepatitis C patients with and without preexisting major depressive disorder. Psychosomatics. 2009;50:500–505. doi: 10.1176/appi.psy.50.5.500. http://dx.doi.org/10.1176/appi.psy.50.5.500 [PubMed PMID: 19855036; PubMed Central PMCID: PMC2987665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Hartung D, Rahman B, Wasson N, Cottrell EB, Fu R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med. 2013;158:114–123. doi: 10.7326/0003-4819-158-2-201301150-00576. [PubMed PMID: 23437439]. [DOI] [PubMed] [Google Scholar]
- 6.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. http://dx.doi.org/10.1056/NEJMoa1010494 [PubMed PMID: 21449783; PubMed Central PMCID: PMC3766849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. http://dx.doi.org/10.1056/NEJMoa1014463 [PubMed PMID: 21916639; PubMed Central PMCID: PMC3809077]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. http://dx.doi.org/10.1016/j.jad.2004.04-002 [PubMed PMID: 15488246]. [DOI] [PubMed] [Google Scholar]
- 9.Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847–854. doi: 10.1017/S1355617703960048. [PubMed PMID: 14632243]. [DOI] [PubMed] [Google Scholar]
- 10.Amodio P, De Toni EN, Cavalletto L, Mapelli D, Bernardinello E, Del Piccolo F, et al. Mood, cognition and EEG changes during interferon alpha (alpha-IFN) treatment for chronic hepatitis C. J Affect Disord. 2005;84:93–98. doi: 10.1016/j.jad.2004.09.004. http://dx.doi.org/10.1016/j.jad.2004.09.004 [PubMed PMID: 15620390]. [DOI] [PubMed] [Google Scholar]
- 11.Baranyi A, Meinitzer A, Stepan A, Putz-Bankuti C, Breitenecker RJ, Stauber R, et al. A biopsychosocial model of interferon-alpha-induced depression in patients with chronic hepatitis C infection. Psychother Psychosom. 2013;82:332–340. doi: 10.1159/000348587. http://dx.doi.org/10.1159/000348587 [PubMed PMID: 23942342]. [DOI] [PubMed] [Google Scholar]
- 12.Byrnes V, Miller A, Lowry D, Hill E, Weinstein C, Alsop D, et al. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56:549–556. doi: 10.1016/j.jhep.2011.09.015. http://dx.doi.org/10.1016/j.jhep.2011.09.015 [PubMed PMID: 22027578]. [DOI] [PubMed] [Google Scholar]
- 13.Fontana RJ, Bieliauskas LA, Back-Madruga C, Lindsay KL, Litman HJ, Lok AS, et al. Cognitive function does not worsen during long-term low-dose peginterferon therapy in patients with chronic hepatitis C. Am J Gastroenterol. 2010;105:1551–1560. doi: 10.1038/ajg.2010.3. http://dx.doi.org/10.1038/ajg.2010.3 [PubMed PMID: 20104219; PubMed Central PMCID: PMC3772520]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana RJ, Bieliauskas LA, Lindsay KL, Back-Madruga C, Wright EC, Snow KK, et al. Cognitive function does not worsen during pegylated interferon and ribavirin retreatment of chronic hepatitis C. Hepatology. 2007;45:1154–1163. doi: 10.1002/hep.21633. http://dx.doi.org/10.1002/hep.21633 [PubMed PMID: 17465000]. [DOI] [PubMed] [Google Scholar]
- 15.Hilsabeck RC, Hassanein TI, Ziegler EA, Carlson MD, Perry W. Effect of interferon-alpha on cognitive functioning in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2005;11:16–22. doi: 10.1017/S1355617705050022. http://dx.doi.org/10.1017/S1355617705050022 [PubMed PMID: 15686604]. [DOI] [PubMed] [Google Scholar]
- 16.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, et al. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology. 2000;152:383–389. doi: 10.1007/s002130000549. [PubMed PMID: 11140330]. [DOI] [PubMed] [Google Scholar]
- 17.Kraus MR, Schafer A, Wissmann S, Reimer P, Scheurlen M. Neurocognitive changes in patients with hepatitis C receiving interferon alfa-2b and ribavirin. Clin Pharmacol Ther. 2005;77:90–100. doi: 10.1016/j.clpt.2004.09.007. http://dx.doi.org/10.1016/j.clpt.2004.09.007 [PubMed PMID: 15637534]. [DOI] [PubMed] [Google Scholar]
- 18.Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, et al. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNalpha): results from a prospective study. Eur Psychiatry. 2006;21:204–210. doi: 10.1016/j.eurpsy.2004.09.030. http://dx.doi.org/10.1016/j.eurpsy.2004.09.030 [PubMed PMID: 16632167]. [DOI] [PubMed] [Google Scholar]
- 19.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. http://dx.doi.org/10.1016/j.bbi.2007.12.009 [PubMed PMID: 18258414; PubMed Central PMCID: PMC2497339]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattullo V, McAndrews MP, Damyanovich A, Heathcote EJ. Influence of hepatitis C virus on neurocognitive function in patients free from other risk factors: validation from therapeutic outcomes. Liver Int. 2011;31:1028–1038. doi: 10.1111/j.1478-3231.2011.02549.x. http://dx.doi.org/10.1111/j.1478-3231.2011.02549.x [PubMed PMID: 21733093]. [DOI] [PubMed] [Google Scholar]
- 21.Pawelczyk T, Pawelczyk A, Strzelecki D, Rabe-Jablonska J. Pegylated interferon alpha and ribavirin therapy may induce working memory disturbances in chronic hepatitis C patients. Gen Hosp Psychiatry. 2008;30:501–508. doi: 10.1016/j.genhosppsych.2008.03.001. http://dx.doi.org/10.1016/j.genhosppsych.2008.03.001 [PubMed PMID: 19061675]. [DOI] [PubMed] [Google Scholar]
- 22.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, et al. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. http://dx.doi.org/10.1016/j.biopsych.2010.04.019 [PubMed PMID: 20537611; PubMed Central PMCID: PMC2937202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thein HH, Maruff P, Krahn MD, Kaldor JM, Koorey DJ, Brew BJ, et al. Improved cognitive function as a consequence of hepatitis C virus treatment. HIV Med. 2007;8:520–528. doi: 10.1111/j.1468-1293.2007.00505.x. http://dx.doi.org/10.1111/j.1468-1293.2007.00505.x [PubMed PMID: 17944685]. [DOI] [PubMed] [Google Scholar]
- 24.Wobrock T, Mihm U, Lohr C, Hofmann WP, Sarrazin C, Zeuzem S, et al. Cognition in hepatitis C patients treated with pegylated interferon. World J Biol Psychiatry. 2009;10:819–826. doi: 10.1080/15622970701714362. http://dx.doi.org/10.1080/15622970701714362 [PubMed PMID: 19995219]. [DOI] [PubMed] [Google Scholar]
- 25.Manos MM, Ho CK, Murphy RC, Shvachko VA. Physical, social, and psychological consequences of treatment for hepatitis C: a community-based evaluation of patient-reported outcomes. Patient. 2013;6:23–34. doi: 10.1007/s40271-013-0005-4. http://dx.doi.org/10.1007/s40271-013-0005-4 [PubMed PMID: 23420134; PubMed Central PMCID: PMC3619379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:1128–1138. doi: 10.4088/JCP.12r07694. http://dx.doi.org/10.4088/JCP.12r07694 [PubMed PMID: 22967776]. [DOI] [PubMed] [Google Scholar]
- 27.Galvao-de Almeida A, Quarantini LC, Batista-Neves S, Lyra AC, Parana R, de Oliveira IR, et al. Is the interferon-alpha-triggered depressive episode a self-limited kind of depression? Four cases of persistent affective symptoms after antiviral treatment in HCV-infected individuals. World J Biol Psychiatry. 2010;11:914–918. doi: 10.3109/15622975.2010.504282. http://dx.doi.org/10.3109/15622975.2010.504282 [PubMed PMID: 20642400]. [DOI] [PubMed] [Google Scholar]
- 28.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [PubMed PMID: 7989716]. [DOI] [PubMed] [Google Scholar]
- 29.Kajitani K, Kanba S. Escitalopram ameliorates prolonged depression with psychotic features induced by interferon therapy for hepatitis C infection: report of 2 cases. Psychosomatics. 2013;54:506–507. doi: 10.1016/j.psym.2013.05.001. http://dx.doi.org/10.1016/j.psym.2013.05.001 [PubMed PMID: 23845321]. [DOI] [PubMed] [Google Scholar]
- 30.Rifflet H, Vuillemin E, Oberti F, Duverger P, Laine P, Garre JB, et al. Suicidal impulses in patients with chronic viral hepatitis C during or after therapy with interferon alpha. Gastroenterol Clin Biol. 1998;22:353–357. [PubMed PMID: 9762223]. [PubMed] [Google Scholar]
- 31.Schaefer M, Capuron L, Friebe A, Diez-Quevedo C, Robaeys G, Neri S, et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol. 2012;57:1379–1390. doi: 10.1016/j.jhep.2012.07.037. http://dx.doi.org/10.1016/j.jhep.2012.07.037 [PubMed PMID: 22878466]. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and statistical manual for mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Text Revision ed.]. [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [quiz 4–57. PubMed PMID: 9881538]. [PubMed] [Google Scholar]
- 34.Psychological Corporation. Wechsler test of adult reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 35.Stern R, White T. Neuropsychological assessment battery. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 36.Beck AT. Beck Depression Inventory. 2nd ed. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 37.Patterson AL, Morasco BJ, Fuller BE, Indest DW, Loftis JM, Hauser P. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33:354–362. doi: 10.1016/j.genhosppsych.2011.04.005. [PubMed PMID: 21762832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argyropoulos SV, Ploubidis GB, Wright TS, Palm ME, Hood SD, Nash JR, et al. Development and validation of the Generalized Anxiety Disorder Inventory (GADI) J Psychopharmacol. 2007;21:145–152. doi: 10.1177/0269881107069944. [PubMed PMID: 17329293]. [DOI] [PubMed] [Google Scholar]
- 39.Ferentinos P, Kontaxakis V, Havaki-Kontaxaki B, Dikeos D, Lykouras L. Psychometric evaluation of the Fatigue Severity Scale in patients with major depression. Qual Life Res. 2011;20:457–465. doi: 10.1007/s11136-010-9769-3. [PubMed PMID: 20953713]. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker C, Chan K, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res. 2000;9:499–508. doi: 10.1023/a:1008960710415. [PubMed PMID: 11190005]. [DOI] [PubMed] [Google Scholar]
- 41.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [PubMed PMID: 2803071]. [DOI] [PubMed] [Google Scholar]
- 42.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed PMID: 8080219]. [PubMed] [Google Scholar]
- 43.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [PubMed PMID: 15322437]. [DOI] [PubMed] [Google Scholar]
- 44.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [PubMed PMID: 15042521]. [DOI] [PubMed] [Google Scholar]
- 45.Loftis JM, Patterson AL, Wilhelm CJ, McNett H, Morasco BJ, Huckans M, et al. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: a 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. http://dx.doi.org/10.1016/j.jpsychores.2012.10.012 [PubMed PMID: 23272989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huckans M, Fuller BE, Olavarria H, Sasaki AW, Chang M, Flora KD, et al. Multi-analyte profile (MAP) analysis of plasma immune proteins: Altered expression of peripheral immune factors is associated with neuropsychiatric symptom severity in adults with and without chronic hepatitis C virus infection. Brain Behav. 2014;4:123–142. doi: 10.1002/brb3.200. [PubMed PMID: 24683507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [PubMed PMID: 22197082]. [DOI] [PubMed] [Google Scholar]
- 48.Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neurosci Lett. 2008;430:264–268. doi: 10.1016/j.neulet.2007.11.001. [PubMed PMID: 18063307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [PubMed PMID: 19319993]. [DOI] [PubMed] [Google Scholar]
- 50.Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Physiol. 2012;27:6–14. doi: 10.1002/hup.1259. [PubMed PMID: 22213434]. [DOI] [PubMed] [Google Scholar]
- 51.Arnett SV, Clark IA. Inflammatory fatigue and sickness behaviour — lessons for the diagnosis and management of chronic fatigue syndrome. J Affect Disord. 2012;141:130–142. doi: 10.1016/j.jad.2012.04.004. [PubMed PMID: 22578888]. [DOI] [PubMed] [Google Scholar]
- 52.Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain. 2012;13:10–20. doi: 10.1016/j.jpain.2011.10.003. [PubMed PMID: 22172450]. [DOI] [PubMed] [Google Scholar]
- 53.de Oliveira CM, Sakata RK, Issy AM, Gerola LR, Salomao R. Cytokines and pain. Rev Bras Anestesiol. 2011;61:255–259. doi: 10.1016/S0034-7094(11)70029-0. [60–5, 137–42. PubMed PMID: 21474032]. [DOI] [PubMed] [Google Scholar]
- 54.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, et al. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152:2802–2812. doi: 10.1016/j.pain.2011.09.005. [PubMed PMID: 22000099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starkweather AR, Lyon DE, Schubert CM. Pain and inflammation in women with early-stage breast cancer prior to induction of chemotherapy. Biol Res Nurs. 2013;15:234–241. doi: 10.1177/1099800411425857. [PubMed PMID: 22084403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stedman CA. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol. 2013;28:38–45. doi: 10.1111/jgh.12028. http://dx.doi.org/10.1111/jgh.12028 [PubMed PMID: 23137126]. [DOI] [PubMed] [Google Scholar]
- 57.Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Mullhaupt B, et al. Faldaprevir (BI 201335), deleobuvir (BI 207127) and ribavirin oral therapy for treatment-naive HCV genotype 1: SOUND-C1 final results. Antivir Ther. 2013;18:1015–1019. doi: 10.3851/IMP2567. http://dx.doi.org/10.3851/IMP2567 [PubMed PMID: 23558093]. [DOI] [PubMed] [Google Scholar]
- 58.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Mullhaupt B, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630–639. doi: 10.1056/NEJMoa1213557. http://dx.doi.org/10.1056/NEJMoa1213557 [PubMed PMID: 23944300]. [DOI] [PubMed] [Google Scholar]
- 59.Bacon BR. Managing hepatitis C. Am J Manage Care. 2004;10:S30–S40. [PubMed PMID: 15084065]. [PubMed] [Google Scholar]
- 60.Loftis JM, Hauser P. Risk and protective factors in interferon-induced depression: treatment strategies and clinical outcomes. Dir Psychiatry. 2008;28:227–242. [Google Scholar]
- 61.Rifai MA, Loftis JM, Hauser P. Interferon-alpha treatment of patients with Hepatitis C: the role of a comprehensive risk-benefit assessment. CNS Drugs. 2005;19:719–721. doi: 10.2165/00023210-200519080-00009. [author reply 21–2. PubMed PMID: 16097855]. [DOI] [PubMed] [Google Scholar]
- 62.Schaefer M, Sarkar R, Diez-Quevedo C. Management of mental health problems prior to and during treatment of hepatitis C virus infection in patients with drug addiction. Clin Infect Dis. 2013;57:S111–S117. doi: 10.1093/cid/cit266. http://dx.doi.org/10.1093/cid/cit266 [PubMed PMID: 23884058]. [DOI] [PubMed] [Google Scholar]
- 63.Jiang HY, Deng M, Zhang YH, Chen HZ, Chen Q, Ruan B. Specific serotonin reuptake inhibitors prevent interferon-alpha-induced depression in patients with hepatitis C: a meta-analysis. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.04.035. http://dx.doi.org/10.1016/j.cgh.2013.04.035 [PubMed PMID: 23648373]. [DOI] [PubMed] [Google Scholar]
- 64.Morasco BJ, Loftis JM, Indest DW, Ruimy S, Davison JW, Felker B, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51:401–408. doi: 10.1176/appi.psy.51.5.401. http://dx.doi.org/10.1176/appi.psy.51.5.401 [PubMed PMID: 20833939; PubMed Central PMCID: PMC2994596]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morasco BJ, Rifai MA, Loftis JM, Indest DW, Moles JK, Hauser P. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients with hepatitis C. J Affect Disord. 2007;103:83–90. doi: 10.1016/j.jad.2007.01.007. http://dx.doi.org/10.1016/j.jad.2007.01.007 [PubMed PMID: 17292481]. [DOI] [PubMed] [Google Scholar]
- 66.Schaefer M, Sarkar R, Knop V, Effenberger S, Friebe A, Heinze L, et al. Escitalopram for the prevention of peginterferon-alpha2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157:94–103. doi: 10.7326/0003-4819-157-2-201207170-00006. http://dx.doi.org/10.7326/0003-4819-157-2-201207170-00006 [PubMed PMID: 22801672]. [DOI] [PubMed] [Google Scholar]