Abstract
Microglia, the resident immune cells of the brain, are activated in response to any kind of CNS injury, and their activation is critical for maintaining homeostasis within the CNS. However, during inflammatory conditions, sustained microglial activation results in damage to surrounding neuronal cells. β-Glucans are widely recognized immunomodulators, but the molecular mechanisms underlying their immunomodulatory actions have not been fully explored. We previously reported that β-glucans activate microglia through Dectin-1 without inducing significant amount of cytokines and chemokines. Here, we show that particulate β-glucans attenuate cytokine production in response to TLR stimulation; this inhibitory activity of β-glucan is mediated by Dectin-1 and does not require particle internalization. At the molecular level, β-glucan suppressed TLR-mediated NF-κB activation, which may be responsible for the diminished capacity of microglia to produce cytokines in response to TLR stimulation. Overall, these results suggest that β-glucans may be used to prevent or treat excessive microglial activation during chronic inflammatory conditions.
Keywords: Microglia, Toll-like receptors, Neuroimmunology, Cell surface molecules, Cell activation, Glucan
Microglia, the innate immune cells of the CNS are resting under normal conditions, but become activated in case of brain injury resulting from trauma, infection, or neurodegenerative diseases [29]. The activated microglia migrate to the site of injury and secrete cytokines, chemokines, and reactive oxygen and nitrogen species, and they also actively phagocytose apoptotic cells, microbes, and cellular debris in the CNS [9]. Although microglial activation is necessary for the elimination of pathogenic agents and preventing further damage, it can be a double-edged sword during chronic inflammatory conditions, such as neurodegenerative diseases or CNS infections. For example, in Alzheimer’s disease, microglia are unable to phagocytose β-amyloid plaques and are persistently activated [15]. The persistently activated microglia continuously release cytokines, chemokines, and reactive oxygen species. This leads to feed-forward activation of these cells and surrounding astrocytes, resulting in disease progression due to continuous production of substances toxic to the neurons [1]. Similarly, persistent microglial activation during chronic bacterial infections of the CNS is also responsible for the excessive tissue damage seen in brain abscess [16]. Away to resolve this microglial conundrum is to modulate the microglial response by limiting its excessive activation. Therefore, there is a need for therapeutic approaches that modulate such microglial responses.
Immunomodulators are compounds that are capable of modifying the host response by upregulating or downregulating specific aspects of the immune response. Polysaccharides like β-glucans have evoked lot of interest lately because of their immunomodulatory properties. They are known to possess antitumor and anti-infective properties [31,4]. Remarkably, a number of studies have also shown that soluble glucans significantly increase long-term survival in animal models of polymicrobial sepsis as well as ischemia/reperfusion injury, in part through attenuation of the inflammatory pathways and activation of the PI3K survival pathway, although the underlying molecular mechanisms still need to be fully determined [33,34]. In contrast to the aforementioned animal models, relatively little is known about the potential value of glucans in modifying CNS injury outcomes. However, recent identification of Dectin-1 as the major β-glucan receptor in leukocytes as well as in microglia [3,8,24,27] provided us with an opportunity to investigate the effects of glucans on inflammatory responses of microglia at the cellular and molecular level.
We previously demonstrated for the first time that particulate β-glucan-stimulated microglia become phagocytic and secrete reactive oxygen species [27]. Remarkably, unlike in macrophages, particulate β-glucan on its own did not elicit any cytokine or chemokine production in microglia. Intrigued by this observation, we sought to investigate whether β-glucan can modulate microglial production of inflammatory mediators in response to other stimulants. Here, we show that β-glucan attenuates cytokine production induced by both TLR4 and TLR2 ligands and this response is mediated by Dectin-1, the major receptor for β-glucans. This anti-inflammatory activity of β-glucan is in part due to NF-κB inhibition. Thus, our results suggest that β-glucans could serve as immunomodulators of microglial activation.
Anti-NF-κB p65, anti-IκBα, anti-phospho-IκB-α (Ser 32/36) and anti-phospho-NF-κB p65 (Ser 536) antibodies were purchased from Cell Signaling (Beverly, MA). LPS and cytochalasin D were purchased from Sigma–Aldrich, whereas Pam3Csk4 was from InvivoGen.
Particulate β-glucan was isolated and characterized as described by Muller et al. [21], Lowman et al. [19], and colleagues. Glucan phosphate was prepared and characterized as described by Williams et al. [32], Muller et al. [22], and colleagues. Glucan phosphate was selected for this study because it is bound by Dectin-1 with high affinity [2]. The primary structures of the glucans were confirmed by NMR [19].
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed, bred, and euthanized in accordance with protocols reviewed and approved by The Ohio State University Institutional Animal Care And Use Committee. Primary mixed glial cultures were prepared from postnatal day 1–3 mice as described previously [27]. These mixed glial cells were cultured for 18 days, changing medium every 4–5 days. Microglial cells were isolated from the mixed culture using the mild trypsinization method as described previously [26]. The isolated microglial cells were then reseeded in 24 well plates or 60 mm dishes and cultured in astrocyte-conditioned medium for 5–7 days to allow sufficient time for them to become quiescent. This method yielded highly pure microglial cultures, as evident from the morphology and absence of astrocytic and neuronal markers.
For Western blot analysis, primary microglia plated in 60-mm dishes were treated with particulate β-glucan (100 µg/ml) and LPS (1 µg/ml) or Pam3Csk4 (1 µg/ml) for 5 min or 15min. A subset of cells was pre-treated with β-glucan for 2 h before stimulation with LPS or Pam3Csk4. The cells were then lysed in RIPA buffer, and proteins were eluted by boiling with Laemmli buffer and then resolved by SDS-PAGE. Western blotting was essentially performed as described previously [27].
For cytokine measurement, primary microglia were stimulated with LPS (1 µg/ml), Pam3Csk4 (1 µg/ml) or particulate β-glucan (100 µg/ml) for 16 h, and the supernatants were harvested and stored at −80 °C. The supernatants were then analyzed for TNF-α and IL-6 using appropriate ELISA kits purchased from BioLegend. For some experiments, cells were pre-treated with particulate β-glucan for 2 h or 24 h before stimulation with LPS or Pam3Csk4. For experiments involving inhibitors, cells were treated with glucan phosphate (100 µg/ml) or cytochalasin D (5 µM) for 40 min before stimulation, and the inhibitors remained present in the cell medium throughout the 16 h incubation period.
Statistical significance was determined by one-way ANOVA, and the Tukey–Kramer multiple comparison test was used to determine p values.
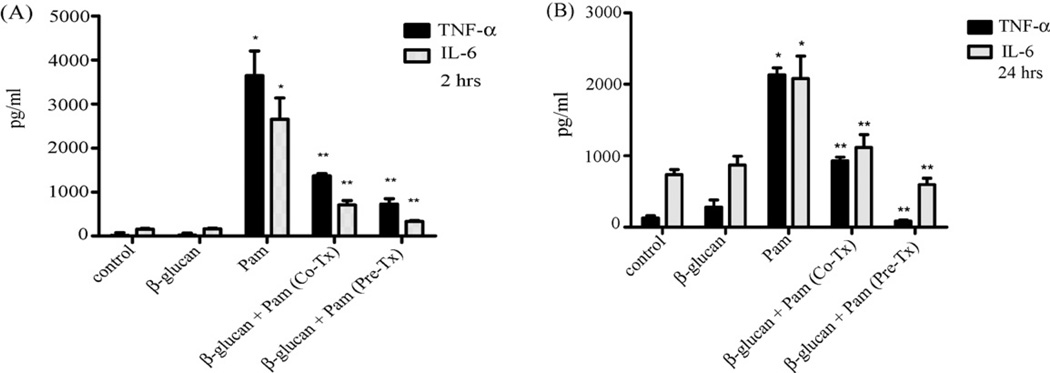
Previously, we reported that forced internalization of Dectin-1 by glucan phosphate, a soluble β-glucan, resulted in slightly increased TNF-α production in response to zymosan stimulation in microglia, suggesting that Dectin-1 may have an inhibitory effect in microglia [27]. Conversely, we observed that co-stimulation of microglia with particulate β-glucan significantly inhibited TNF-α production by Pam3Csk4, a TLR2 ligand. Based on these findings, we hypothesized that particulate β-glucan may be acting as a negative regulator of Toll receptor-mediated cytokine production. To address this hypothesis, we conducted additional experiments in which primary microglia were pre-treated with particulate β-glucan for 2 h (Fig. 1A) or 24 h (Fig. 1B) followed by stimulation with Pam3Csk4 for 16 h prior to determination of TNF-α and IL-6 levels. For comparison, a subset of cells was simultaneously treated with β-glucan and Pam3Csk4. As shown, unlike Pam3Csk4, particulate β-glucan by itself did not induce cytokine production. However, consistent with our previous findings, co-treatment as well as pre-treatment with β-glucan for both 2 h and 24 h significantly reduced Pam3Csk4-induced TNF-α and IL-6 production. Furthermore, pre-treatment withβ-glucan was observed to be more effective than co-treatment in reducing cytokine secretion by microglia. Our results suggest that in contrast to peripheral leukocytes, where particulate glucan is known to stimulate production of pro-inflammatory cytokines [5,25], microglia may be unique in that glucan particles actually inhibit TLR-induced cytokine production.
Fig. 1.
Particulate β-glucan inhibits TLR2-mediated cytokine production by microglia. Primary microglia were left untreated (control) or were stimulated with β-glucan (100 µg/ml), Pam3Csk4 (Pam; 1 µg/ml) or combination of β-glucan and Pam3Csk4 for 16 h (Co-Tx). A subset of cells was pre-treated with β-glucan (Pre-Tx) for either 2 h (A) or 24 h (B) before stimulation with Pam3Csk4 for 16 h in continuous presence of β-glucan. Supernatants were then collected, and levels of TNF-α and IL-6 were measured using ELISA. Data are presented as mean ± SEM, n = 3. *p < 0.05 compared with control. **p < 0.05 compared with Pam alone.
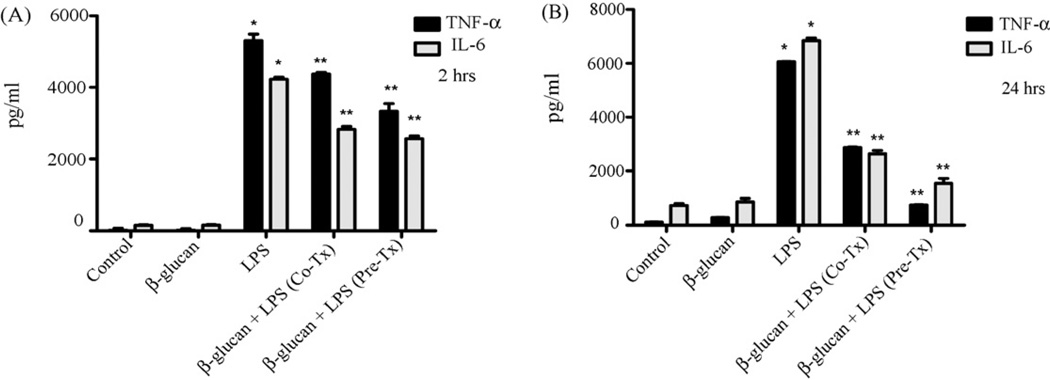
We sought to determine whether β-glucan-induced inhibitory effects were limited to TLR2-induced signaling or were applicable to other Toll-like receptors. To address this, we pre-treated primary microglia with particulateβ-glucan for 2 h (Fig. 2A) or 24 h (Fig. 2B), followed by stimulation with the TLR4 ligand LPS, for 16 h. As shown (Fig. 2), LPS-induced TNF-α and IL-6 production was downregulated by co-treatment and pre-treatment with particulate β-glucan. Thus, the results suggest that β-glucan has a broader inhibitory effect on Toll receptor-mediated inflammatory responses, including those mediated by TLR2 and TLR4.
Fig. 2.
Particulate β-glucan inhibits TLR4-mediated cytokine production by microglia. Primary microglia were left untreated (control) or were stimulated with β-glucan (100 µg/ml), LPS (1 µg/ml) or combination of β-glucan and LPS for 16 h (Co-Tx). A subset of cells was pre-treated with β-glucan (Pre-Tx) for 2 h (A) or 24 h (B) before stimulation with LPS for 16 h in continuous presence of β-glucan. Supernatants were then collected, and levels of TNF-α and IL-6 were measured using ELISA. Data are presented as mean ± SEM, n = 3. *p < 0.05 compared with control. **p < 0.05 compared with LPS alone.
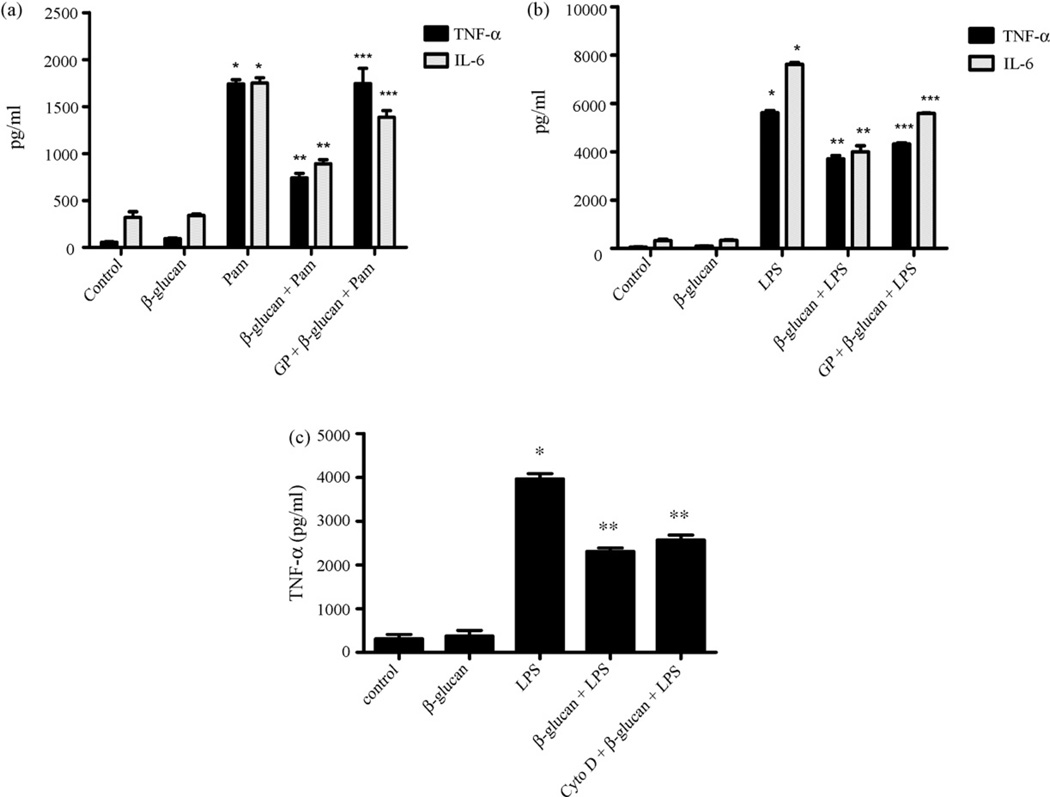
Since β-glucan effected both TLR2 and TLR4 signaling, we asked whether the effects were mediated by the Dectin-1 pathway or were more generic in nature. To determine whether Dectin-1 is required for the inhibitory effects of β-glucan, we tested the effects of β-glucan in microglia that were pre-treated with glucan phosphate, a soluble glucan that is known to deplete Dectin-1 on the cell surface through forced internalization [11,23]. As before, Pam3Csk4-induced TNF-α production was suppressed by co-incubation with particulate β-glucan (Fig. 3A). However, when the cells were pre-treated with glucan phosphate, the inhibitory effect of particulate β-glucan on Pam3Csk4-induced TNF-α production was completely reversed, while the inhibitory effect on IL-6 production was reversed by 60% (Fig. 3A). Similarly, pre-treatment with glucan phosphate reversed the inhibitory effect of particulate β-glucan on LPS-induced TNF-α and IL-6 by about 33% and 45%, respectively (Fig. 3B). Therefore, our results indicate that β-glucan-mediated immunomodulation of microglial inflammatory responses require Dectin-1.
Fig. 3.
β-Glucan-induced downregulation of TLR-mediated cytokine production is through Dectin-1 and does not require particle internalization. Primary microglia were left untreated (control) or were stimulated with β-glucan (100 µg/ml) (A–C), Pam3Csk4 (Pam; 1 µg/ml) (A), LPS (1 µg/ml) (B and C), combination of β-glucan with Pam3Csk4 (A) or combination of β-glucan with LPS (B and C) for 16 h. A subset of cells was pre-treated with glucan phosphate (GP; 100 µg/ml) (A and B) or cytochalasin D (Cyto D; 5 µM) (C) for 40 min before appropriate stimulation. Supernatants were then collected, and levels of TNF-α and IL-6 were measured using ELISA. Data are presented as mean ± SEM, n = 3. *p < 0.05 compared with control. **p < 0.05 compared with Pam alone or LPS alone. ***p < 0.05 compared with β-glucan + Pam or β-glucan + LPS.
Binding of β-glucan to Dectin-1 causes particle internalization through phagocytosis [27,11]. To determine if particle internalization is necessary for the inhibitory effect of β-glucan on TLR-mediated cytokine production, we used cytochalasin D, a potent inhibitor of particle uptake through actin-dependent mechanisms, to inhibit β-glucan internalization. Interestingly, β-glucan suppressed LPS-induced TNF-α production even in cells pre-treated with cytochalasin D (Fig. 3C). This strongly suggests that internalization of β-glucan is not required for the immunomodulatory actions of β-glucan, and that surface stimulation of Dectin-1 may be sufficient to mediate inhibitory signals.
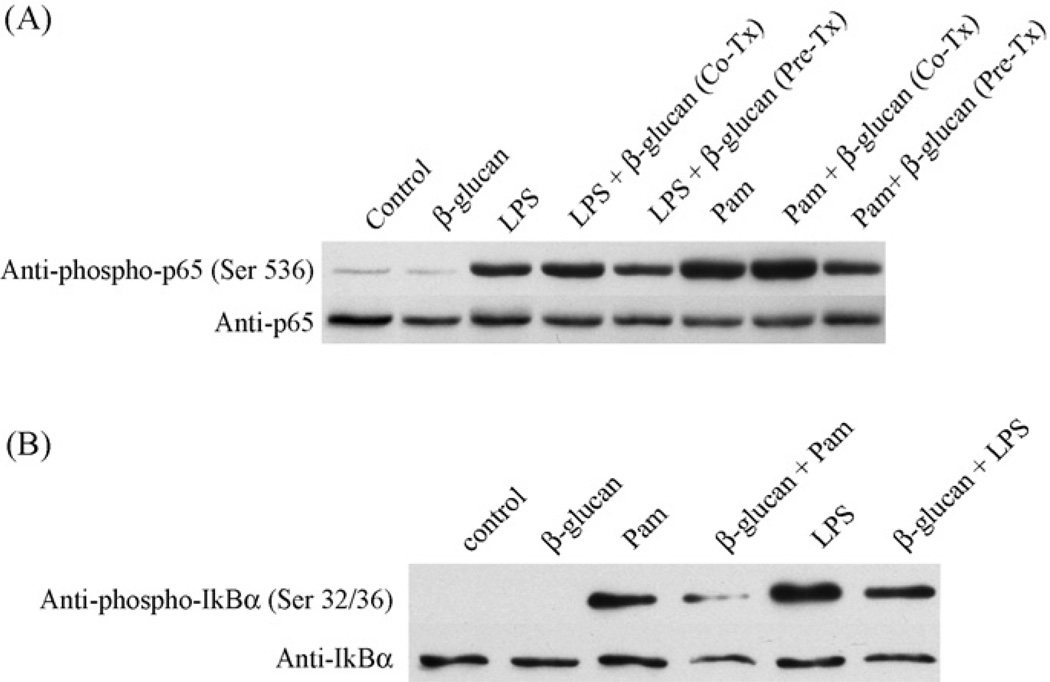
NF-κB is a major transcription factor involved in the regulation of cytokine production [17]. Therefore, we investigated the effect of β-glucan on NF-κB activation in response to LPS and Pam3Csk4. To address this, we left primary microglial cells untreated, or treated them either with LPS, Pam3Csk4, or β-glucan. As shown in Fig. 4A, both LPS and Pam3Csk4, but not β-glucan, induced phosphorylation of NF-κB on Ser 536, a marker for NF-κB activation. While co-treatment of cells with β-glucan and LPS or Pam3Csk4 did not alter NF-κB activation, pre-treatment with β-glucan for 2 h inhibited both LPS- and Pam3Csk4-induced NF-κB activation (Fig. 4A). To confirm our findings, we also measured phosphorylation of IκB-α on Ser 32 and 36 as an additional indicator of NF-κB activation. As with NF-κB activation, both LPS- and Pam3Csk4-induced IκB-α phosphorylation was inhibited by pre-treatment with β-glucan (Fig. 4B). Taken together, our results suggest that β-glucan-induced immunomodulation in microglia is likely mediated through a signaling pathway that ultimately interferes with TLR-mediated NF-κB activation.
Fig. 4.
Particulate β-glucan suppresses activation of NF-κB by TLR ligands. Primary microglia (A and B) were left untreated (control) or were stimulated with LPS (1 µg/ml) or Pam3Csk4 (Pam; 1 µg/ml) for 15 min (A) or 5 min (B). Subset of cells was stimulated with β-glucan alone for 2 h (A and B) or co-stimulated with β-glucan + LPS or β-glucan + Pam3Csk4 for 15 min (A). In some experiments cells were pre-treated with β-glucan for 2 h followed by stimulation with LPS or Pam3Csk4 for 15 min (A) or 5 min (B). Cells were lysed and whole cell lysates were used to determine phospho-Ser 536 levels of NF-κB (p65) (A) or phospho-Ser 32/36 levels of IκBα (B). Equal levels of p65 and IκBα were then confirmed by stripping and reprobing the membrane using anit-p65 antibodies and IκBα antibodies respectively.
In this study we show that particulate β-glucan modulates TLR-mediated inflammatory response in microglia. The TLR2 ligand Pam3Csk4 and the TLR4 ligand LPS induced robust TNF-α and IL-6 production by microglia. However, TLR2 is known to be a relatively weaker inducer of pro-inflammatory cytokines compared to TLR4 [12], and in our hands it consistently induced low levels of cytokines. Interestingly, co- and pre-treatment of microglia with particulate β-glucan diminished both TNF-α and IL-6 production by LPS and Pam3Csk4. Our data suggest that it may be possible to harness this property of β-glucan to modulate microglial activation during chronic inflammatory conditions of the brain. Previously, we reported that particulate β-glucan activates microglia by stimulating their phagocytic activity and inducing ROS production [27]. Remarkably, particulateβ-glucan did not induce production of cytokines or chemokines by microglia, which was in sharp contrast to macrophages, where zymosan as well as glucan particles trigger induction of several cytokines and chemokines [25,13,30]. Unlike in macrophages and dendritic cells, where Dectin-1 has been implicated in induction of several cytokines either alone or in combination with TLR2 [8,24,2,28], we consistently found that zymosan-induced cytokine production was augmented when cells were pre-treated with glucan phosphate to internalize Dectin-1 [27]. Therefore, Dectin-1 may have a unique function in microglia, as shown by our results with glucan phosphate. Interestingly, glucan phosphate was previously shown to increase long-term survival of animal models of polymicrobial sepsis as well as ischemia/reperfusion injury [33,18]. Moreover, following in vivo injection into the animals, the immunomodulatory property of glucan phosphate correlated negatively with NF-κB activity but positively with PI3K activity. In contrast, we did not observe any discernible effects of soluble glucans, such as glucan phosphate, alone on cytokine production or NF-κB or PI3K activity (data not shown) in microglia. Rather, glucan phosphate effectively antagonized the effects mediated by particulate glucan. Thus, at least at the cellular level, microglial responses to glucans are dependent upon the physical form of glucans, whereby particulate glucan is actively phagocytosed, induces ROS production, but inhibits TLR-induced cytokine production, whereas soluble glucan counters the effects of particulate glucan but has no discernible effect of its own. In macrophages and monocytes, Dectin-1 was shown to synergistically augment TLR2- and TLR4-mediated cytokine production [7]. Thus, the contrasting results in microglia suggest yet another important functional difference between peripheral macrophages and brain microglia, which are considered akin to each other because of their myeloid origin [10].
Purified particulate β-glucan prepared from various sources range in size from 2 µm to 8 µm [20] and we previously demonstrated that the interaction of purified particulate β-glucan with microglia leads to their internalization in an actin-dependent manner [27]. Herein, our results suggest that binding of β-glucan to the surface receptors is sufficient to induce an inhibitory effect on TLR-mediated cytokine production. Further lending support to our findings, a recent study reported that internalization of β-glucan is not necessary for inducing cytokine production in macrophages [20]. Thus, the signaling pathway mediating the inhibitory effect of β-glucan is likely mediated by surface activation of Dectin-1.
NF-κB is a major transcription factor that is involved in TLR-mediated cytokine production [17]. Studies showing effect of β-glucan on NF-κB activation are contrasting. While β-glucan devoid of TLR2 stimulatory activity induced NF-κB activation in alveolar epithelial cells [6], it did not do so in macrophages [8]. In our studies, particulate β-glucan did not directly activate NF-κB in primary microglia, indicating that the actions of β-glucan may vary depending on the cell types involved. However, it consistently blunted LPS- and Pam3Csk4-induced NF-κB activation, suggesting that β-glucan stimulation likely interferes with TLR signaling rather than directly affect NF-κB activity.
While our studies were ongoing, a study by Jung et al. reported similar findings in BV-2 cells, where inhibition of LPS-induced NF-κB activation was attenuated by pre-treatment with particulate β-glucan [14]. Thus, our results are consistent with these observations and suggest that β-glucans are important modulators of microglial activation. Future studies addressing the molecular mechanisms governing this anti-inflammatory action may provide important insights into novel approaches for countering excessive CNS inflammation.
Acknowledgements
We thank Dr. Kari Hoyt and Michael Enzerra for help with ELISA.
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer’s disease. Microsc. Res. Tech. 2001;54:59–70. doi: 10.1002/jemt.1121. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Seviour R. Medicinal importance of fungal beta-(1 → 3), (1 → 6)-glucans. Mycol. Res. 2007;111:635–652. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Czop JK. The role of beta-glucan receptors on blood and tissue leukocytes in phagocytosis and metabolic activation. Pathol. Immunopathol. Res. 1986;5:286–296. doi: 10.1159/000157022. [DOI] [PubMed] [Google Scholar]
- 6.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 2005;32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 8.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garden GA, Moller T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 10.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 11.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 12.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman OA, Standing JE, Limper AH. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J. Immunol. 1993;150:3932–3940. [PubMed] [Google Scholar]
- 14.Jung KH, Kim MJ, Ha E, Kim HK, Kim YO, Kang SA, Chung JH, Yim SV. The suppressive effect of beta-glucan on the production of tumor necrosis factor-alpha in BV2 microglial cells. Biosci. Biotechnol. Biochem. 2007;71:1360–1364. doi: 10.1271/bbb.60608. [DOI] [PubMed] [Google Scholar]
- 15.Khoury JE, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol. Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kielian T. Immunopathogenesis of brain abscess. J. Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuprash DV, Udalova IA, Turetskaya RL, Rice NR, Nedospasov SA. Conserved kappa B element located downstream of the tumor necrosis factor alpha gene: distinct NF-kappaB binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene. 1995;11:97–106. [PubMed] [Google Scholar]
- 18.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. Modulating Toll-like receptor mediated signaling by (1 → 3)-beta-d-glucan rapidly induces cardioprotection. Cardiovasc. Res. 2004;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Lowman DW, Ferguson DA, Williams DL. Structural characterization of (1 → 3)-beta-d-glucans isolated from blastospore and hyphal forms of Candida albicans. Carbohydr. Res. 2003;338:1491–1496. doi: 10.1016/s0008-6215(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 20.McCann F, Carmona E, Puri V, Pagano RE, Limper AH. Macrophage internalization of fungal beta-glucans is not necessary for initiation of related inflammatory responses. Infect. Immun. 2005;73:6340–6349. doi: 10.1128/IAI.73.10.6340-6349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller A, Ensley H, Pretus H, McNamee R, Jones E, McLaughlin E, Chandley W, Browder W, Lowman D, Williams D. The application of various protic acids in the extraction of (1 → 3)-beta-d-glucan from Saccharomyces cerevisiae. Carbohydr. Res. 1997;299:203–208. doi: 10.1016/s0008-6215(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 22.Muller A, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. Comparison of the carbohydrate biological response modifiers Krestin, schizophyllan and glucan phosphate by aqueous size exclusion chromatography with in-line argon-ion multi-angle laser light scattering photometry and differential viscometry detectors. J. Chromatogr. B: Biomed. Appl. 1995;666:283–290. doi: 10.1016/0378-4347(94)00575-p. [DOI] [PubMed] [Google Scholar]
- 23.Ozment-Skelton TR, Goldman MP, Gordon S, Brown GD, Williams DL. Prolonged reduction of leukocyte membrane-associated Dectin-1 levels following beta-glucan administration. J. Pharmacol. Exp. Ther. 2006;318:540–546. doi: 10.1124/jpet.106.102293. [DOI] [PubMed] [Google Scholar]
- 24.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 26.Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- 27.Shah VB, Huang Y, Keshwara R, Ozment-Skelton T, Williams DL, Keshvara L. Beta-glucan activates microglia without inducing cytokine production in Dectin-1-dependent manner. J. Immunol. 2008;180:2777–2785. doi: 10.4049/jimmunol.180.5.2777. [DOI] [PubMed] [Google Scholar]
- 28.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rossum D, Hanisch UK. Microglia. Metab. Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- 30.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J. Immunol. 2000;164:3755–3763. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 31.Williams DL, Cook JA, Hoffmann EO, Di Luzio NR. Protective effect of glucan in experimentally induced candidiasis. J. Reticuloendoth. Soc. 1978;23:479–490. [PubMed] [Google Scholar]
- 32.Williams DL, McNamee RB, Jones EL, Pretus HA, Ensley HE, Browder IW, Di Luzio NR. A method for the solubilization of a (1–3)-beta-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991;219:203–213. doi: 10.1016/0008-6215(91)89052-h. [DOI] [PubMed] [Google Scholar]
- 33.Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-kappa B and nuclear factor interleukin 6 with (1 → 3)-beta-d-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65. doi: 10.1067/msy.1999.99058. [DOI] [PubMed] [Google Scholar]
- 34.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]