Abstract
Background
Malignant infarction is characterized by the formation of cerebral edema, and medical treatment is limited. Preclinical data suggest that glyburide, an inhibitor of SUR1-TRPM4, is effective in preventing edema. We previously reported feasibility of the GAMES-Pilot study, a two-center prospective, open label, phase IIa trial of 10 subjects at high risk for malignant infarction based on diffusion weighted imaging (DWI) threshold of 82 cm3 treated with RP-1127 (glyburide for injection). In this secondary analysis, we tested the hypothesis that RP-1127 may be efficacious in preventing poor outcome when compared to controls.
Methods
Controls suffering large hemispheric infarction were obtained from the EPITHET and MMI-MRI studies. We first screened subjects for controls with the same DWI threshold used for enrollment into GAMES-Pilot, 82 cm3. Next, to address imbalances, we applied a weighted Euclidean matching. Ninety day mRS 0–4, rate of decompressive craniectomy, and mortality were the primary clinical outcomes of interest.
Results
The mean age of the GAMES cohort was 51 years and initial DWI volume was 102 ± 23 cm3. After Euclidean matching, GAMES subjects showed similar NIHSS, higher DWI volume, younger age and had mRS 0–4—90 % versus 50 % in controls p = 0.049; with a similar trend in mRS 0–3 (40 vs. 25 %; p = 0.43) and trend toward lower mortality (10 vs. 35 %; p = 0.21).
Conclusions
In this pilot study, RP-1127-treated subjects showed better clinical outcomes when compared to historical controls. An adequately powered and randomized phase II trial of patients at risk for malignant infarction is needed to evaluate the potential efficacy of RP-1127.
Keywords: Malignant edema, Stroke, Cerebral edema, Brain swelling, Hemorrhage, Hemorrhagic transformation, Clinical trial, Decompressive craniectomy
Introduction
The sulfonylurea receptor 1 (SUR1) and transient receptor potential melastatin 4 (SUR1-TRPM4) [1] channel is a potential target for early treatment in stroke. The channel is upregulated in neurons, astrocytes, and capillary endothelium in the setting of ATP depletion [2]. Channel opening leads to cell and tissue swelling and is associated with hemorrhagic transformation [3].
Preclinical studies in rodent models of malignant cerebral edema have shown that pharmacological blockade using a low dose of the selective SUR1 inhibitor, glyburide, decreases infarct volume, reduces mortality, and improved neurological function, both with and without co-treatment with recombinant tissue plasminogen activator (rt-PA) [2, 4–7]. Treatment with glyburide is effective up to 10 h after onset of ischemia in these studies [6]. In support of the preclinical data, retrospective studies of diabetic patients taking sulfonylurea drugs and presenting with stroke have shown significantly improved clinical outcomes [8], with a reduced incidence of symptomatic hemorrhagic transformation [9].
Patients suffering a large stroke, especially those with delayed recanalization, are at high risk for progressive edema and hemorrhagic transformation, which independently contribute to increased mortality [10]. In addition, in patients with large infarction, IV rt-PA [11] may be futile [12–14] or even potentially harmful by some analyses [10]. Currently, there is no proven pharmacotherapy for preventing brain swelling in patients suffering a large hemispheric stroke.
We recently reported the feasibility and safety results of an open-label, prospective, phase IIa study of patients with severe ischemic stroke who were at high risk for developing clinically significant brain swelling (Glyburide Advantage in Malignant Edema and Stroke-Pilot; Clini-calTrials.gov identifier: NCT01268683) [15]. We have also shown that intravenous glyburide has a salutary effect on several biomarkers of cerebral edema compared to matched controls [16]. GAMES-Pilot was designed to test the safety and feasibility of RP-1127 administration in patients at high risk for malignant infarction. Here, we report the results of a post hoc exploratory efficacy analysis of RP-1127 (Glyburide for injection) administration in GAMES-Pilot subjects compared to control stroke patients who suffered a large infarction, derived from similar patient populations. A concurrent placebo group was not employed in the GAMES-Pilot study in order to facilitate the rapid clinical experience of RP-1127 in the target patient population. The motivation for this analysis was to compare clinical outcomes from treated patients in a single arm study to similar patients suffering from a large stroke to obtain preliminary information regarding potential efficacy.
Methods
Study Design and Patient Enrollment
The study design for GAMES-Pilot has been previously reported [15]. Briefly, we enrolled ten subjects from the University of Maryland and the Massachusetts General Hospital in an open-label, phase IIa study to study the safety and feasibility of RP-1127 in patients who suffered a large stroke. The major inclusion criteria were as follows: baseline diffusion weighted image (DWI) lesion between 82 and 210 cm3, age 18–80 years, and time from symptom onset to start of study drug infusion of ≤10 h [6]. Subjects were eligible with or without IV rt-PA up to 4.5 h per established criteria [17]. DWI volume of ≥82 cm3 was selected for enrollment because it is specific for malignant infarction and has been independently prospectively validated [18]. Exclusion criteria included the use of endovascular treatment, commitment to decompressive craniectomy (DC) prior to enrollment, clinical signs of herniation prior to RP-1127 administration, inability to undergo MRI evaluation, severe renal or liver disease, admission blood glucose ≤55 mg/dL, and known sulfonylurea treatment in the prior 30 days. Patients were screened as potential study candidates if they presented with National Institutes of Health Stroke Scale (NIHSS) scores ≥10 less than 8 h after time last known at baseline. DWI infarct volume assessment was made at local sites in real time using the ABC/2 method [19].
Procedures for Selecting Controls
Patients with incomplete data from the EPITHET + MMI comparison group were excluded. Comparisons were made between GAMES-Pilot subjects and subjects from two other studies where subjects who suffered from large infarction and underwent early MRI were enrolled. EPITHET was a phase II randomized controlled trial of ischemic stroke patients who were treated with IV rt-PA or placebo between 3 and 6 h [20] and who underwent MRI at baseline and at 3–5 days [21]. MMI-MRI was a prospective observational study of ischemic stroke patients with MCA occlusion who underwent MRI within 6 h of symptom onset [18]; the study objective was to identify early those patients at high risk for developing malignant MCA infarction. Functional outcomes, using modified Rankin Scale scores, were evaluated in GAMES-Pilot, EPITHET, and MMI studies at 90 days.
Treatment
All subjects enrolled in GAMES-Pilot received a 0.13 mg IV bolus of RP-1127 over 2 min, followed by IV drug infusion for 72 h. Blood glucose measurements were performed every 15 min for the first hour, hourly for the first 24 h, every 2 h for the subsequent 48 h of drug infusion, and every 4 h up to 16 h after completion of drug infusion. A pre-specified protocol required that any blood glucose level <70 mg/dL be treated with a bolus of D50W. Insulin therapy for hyperglycemia was permitted for critically ill subjects; however, insulin therapy was not permitted for glucose levels <120 mg/dL. Pre-specified stopping rules were: (i) the RP-1127 dose would be reduced by 30 % for glucose less than 55 mg/dL or three consecutive values less than 70 mg/dL and (ii) a second episode of hypoglycemia would result in cessation of study drug. Osmotherapy and DC were instituted only in the setting of pre-defined criteria for neurological deterioration [22, 23] with the major criterion being a diminished level of arousal attributed to progressive swelling, as determined by the treating physician [22, 23]. These guidelines are consistent with criteria used for DC in the pooled analysis of randomized DC trials [24].
Statistical Analysis
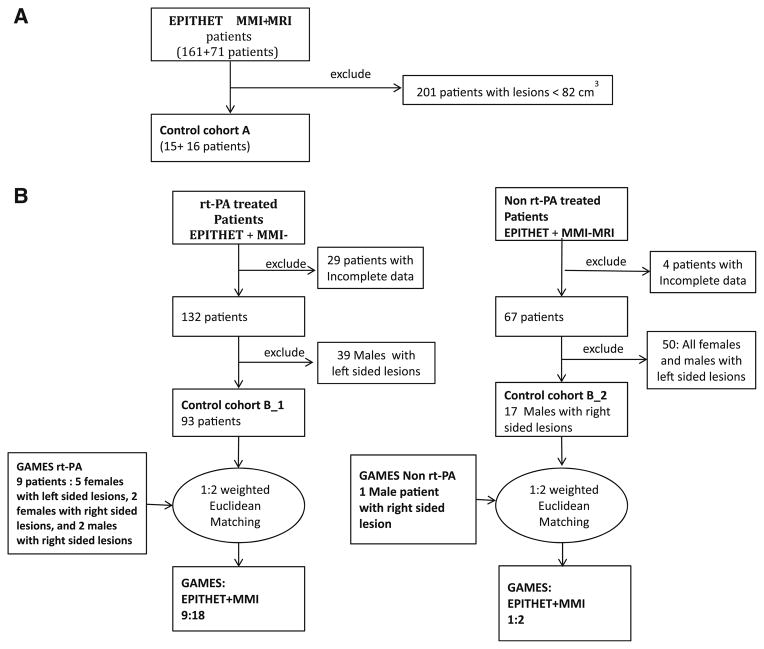
The GAMES-Pilot patients were first compared with the subgroup of patients in EPITHET and MMI-MRI having DWI greater than 82 cm3 (Fig. 1a, control cohort A). Because of resulting imbalances, as a second approach, we applied a weighted Euclidean matching methodology optimized for small sample size (pPAIRS© [9, 25, 26]) to the combined EPITHET and MMI-MRI cohorts to identify historical controls that were best matched to the GAMES-Pilot subjects based on several baseline characteristics (Fig. 1b, control cohort B). Patients from the combined EPITHET and MMI-MRI group were matched to the GAMES-Pilot group on dichotomous factors of hemispheric side of lesion (right/left), gender (male/female), use of rt-PA (yes/no), and continuous factors including baseline NIHSS, age, baseline glucose, and size of DWI lesion at baseline.
Fig. 1.
Flow chart for the matching process. a Matching based on DWI threshold volume >82 cm3. b Euclidean matching
Matching was performed by PM and TAK without knowledge of the clinical outcomes. The K-nn [27] algorithm was used to find the nearest neighbor in terms of a four-dimensional weighted distance. The K-nn algorithm makes no assumptions regarding the distribution of the data and is guaranteed to find the nearest neighbor in up to 12 dimensions [27, 28] The 4D distance was defined here as
Since glucose and DWI lesion volume have larger ranges compared to NIHSS and age, weighted Euclidean matching was adopted.
Subjects from the control group did not enter the matching process if 90 day outcome data or baseline glucose was missing (Fig. 1b). Further, since there are no rt-PA-treated males with left sided lesions in the GAMES group this particular group was eliminated from the control group (n = 39) before matching. Due to an excess of patients in the control group after the first round of dichotomous matching for hemispheric side of lesion, gender, and use of rt-PA, (10:110::GAMES:EPI-THET + MMI), 1:2 matching ratio was used to increase the control group sample size [9]).
Baseline characteristics of the RP-1127-treated group and matched control group were compared with two-sample t tests or Wilcoxon rank-sum test (for NIHSS). The proportions of dichotomous functional measures (mRS 0–4), mortality, and symptomatic hemorrhages were compared between the RP-1127-treated group and the matched control group using Clopper Pearson. The treated group and the cohort B 1:2 matched group functional measures were compared using MacNemar’s test of discordant pairs.
Results
GAMES-Pilot Subjects
Subjects in GAMES-Pilot were often younger than age 60, and IV rt-PA was more commonly used [15] than in historical subjects. As reported in the primary results, ten subjects were enrolled between February 2011 and May 2012 [15]. There were no episodes of hypoglycemia, no instances of dose reduction, and serious adverse events are reported here in Table 1. In Fig. 2, we demonstrate the major sources of screen failures. Other than two subjects who underwent DC in GAMES-Pilot, there were no additional instances of endotracheal intubation.
Table 1.
Frequency of severe adverse events
| Event | Frequency (%) | Relationship |
|---|---|---|
| Herniation | 20 | Unrelated |
| Ischemic stroke | 10 | Unrelated |
| Respiratory failure | 10 | Unrelated |
| Hypotension | 10 | Unrelated |
| Myocardial infarction | 10 | Unrelated |
Fig. 2.
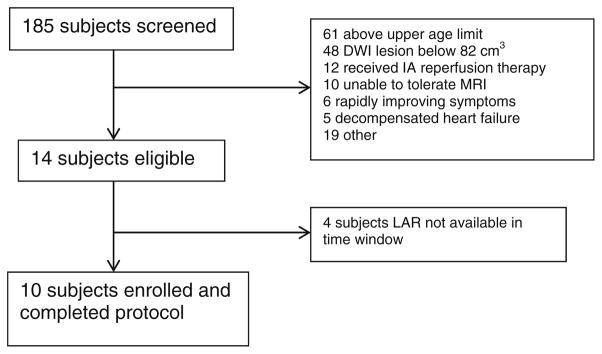
Screen failure and enrollment summary
GAMES-Pilot Subjects Versus Untreated Historical Controls Defined by Initial DWI Lesion Volume
We compared baseline characteristics, clinical outcomes, and neuroimaging metrics of cerebral edema [21] for GAMES-Pilot subjects to those of patients from EPITHET [20] and MMI-MRI [18] with baseline DWI lesion volume >82 cm3 (control cohort A in Fig. 1) (Table 2). GAMES-Pilot subjects were younger and more commonly received rt-PA, while initial DWI lesion volumes were larger in the historical controls (when controls were defined by DWI volume). A 90 day mRS of 0–4 was observed significantly more frequently in GAMES-Pilot patients (90 %) compared to 33 % in EPITHET [20] and 33 % in the MMI-MRI [18] subsets (two-sided p = 0.012, Fisher’s exact test comparing GAMES-Pilot vs. each control group). This trend was consistent across mRS cutoffs of 0–3 (not significant) and the composite outcome of mRS 0–4 without DC, the original pre-specified functional outcome measure (two-sided p = 0.015, Fisher’s exact test comparing GAMES-Pilot vs. each control group). Although not statistically significantly different, GAMES-Pilot subjects had lower mortality.
Table 2.
GAMES-Pilot patients versus historical controls (subset of EPITHET and MMI-MRI subjects with baseline DWI lesion volume >82 cm3)
| Patient characteristic | GAMES-Pilot (n = 10)
|
Subset of EPITHET with DWI > 82 cm3 (n = 15)
|
Subset of MMI-MRI with DWI > 82 cm3 (n = 16)
|
|||
|---|---|---|---|---|---|---|
| Mean or % | 95 % CIa | Mean or % | 95 % CIa | Mean or % | 95 % CIa | |
| Age, mean (SD) | 50.5 (15.3) | (39.6, 61.4) | 70 (13) | (62.8, 77.6) | 59.6 (14.5) | (51.8, 67.3) |
| Onset to MRI (h), mean (SD) | 5.3 (1.9) | (3.9, 6.7) | 4.1 (0.9) | (3.6, 4.6) | 3.0 (1.1) | (2.4, 3.6) |
| Baseline NIHSS, median (IQR) | 18 (8) | (13.6, 22.0) | 19 (5.5) | (16.7, 21.6) | 20 (4.5) | (18.5, 22.8) |
| Baseline glucose, mean (SD) | 7.0 (1.7) | (5.7, 8.2) | 8 (3.4) | (6.6, 10.3) | 8.3 (4.9) | (5.3, 11.2) |
| Initial DWI lesion volume, mean (SD) | 102 (22.6) | (84.4, 119.2) | 141.9 (34.1) | (123.0, 160.8) | 125.3 (43.8) | (102.0, 148.7) |
| Initial hemisphere volume, mean (SD) | 468.2 (73.7) | (420.1, 516.3) | 460.3 (62.7) | |||
| Final visit hemisphere volume, mean (SD) | 507.9 (59.2) | (469.2, 546.6) | 531.8 (59.6) | |||
| IV rt-PA, % (n) | 90 % (9) | (55.5 %, 99.8 %) | 53.3 % (8) | (26.6 %, 78.7 %) | 37.5 % (6) | (15.2 %, 64.6 %) |
| Vessel occlusion | 0 % | (0 %, 30.1 %) | N/A | 0 % | (0 %, 20.6 %) | |
| ICA alone, % (n) | 30 % (3) | (0.3 %, 44.5 %) | N/A | 62.5 % | (35.4 %, | |
| Tandem ICA and MCA, % (n) | 100 % (10) | (69.2 %, 100 %) | N/A | (10) | 84.8 %) | |
| MCA, % (n) | 37.5 % (6) | (15.2 %, 64.6 %) | ||||
| Clinical outcomes | ||||||
| Post-baselineb NIHSS improvement of 4 or more points, % (n) | 40 % (4) | (12.2 %, 73.8 %) | 16.7 % (2/12) | (2.1 %, 48.4 %) | 33.3 % (3/9) | (7.5 %, 70.1 %) |
| 90 day mRSc, median (IQR) | 4 (1) | (2.9, 4.5) | 5 (2) | (4.0, 5.6) | 5 (2) | (4.2, 5.7) |
| 90 day mRSc (0–3), % (n) | 40 % (4) | (12.2 %, 73.8 %) | 13.3 % (2) | (1.7 %, 40.5 %) | 16.7 % (2/12) | (2.1 %, 48.4 %) |
| 90 day mRSc (0–4), % (n) | 90 % (9) | (55.5 %, 99.8 %) | 33.3 % (5) | (11.8 %, 61.6 %) | 33.3 % (4/12) | (9.9 %, 65.1 %) |
| 90 day mRSc (0–4 without DC), % (n) | 80 % (8) | (44.4 %, 97.5 %) | 26.7 % (4) | (7.8 %, 55.1 %) | 26.7 % (4/15) | (7.8 %, 55.1 %) |
| DC, % (n) | 20 % (2) | (2.5 %, 55.6 %) | 6.7 % (1) | (0.2 %, 32.0 %) | 57.1% (8/14) | (28.9 %, 82.3 %) |
| Mortality, % (n) | 10 % (1) | (0.3 %, 44.5 %) | 40 % (6) | (16.3 %, 67.7 %) | 41.7 % (5/12) | (15.2 %, 72.3 %) |
| PH1/PH2, % (n) | 0 % | (0 %, 30.1 %) | 26.7 % (4) | (7.8 %, 55.1 %) | 8.3 %d (1/12) | (0.2 %, 38.5 %) |
Results shown for proportions are based on Clopper–Pearson (exact) methods
Post-baseline NIHSS was at day 7 for GAMES-Pilot and MMI-MRI and at day 3 for EPITHET
Summary includes the 90 day mRS score for one subject who was deemed lost to follow up but was later determined (external to the study) to have an mRS of 3
Based on symptomatic hemorrhage
GAMES-Pilot Subjects Versus Matched Controls
The baseline imbalances observed in the above comparison, in particular the larger baseline DWI lesion volume in controls, could have favored better outcomes by comparison in GAMES-Pilot subjects. We performed weighted Euclidean matching to better match baseline characteristics (control cohort B in Fig. 1). The 10 GAMES-Pilot patients were matched with 20 control group patients (Table 3; Fig. 1b). Comparison of baseline variables of the GAMES-Pilot subjects and EPITHET-MMI subjects and outcomes are shown in Table 3. Matching on baseline NIHSS and glucose was excellent. Following matching, GAMES-Pilot subjects were younger with large DWI lesion volumes compared to the post-match control group (Table 3; p < 0.05).
Table 3.
GAMES-Pilot subjects matched 1:2 against EPITHET and MMI-MRI. Outcomes presented as proportions
| GAMES | EPITHET-MM | p | |
|---|---|---|---|
| NIHSS (median) | 18.0 | 19.0 | 0.531 |
| NIHSS (mean + SD) | 17.8 ± 5.92 | 18.4 ± 3.66 | 0.734 |
| Age (mean + SD) | 50.5 ± 15.3 | 69.5 ± 14.1 | 0.002 |
| Glucose (mean + SD) | 126 ± 31.00 | 119 ± 30.05 | 0.554 |
| DWI (mean + SD) | 102 ± 22.57 | 63 ± 32.05 | 0.003 |
| Number | 10 | 20 | |
| mRS 0–1 | 0.000 | 0.050 | 1.000 |
| mRS 0–2 | 0.100 | 0.100 | 1.000 |
| mRS 0–3 | 0.400 | 0.250 | 0.431 |
| mRS 0–4 | 0.900 | 0.500 | 0.049 |
| mRS 0–5 | 0.900 | 0.650 | 0.213 |
| Mortality | 0.100 | 0.350 | 0.210 |
| Symptomatic hemorrhage | 0.000 | 0.250 | 0.140 |
| Decompression | 0.100 | 0.050 | 1.00 |
Outcome comparison in the two groups showed that despite higher baseline DWI lesion volume (Table 3), GAMES-Pilot subjects had a higher proportion of 90 day mRS of 0–4 (two-sided p = 0.049, Fisher’s exact test). Mortality, symptomatic hemorrhage, and the need for DC were equivalent in both groups. Restricting the matching to patients who received rt-PA (control cohort B_1 in Fig. 1b) confirmed that the pre-specified efficacy measure of mRS 0–4 was improved in GAMES-treated subjects (Table 4; p = 0.013). Mortality in the GAMES rt-PA group demonstrated a trend toward increased survival (p = 0.059). In both cases, similar trends, although not significant, were seen with respect to a higher percentage achieving mRS 0–3, and notably, treatment did not increase the number of subjects with mRS 5 compared to matched controls.
Table 4.
GAMES-Pilot subjects matched 1:2 against EPITHET and MMI-MRI. Outcomes presented as proportions
| GAMES | EPITHET-MM | p | |
|---|---|---|---|
| NIHSS (median) | 19.0 | 19.0 | 0.772 |
| NIHSS (mean + SD) | 18.6 ± 5.75 | 18.4 ± 3.28 | 0.949 |
| Age (mean + SD) | 51.6 ± 15.8 | 69.8 ± 14.2 | 0.006 |
| Glucose (mean + SD) | 128 ± 32.22 | 122 ± 29.89 | 0.646 |
| DWI (mean + SD) | 102 ± 24.13 | 58 ± 30.12 | 0.002 |
| Number | 9 | 18 | |
| mRS 0–1 | 0.000 | 0.056 | 1.000 |
| mRS 0–2 | 0.111 | 0.111 | 1.000 |
| mRS 0–3 | 0.444 | 0.278 | 0.423 |
| mRS 0–4 | 1.000 | 0.500 | 0.013 |
| mRS 0–5 | 1.000 | 0.611 | 0.065 |
| Mortality | 0.000 | 0.389 | 0.059 |
| Symptomatic hemorrhage | 0.000 | 0.278 | 0.136 |
| Decompression | 0.111 | 0.000 | 0.333 |
Matching limited to rt-PA-treated subjects
Discussion
Having previously reported the primary safety data with respect to intravenous glyburide in a prior report of the GAMES-Pilot trial [15], this analysis explored a potential benefit. Our exploratory analysis comparing GAMES-Pilot study outcomes to relevant untreated controls suggests that RP-1127 may improve outcome. Because inevitable imbalances may affect small sample size cohorts disproportionately, we pursued this question with different cohort populations and applied a cohort matching strategy. The first comparison, RP-1127 group compared against a subset of EPITHET-MMI subjects with baseline DWI lesions >82 cm3, without consideration of other baseline variables led to imbalances that favored better outcomes in the treatment group. A separate comparison was then made between the RP-1127-treated group against the combined EPITHET-MMI group matched in the 4D Euclidean space of NIHSS, age, glucose, and DWI lesion volume. This finding led to improved balancing of several baseline factors and confirmed improved outcome in treated subjects.
Weighted Euclidean matching is well suited for studies with small sample sizes where comparison groups have non-overlapping distribution of baseline factors. The K-nn algorithm used here guarantees in identifying the nearest-neighbor matches considering all variables simultaneously [27, 28] and has been demonstrated to provide good matching in relatively small samples [29]. Weighting minimizes the influence of factors that are scaled across wide ranges. Other matching methods such as propensity score generally require very large and overlapping distributions. Matching by hand is difficult when multiple factors are considered. However, use of historical controls does not substitute for a prospective study of subjects treated under more similar circumstances and these findings need to be confirmed.
Patients with large hemispheric stroke and swelling face high case fatality rates [30, 31], with no effective medical treatments available. Our findings in GAMES-Pilot are limited to patients with severe stroke and with criteria similar to those who are at the highest risk of malignant edema, hemorrhagic transformation, and poor outcome. The SUR1-TRPM4 channel is implicated in the pathogenesis of cerebral edema formation [2]. Channel blockade with a constant infusion of low-dose glyburide significantly reduces cerebral edema in several clinically relevant rodent models of stroke [2, 4–7]. SUR1-TRPM4 channels also have been identified in human stroke patients, including those with malignant infarction [32]. Furthermore, glyburide’s penetration of the blood–brain barrier after infarction has been demonstrated in rodent models [2], providing evidence that the agent reaches and engages the target. The molecular mechanism responsible for transcriptional upregulation of SUR1-TRPM4 in the neurovascular unit involves a two-step sequential gene activation process [33], suggesting that an extended time interval will elapse before the channel is in place and available for inhibition.
In accordance with the foregoing preclinical data, we enrolled patients in GAMES-Pilot that were at high risk for brain swelling after ischemia. Patients with a large stroke are most likely to suffer clinically significant swelling. The DWI cutpoint of 82 cm3 has been prospectively validated in an independent cohort [18]. As demonstrated by Thomalla et al. [18], early DWI volume is superior to NIHSS and vessel occlusion for predicting malignant edema. Of note, GAMES-Pilot subjects had median NIHSS of 18 and all had a large vessel occlusion.
Using hemisphere volume as a measure of total brain injury and swelling [21], we found that subjects treated with RP-1127 had mean increases in hemisphere volumes over 3 days of 22.0 cm3. Prior work in hematoma expansion [34] and MRI-based changes in stroke after administration of mannitol [35] suggests that, to be effective, a treatment must reduce volume growth by 8–13 cm3. The smaller growth of infarcts and of hemisphere volumes mirror changes observed in preclinical studies [4–6].
There were no PH1/PH2 class hemorrhages in GAMES-Pilot subjects, despite 90 % of subjects presenting with high NIHSS scores and receiving IV rt-PA [15]. In DEFUSE and EPITHET patients with a large perfusion deficit, PH1/PH2 rates were 30 % [10]. The absence of PH1/PH2 hemorrhage in GAMES-Pilot coincides with our finding that GAMES-Pilot patients had decreased MMP-9 level and attenuated markers of vasogenic edema, compared to matched controls [16]. If confirmed, the decreased hemorrhage rates in GAMES-Pilot would be concordant with a prior retrospective analysis of diabetic stroke patients treated with oral sulfonylureas after the onset of ischemia [9]. These clinical observations also mirror decreased the hemorrhage rates observed in preclinical models of ischemia accompanied by co-treatment with rt-PA and glyburide [6]. In addition, glyburide is reported to be associated with reduced MMP-9 activity [16], as is seen in hemorrhagic transformation [36]. An association between decreased hemorrhagic transformation and improved outcome after human stroke has been reported previously [8, 9].
Managing patients with a large stroke requires neuro-intensive care for mechanical ventilation, osmotherapy, and DC in the event of herniation [37]. RP-1127-treated subjects had low rates of intubation and osmotherapy, despite uniformly large infarct volumes, in contrast to prior observations in patients with a large stroke [38–40]. Whether or not the incidence of critical care interventions is lower in RP-1127-treated patients must be evaluated in future studies since those already committed to DC were excluded.
RP-1127 administration was safe and feasible in GAMES-Pilot. We did not identify any intervention-specific serious adverse events, including hypoglycemia. In addition, we achieved study milestones for subject enrollment. Future studies will need to identify centers where early MRI is readily incorporated into acute stroke evaluation.
In the cohorts selected by Euclidian matching, more favorable outcome was observed in the GAMES-Pilot subjects despite nearly 50 % larger baseline DWI volumes. GAMES-Pilot subjects were younger than pooled control subjects reflected the overall older age of the pooled control subjects and lack of availability for matching of younger subjects. While younger age may have resulted in lower than expected mortality, it may also have predisposed to adverse consequences of edema such as increased intracranial pressure, seen preferentially in younger patients with severe infarction [41].
Our open label study of ten patients was not designed to establish efficacy. Nevertheless, comparison with control cohorts is of heuristic value. The proportion of subjects with mRS ≤4 in GAMES-Pilot was 90 %, compared to 24 % (at 12 months) in control patients from a pooled analysis of DC trials [24], and 19 % (at 90 days) in DEFUSE 2 patients with the malignant profile [13]. The clinical efficacy measure of the proportion of subjects with mRS 0–4 used in GAMES-Pilot is appropriate in clinical trials with high baseline NIHSS [24] and where the natural history has a high mortality as in large hemispheric stroke. It was, however, reassuring that it was a similar trend for a higher percentage of GAMES-Pilot subjects achieving an mRS 0–3 without an increase in the severely affected group at mRS 5 or that died. While there may be some controversy with respect to benefit of increasing the number of patients at mRS 0–4, one advantage of using mRS 0–4 is that we have shown it to contribute the smallest error to the outcome measurements and hence does not require an increase in sample size to make up for these errors as is needed when using other intermediate mRS score ranges [42].
Note also that the earlier reports suggest that as many as 40 % of large disabling stroke patient who present with a MRS of 4 will recover to MRS <3 and as many as 17 % who present with MRS of 5 will recover to an MRS <3 [43]. Motivated by these observations, while the primary endpoint in the ongoing phase II blinded randomized trial of RP-1127 in large stroke, GAMES-RP, includes modified Rankin assessment at 90 days, follow up will occur up to 12 months.
Preclinical work suggests that the prevention of cerebral swelling is preferable to decompressing the already swollen brain [5]. This is the first study that has examined a novel treatment strategy for preventing cerebral edema in patients with a large stroke at high risk for malignant swelling. The approach here aims to shift current practice from unproven, and largely reactive, drug interventions (e.g., mannitol, hypertonic saline) for the treatment of malignant infarction to a preventative treatment paradigm. The findings reported here support a larger prospective test of this strategy.
Acknowledgments
Funding
The sponsor for this study was Remedy Pharmaceuticals, Inc.
The study PI (KNS) made all decisions regarding analysis and publication and assumes responsibility for the manuscript. The Stroke Outcomes Laboratory (TAK, PM) was supported by a Pilot Grant from the Institute for Clinical and Translational Research at the Baylor College of Medicine.
Footnotes
Clinical Trial Registration
URL: www.clinicaltrials.gov Identifier: NCT01268683.
Contributor Information
Kevin N. Sheth, Email: kevin.sheth@yale.edu, Departments of Neurology and Neurosurgery, Yale University School of Medicine, 15 York Street, LCI 710A, New Haven, CT 06520, USA
W. Taylor Kimberly, Division of Neurocritical Care and Emergency Neurology, Department of Neurology, Massachusetts General Hospital, Boston, MA, USA.
Jordan J. Elm, Medical University of South Carolina, Columbia, SC, USA
Thomas A. Kent, Michael E. DeBakey VA Medical Center Stroke Program and the Stroke Outcomes Laboratory, Department of Neurology, Baylor College of Medicine, Houston, TX, USA
Albert J. Yoo, Division of Neuroradiology, Department of Radiology, Massachusetts General Hospital, Boston, MA, USA
Götz Thomalla, Klinik Und Poliklinik für Neurologie, Kopf- Und Neurozentrum, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany.
Bruce Campbell, Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia.
Geoffrey A. Donnan, Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia
Stephen M. Davis, Melbourne Brain Centre at the Royal Melbourne Hospital, University of Melbourne, Parkville, VIC, Australia
Gregory W. Albers, Department of Neurology, Stanford University School of Medicine, Stanford, CA, USA
Sven Jacobson, Remedy Pharmaceuticals, Inc, New York, NY, USA.
Gregory del Zoppo, Department of Hematology, University of Washington School of Medicine, Seattle, WA, USA.
J. Marc Simard, Departments of Neurology, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD, USA. Department of Neurosurgery, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Barney J. Stern, Departments of Neurology, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD, USA
Pitchaiah Mandava, Michael E. DeBakey VA Medical Center Stroke Program and the Stroke Outcomes Laboratory, Department of Neurology, Baylor College of Medicine, Houston, TX, USA.
References
- 1.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013;288(5):3655–67. doi: 10.1074/jbc.M112.428219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12(4):433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6(3):258–68. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40(2):604–9. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41(3):531–7. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, Yurovsky V, Ivanova S, Lee R, Gerzanich V. Glibenclamide-10-h treatment window in a clinically relevant model of stroke. Transl Stroke Res. 2012;3(2):286–95. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wali B, Ishrat T, Atif F, Hua F, Stein DG, Sayeed I. Glibenclamide administration attenuates infarct volume, hemispheric swelling, and functional impairments following permanent focal cerebral ischemia in rats. Stroke Res Treat. 2012;2012:460909. doi: 10.1155/2012/460909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F, Weih M, Dirnagl U. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38(9):2526–30. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunte H, Busch M, Trostdorf K, Vollnberg B, Harms L, Mehta R, Castellani R, Kent T, Mandava P, Simard J. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol. 2012;72:799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, Campbell BC, Bammer R, Olivot JM, Desmond P, Donnan GA, Davis SM, Albers GW DEFUSE-EPITHET Investigators. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42(5):1270–5. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The national institute of neurological disorders and stroke rt-PA stroke study group. . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 12.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40(6):2046–54. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mlynash M, Lansberg MG, Straka M, Kemp S, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski T, Bernstein RA, Chang CW, Warach S, Fazekas F, Thai D, Inoue M. The malignant MRI profile: implications for endovascular therapy. Stroke. 2012;43:A53. [Google Scholar]
- 14.Sanak D, Nosal’ V, Horak D, Bartkova A, Zelenak K, Herzig R, Bucil J, Skoloudik D, Burval S, Cisarikova V, Vlachova I, Kocher M, Zapletalova J, Kurca E, Kanovsky P. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48(9):632–9. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 15.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Mandava P, Yoo AJ, Thomalla G, Campbell B, Donnan GA, Davis SM, Albers GW, Jacobson S, Simard JM, Stern BJ. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014;45(1):281–3. doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, Singhal AB, Elm JJ, Stern BJ, Sheth KN. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20(2):193–201. doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 18.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Kohrmann M, Kersten JF, Krutzelmann A, Humpich MC, Sobesky J, Gerloff C, Villringer A, Fiehler J, Neumann-Haefelin T, Schellinger PD, Rother J Clinical Trial Net of the German Competence Network Stroke. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68(4):435–45. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 19.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM EPITHET investigators. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 21.Yoo AJ, Sheth KN, Kimberly WT, Chaudhry ZA, Elm JJ, Jacobson S, Davis SM, Donnan GA, Albers GW, Stern BJ, Gonzalez RG. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis. 2012;22(6):742–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimberly WT, Sheth KN. Approach to severe hemispheric stroke. Neurology. 2011;76(7 Suppl 2):S50–6. doi: 10.1212/WNL.0b013e31820c35f4. [DOI] [PubMed] [Google Scholar]
- 23.Simard JM, Sahuquillo J, Sheth KN, Kahle KT, Walcott BP. Managing malignant cerebral infarction. Curr Treat Options Neurol. 2011;13(2):217–29. doi: 10.1007/s11940-010-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W DECIMAL, DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215–22. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 25.Bergstralh EJ, Kosanke JL. Computerized matching of cases to controls. [Accessed 10 Mar 2014];Technical report 56. 1995 http://www.mayo.edu/research/documents/biostat-56pdf/doc-10026923.
- 26.Mandava P, Kalkonde YV, Rochat RH, Kent TA. A matching algorithm to address imbalances in study populations: application to the national institute of neurological diseases and stroke recombinant tissue plasminogen activator acute stroke trial. Stroke. 2010;41(4):765–70. doi: 10.1161/STROKEAHA.109.574103. [DOI] [PubMed] [Google Scholar]
- 27.Friedman JH, Bentley JL, Finkel RA. An algorithm for finding best matches in logarithmic time. ACM Trans Math Softw. 1977;3(3):209–26. [Google Scholar]
- 28.Matlab ®. Classification using nearest neighbors. [Accessed 10 Mar 2014];Statistics toolbox. 2013 (homepage on the internet) http://www.mathworks.com/help/stats/classification-using-nearest-neighbors.html#bsehylk.
- 29.Mandava P, Dalmeida W, Anderson JA, Thiagarajan P, Fabian RH, Weir RU, Kent TA. A pilot trial of low-dose intravenous abciximab and unfractionated heparin for acute ischemic stroke: translating GP IIb/IIIa receptor inhibition to clinical practice. Transl Stroke Res. 2010;1(3):170–7. doi: 10.1007/s12975-010-0026-4. [DOI] [PubMed] [Google Scholar]
- 30.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 31.Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensiv Care Med. 1998;24(6):620–3. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 32.Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, Colucci M, Gerzanich V. Does inhibiting Sur1 complement rt-PA in cerebral ischemia? Ann N Y Acad Sci. 2012;1268:95–107. doi: 10.1111/j.1749-6632.2012.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, Gerzanich V, Simard JM. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab. 2011;32(3):525–36. doi: 10.1038/jcbfm.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238–44. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Videen TO, Zazulia AR, Manno EM, Derdeyn CP, Adams RE, Diringer MN, Powers WJ. Mannitol bolus preferentially shrinks non-infarcted brain in patients with ischemic stroke. Neurology. 2001;57(11):2120–2. doi: 10.1212/wnl.57.11.2120. [DOI] [PubMed] [Google Scholar]
- 36.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35(2):354–6. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 37.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326–33. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 38.Steiner T, Mendoza G, De Georgia M, Schellinger P, Holle R, Hacke W. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997;28(4):711–5. doi: 10.1161/01.str.28.4.711. [DOI] [PubMed] [Google Scholar]
- 39.Rabinstein AA, Wijdicks EF. Outcome of survivors of acute stroke who require prolonged ventilatory assistance and tracheostomy. Cerebrovasc Dis. 2004;18(4):325–31. doi: 10.1159/000080771. [DOI] [PubMed] [Google Scholar]
- 40.Golestanian E, Liou JI, Smith MA. Long-term survival in older critically ill patients with acute ischemic stroke. Crit Care Med. 2009;37(12):3107–13. doi: 10.1097/CCM.0b013e3181b079b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology. 1995;45(7):1286–90. doi: 10.1212/wnl.45.7.1286. [DOI] [PubMed] [Google Scholar]
- 42.Mandava P, Krumpelman CS, Shah JN, White DL, Kent TA. Quantification of errors in ordinal outcome scales using Shannon entropy: effect on sample size calculations. PLoS One. 2013;8(7):e67754. doi: 10.1371/journal.pone.0067754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68(19):1583–7. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]