Abstract
Arterioles are the blood vessels in the arterial side of the vascular tree that are located proximal to the capillaries and, in conjunction with the terminal arteries, provide the majority of resistance to blood flow. Consequently, arterioles are important contributors to the regulation of mean arterial pressure and tissue perfusion. Their wall consists of cellular and extracellular components that have been traditionally classified as conforming three layers: an intima containing endothelial cells sited on a basement membrane; a media made of an internal elastic lamina apposed by 1 or 2 layers of smooth muscle; and an adventitia composed mostly of collagen bundles, nerve endings and some fibroblasts. These components of the arteriolar wall are dynamically interconnected providing a level of plasticity to the arteriolar wall that blurs the traditional boundaries of a rigid layered classification. This review focuses on the structural conformation of the arteriolar wall and shows how wall components interact spatially, functionally, and temporally to control vascular diameter, regulate blood flow, and maintain vascular permeability.
Introduction
In general arterioles are defined as the primary resistance vessels that enter an organ to distribute blood flow into capillary beds. These blood vessels vary significantly in diameter depending on species, vascular bed, and state of contraction. Therefore, their main identifying feature is not size, but the fact that their wall consists of only one or two layers of smooth muscle [1, 2]. Arterioles are considered part of the resistance vasculature that provides in excess of 80% of the resistance to blood flow in the body. Consequently, they are vital to the regulation of hemodynamics, contributing to the control of blood pressure and the regional distribution of blood [3, 4].
Traditionally, the wall composition of arterioles has been described as consisting of three structurally distinct layers: intima, media and adventitia [1]. However, evidence indicates that the cellular and extracellular components of these layers are interconnected in multiple fashions such that their traditionally distinct boundaries are blurred. This is particularly apparent when considering that these interconnections play major roles in the functional capabilities of the vascular wall. For example, fenestrae present in the internal elastic lamina of arterioles provide avenues for the direct contact and communication between endothelial and vascular smooth muscle cells. These cell-to-cell interactions are considered essential for integrating local vasoconstriction and vasodilation effects, and for the coordination of vascular responses in a network of interconnected vessels. Furthermore, the structural characteristics of the vascular wall including the fenestrae, as well as other extracellular matrix and cellular components, change relatively rapidly in response to a myriad of physiological and pathological stimuli. This review is focused on describing the structural and anatomical features of arterioles, most of which also apply to small resistance arteries. Then, emphasis is given to the features that functionally interconnect the traditional intima, media, and adventitia subdivisions of the vascular wall and challenge the restrictive view of traditional anatomical boundaries.
The Intima: a blood barrier with fine-tuning control of vascular tone
Traditionally viewed as a physical barrier between blood components and extravascular tissues, the intimal layer in resistance arteries consists predominantly of endothelial cells and an abluminal basement membrane. Functionally, endothelial cells participate in controlling vessel tone via the production and release of vasoactive factors that exert their action on neighboring smooth muscle cells. These functional properties of endothelial cells are in part linked to their structure. Evidence indicates that endothelial deformation caused by blood flow, pressure or vasoconstriction initiates intracellular signaling pathways that result in the synthesis and release of vasoactive factors that ultimately fine-tune the contractile state of smooth muscle cells for the appropriate control of vascular diameter [5]. In addition, disruption of specific cytoskeletal components severely affects endothelial cell function [6–10], suggesting that endothelial cell structure plays an important role on the capacity of these cells to sense and transduce mechanical forces and produce vasoactive compounds.
Endothelial cells are arranged longitudinally in the direction of flow with an overall endothelial thickness of 0.2 to 0.5 µm, except at the location of the cell nucleus [2]. In feed arterioles, endothelial cells are ~100 µm in length by ~10 µm in width [11]. This 10:1 ratio is reduced as downstream arterioles get smaller in diameter. Endothelial cells present a continuous F-actin filament bordering the cellular edge and F-actin bundles traversing the cell longitudinally as short stress fibers [11, 12]. In the retinal microcirculation, these bundles of stress fibers become less evident, and eventually disappear, as arteriolar branches get smaller in diameter [11]. It is shear forces that induce stress fiber formation and the stabilization of microtubules allowing for the polarization of endothelial cells with the microtubule-organizing center redistributed to the downstream side of the nucleus [13]. All this clearly indicates that endothelial cells modify their intracellular architecture in response to mechanical forces and that cytoskeletal remodeling modulates endothelial function i.e., its capacity to sense and transduce mechanical forces and produce vasoactive compounds [7–10, 14–18].
An additional function of endothelial cells directly associated with their structure is vascular permeability. Changes in endothelial cell morphology occurring in response to toxemia and inflammation promote alterations in cellular junctions and endothelial cytoskeletal structures that allow for variations in microvascular permeability, with permeability being most greatly affected in venules [19, 20]. A functional heterogeneity among endothelial cells has been linked to the variations in response to inflammatory signals and toxins that occur from arterioles to venules. Furthermore, structural differences in endothelial cells from the mesenteric, renal, retinal, and pulmonary circulations suggest that an association exists between endothelial phenotype and regional functional heterogeneity [11, 21–23]. These observations stress the need to determine the origin of these regional variations in endothelial cell phenotype, as identification of the mechanisms that control endothelial cell heterogeneity should provide insights for strategies aimed at preventing and treating endothelial dysfunction.
Endothelial cells within the intima may not only vary phenotypically, some cells may also possess multipotent characteristics. Recent evidence suggests that endothelial progenitor cells are able to transdifferentiate into smooth muscle cells [24, 25]. Endothelial progenitor cells (EPC) are stem-like cells that aid in vascular repair and neovascularization. When stimulated with transforming growth factor (TGF) beta, a common cytokine produced by multiple cells and found in close association with a number of extracellular matrices, EPCs undergo an endothelial to mesenchymal transdifferentiation that produces cells with smooth muscle phenotype. Cells with this multipotent characteristic appear to reside also within the vascular intima [26]. Therefore, this cellular transdifferentiation indicates there may be less of a defined boundary between the endothelial and medial layers and suggests that progenitor cells present in the circulation or within the vascular intima are able to become specialized smooth muscle cells when exposed to specific stimuli.
The basement membrane underneath endothelial cells in arterioles is ~ 0.1 µm in thickness and composed primarily of collagen type IV, laminin, and heparan sulfate proteoglycans [1, 27]. Additional components include collagens type I, III, and V, and fibronectin [28]. The primary function of this membrane is to provide anchoring support for the endothelium. However, during endothelial damage its exposure to blood components and vascular smooth muscle provides signals for the anchoring and migration of cells across the vascular wall. Traditionally this basement membrane has been considered an intrinsic part of the intima produced by endothelial cells. However, recent results indicate that a close interaction between cells from the media and intima is needed for its proper formation and maintenance. For example, during the process of vasculogenesis, interaction between pericytes and endothelial cells is needed for the formation and stabilization of vascular tubes and the endothelial basement membrane [29]. This heterotypic cellular interaction induces not only the production, secretion and remodeling of extracellular matrix proteins, but also the production and sequestration of growth factors that later participate in signal transduction events that control vascular function. Vascular smooth muscle cells in the media also secrete extracellular components not normally produced by endothelial cells that get integrated into the basement membrane and function as anchoring substrates for both endothelial and smooth muscle cells. These anchoring molecules serve to maintain and regulate the characteristics of the basement membrane and as such are intrinsically involved in vessel function. Thus, heterotypic interactions between endothelial cells and pericytes or smooth muscle are needed for the appropriate formation and function of the intimal basement membrane indicating that from vessel development to vascular performance the arteriolar wall is a highly integrated and interdependent structure.
Media: the mechanical regulator of vascular diameter
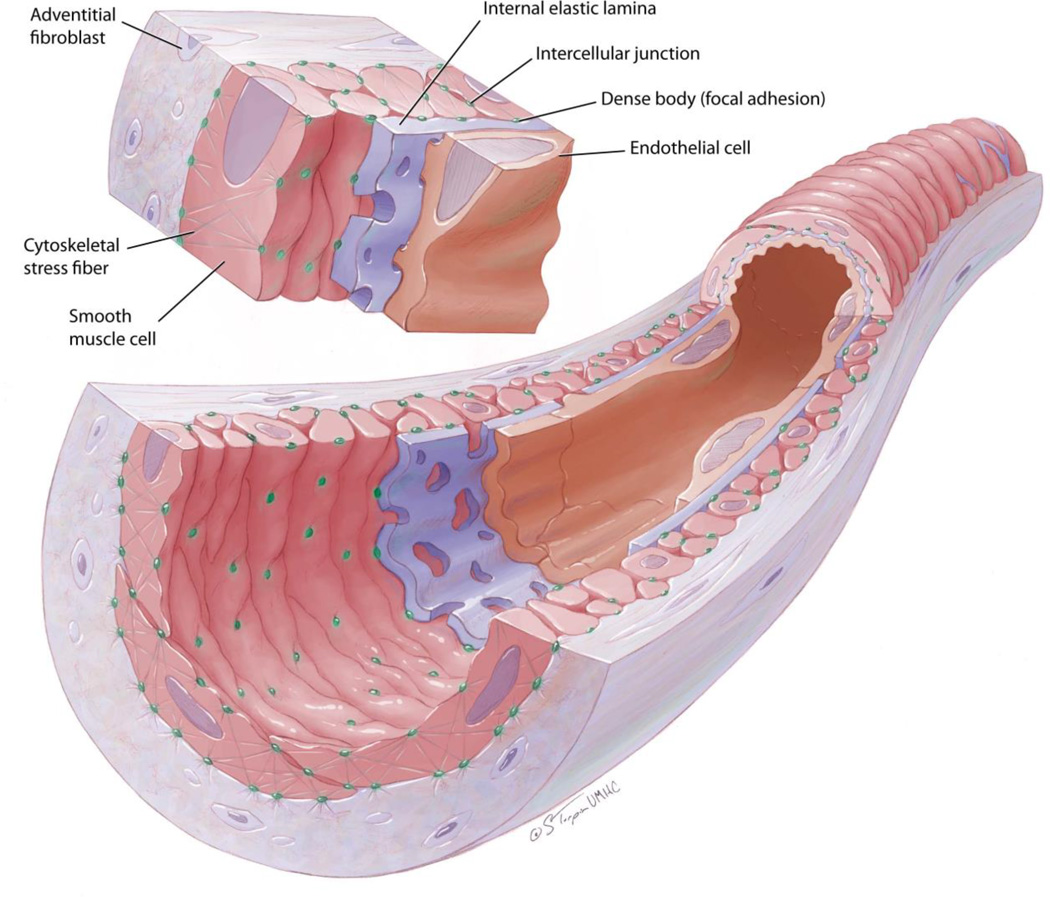
The medial layer in arterioles consists predominantly of vascular smooth muscle cells and an internal elastic lamina. The internal elastic lamina is a sheet of ~0.3 µm in thickness consisting primarily of degradation-resistant elastin molecules. It is not always found at all levels of the arteriolar tree, but it is present in feed arterioles of skeletal muscle, mesentery, and cerebrum [1, 2, 30]. The high elastin content of elastic lamina is known to provide the recoil properties in the vessel wall that are important in dealing with pulsatile blood pressure [30]. This function is clear in conduit arteries, but it is less evident in arterioles where pulsatile pressure is less pronounced. In addition, the internal elastic lamina in arterioles with tone is not smooth/fully stretched and appears wavy with evenly distributed ridges [31] (Figure). This observation suggests that under normal physiological conditions the internal elastic lamina does not contribute significantly to the viscoelastic characteristics of the vessel wall. However, a more comprehensive understanding of the structural role the internal elastic lamina plays in arteriolar function is warranted.
Figure.
Schematic representation of an arteriole with tone. The wall of the vessel consists of cellular and extracellular components. Depicted are endothelial cells that run parallel to the longitudinal axis of the vessel in the luminal side and sit on a basement membrane apposed by an internal elastic lamina. Fenestrae in the internal elastic lamina allow for endothelial cells to make contact with smooth muscle cells that sit on the abluminal side of the lamina. Smooth muscle cells are spindled shaped and arranged transversal to the longitudinal axis of the vessel. Notice that smooth muscle cells have, on the luminal side, evenly distributed dense bodies that make contact with the internal elastic lamina at points where ridges on the lamina are made as the vessel is constricted. These dense bodies are connected to cytoskeletal stress fibers within the smooth muscle cells for the transmission of forces in and out of the cell. Abluminally, smooth muscle cells connect through dense bodies with extracellular components of the adventitia and external elastic lamina whenever one is present. Within the adventitia notice the presence of fibroblasts that are arranged mostly parallel to the longitudinal axis of the vessel.
On scanning electron micrographs, the internal elastic lamina appears as a solid sheet with small holes or fenestrae [32]. These fenestrae grow in size and number during development [33]. Their presence has been shown to influence the transport of molecules from the blood to the media and extravascular tissues [34]. Therefore, it is believed that remodeling of the fenestrae may be modulated by vascular requirements of permeability [33, 35]. Fenestrae also allow for direct contact between endothelial and vascular smooth muscle cells. However, not all fenestrae possess myoendothelial junctions [35]. Importantly, the size and number of these holes have been shown to change in response to physiological and pathological stimuli. For example, chronic blood flow augmentation causes their enlargement [33], while hypertension is associated with their reduction in density and size [32, 35, 36]. The mechanisms that control the remodeling of fenestrae are largely unknown, but the above evidence clearly indicates that the internal elastic lamina, which is usually considered highly resistant to degradation, may be rapidly remodeled.
The most abundant component of the media in arterioles is smooth muscle. The primary function of vascular smooth muscle cells within the media is to control vascular diameter via cell contraction and relaxation processes. To this end, vascular smooth muscle cells are arranged perpendicular to the longitudinal axis of the vessel in a circumferential fashion. They are spindle shaped with an average length of ~100 um (Martinez-Lemus unpublished results) [37, 38], but occasionally, cellular ends are bifurcated [39]. In feed arterioles, average cell width on the transversal axis of the vessel augments as vessels constrict, whereas cell width in the longitudinal axis of the vessel is ~4 um, and does not change with vasoconstriction or vasodilation [40]. This is consistent with the vascular smooth muscle cytoskeleton being arranged in a fashion that allows for the vascular wall to increase or decrease in thickness as vessel tone changes and without affecting vascular length. As mentioned above, feed arterioles consist of one to two layers of smooth muscle. Cells within each layer appear to maintain their position as they wrap around the vascular circumference, but occasionally, cells traverse across layers of smooth muscle and overlap each other transversally to the longitudinal axis of the vessel wall (Martinez-Lemus unpublished observation). Vascular smooth muscle cells are arranged at a pitch that can vary ± 20 degrees perpendicularly to the longitudinal axis of the vessel, and some studies suggest this angle changes as the vessel undergoes vasoconstriction [1]. Intercellular connections between vascular smooth muscle cells are not continuous and occur in the form of simple appositions, interdigitations, intermediate junctions and nexus junctions [41, 42]. Intercellular contacts often involve the presence of rake-like cellular projections [41]. These cellular projections are likely highly plastic and flexible, as results indicate that during the process of vasoconstriction a certain degree of cell slippage occurs when the diameter of the vessel is reduced [40]. In the coronary microcirculation a number of vascular smooth muscle cells in the outermost layer of the media run parallel to the vessel length presumably to provide resistance against the stretching forces produced by the beating heart [43]. Intercellular connections likely involve a number of attachment and junctional molecules such as integrins, cadherins and connexins, but a more detailed description of the in situ structural contacts among arteriolar smooth muscle cells is needed in order to have a more comprehensive understanding of the roles these cellular interconnections play on vascular function.
An important feature of vascular smooth muscle cells associated with their role in controlling vascular diameter is their ability to detect and react to mechanical forces through the process of mechanotransduction. Each smooth muscle cell is surrounded by a basement membrane containing collagen type IV, fibronectin, and some collagenous fibrils [1, 30, 44]. In addition, small elastic fibrils also envelope individual smooth muscle cells [30]. A close association between extracellular matrix structures and cells is expected for the transduction of mechanical forces into cellular responses, and extracellular matrices including interlamellar elastic fibers are hypothesized to aid in transferring stress to smooth muscle cells within the vascular wall [45]. Cellular mechanotransduction is a highly studied phenomenon, and ample molecular information exists on this process as it occurs in cultured cells. However, less is known about how smooth muscle cells detect and transform mechanical forces within the context of the vascular wall.
Under physiological conditions, arteriolar smooth muscle cells are partially contracted to exert tone, while elastic fibers have been described as protruded and wavy [31]. This suggests that under in vivo levels of vascular tone, extracellular matrix components of arterioles, such as collagen and elastic fibers, are in part under a state of compression while cells are under tension. However, the structural mechanisms by which cells transmit tension and compress extracellular matrix elements to induce vasoconstriction are not completely understood. The apparent periodical distribution of the ridges or folds in the internal elastic lamina suggests that contracted smooth muscle cells compress a portion of extracellular matrix components, but concurrently other components may be under tension. Therefore, a more precise description of matrix microarchitecture mechanics within the arteriolar wall is needed for a better understanding of the process of vascular smooth muscle cell mechanotransduction.
Physical forces are primarily transmitted to cells of the vascular wall through their adhesive contacts with the extracellular matrix and one another [46, 47]. We have previously shown that blockade of integrins or cadherins inhibits myogenic vasoconstriction, the process by which arteriolar diameter is reduced in response to intraluminal pressure augmentation [48, 49]. These results indicate that intercellular contacts and cell-extracellular matrix interactions within the arteriolar wall are essential components of myogenic phenomena and mechanotransduction. However, the mechanosensory process remains to be fully elucidated.
Dense bodies are electron-dense regions within vascular smooth muscle in situ, where actomyosin interactions occur or actin filaments bundle with focal adhesion components to form connections with the extracellular matrix. Anatomical descriptions of the distribution of dense bodies indicate that smooth muscle cellular attachments to extracellular matrix components are more prominent at the abluminal surface of the cell membrane [31, 50]. At the luminal side, dense bodies have a distribution pattern consistent with more dense cellular attachments occurring at periodical points where the cell presumably pulls on the internal elastic lamina to form the characteristic folding of the intima seen in constricted arterioles [31]. How the distribution of adhesive sites and the cytoskeleton is functionally linked to mechanosensation and mechanotransduction is not completely understood, but evidence indicates that as vessels undergo vasoconstriction, dense bodies become shorter and thicker, suggesting that adhesion clusters are remodeled as cells contract. In addition, it has been shown that the overall extension of dense bodies in vascular smooth muscle cells change in vessels from hypertensive rats [51, 52]. These observations are consistent with results showing that the expression of integrins changes in hypertensive vessels [53, 54], and with results indicating that in the early stages of vasoconstriction-induced arteriolar remodeling, vascular smooth muscle cells undergo repositioning within the vascular wall [40, 55]. Overall, the aforementioned observations indicate that cell-extracellular matrix interactions in the arteriolar wall are highly dynamic and participate in acute and chronic vascular events such as vasoconstriction and vascular remodeling.
Interestingly, in response to exposure to vasoconstrictor agonists, vascular smooth muscle cells produce and activate a number of matrix metalloproteinases (MMPs) [56–58]. Acutely, activation of MMPs induces vasoconstriction via the transactivation of epidermal growth factor receptors and the production of reactive oxygen species (ROS) [56, 57, 59]. More chronically, MMPs and ROS participate in inward remodeling processes occurring in response to prolonged vasoconstriction [58]. Putatively MMPs aid in allowing vascular smooth muscle cells to reposition during the remodeling process by inducing partial degradation of the basement membrane and extracellular matrix proteins that envelop these cells in the vascular wall [55]. Subsequently new extracellular components and cellular attachments are formed in conjunction with an upregulation of integrin synthesis [54, 60]. Bakker et al. documented that activation of tissue type transglutaminase is also needed in the inward remodeling process occurring in response to prolonged arteriolar constriction [61]. One of the major activities of transglutaminases is the crosslinking of matrix proteins such as collagen. Therefore, Bakker et al. have proposed that cross-linking of collagen by transglutaminases encroaches the arteriolar lumen resulting in inward eutrophic remodeling [61, 62]. The involvement of both MMPs and transglutaminases in the remodeling process suggests that a rapid and dynamic change in extracellular matrix structure and cellular attachments occurs as the arteriolar wall remodels. An association between MMPs, tissue type transglutaminase and integrins has been previously reported in a number of cellular systems [63, 64]. However, although abundant information exists on the characteristics and composition of focal adhesions in cultured cells, little is known about the precise structural characteristics of arteriolar dense bodies in situ and the effects that MMPs and transglutaminases may have on these structures. What are the specific cellular receptors associated with dense bodies at the luminal and abluminal sides of the cells? What is the composition and specific configuration of the extracellular matrices associated with dense bodies? What is the level of tension present at dense bodies under vascular constriction or dilation? Do MMPs or transglutaminases affect the structural characteristics and dynamics of dense bodies within the arteriolar wall? Answers to these questions should provide insights as to how vascular smooth muscle cells respond to mechanical forces and vasoconstriction, and how cellular repositioning occurs within the vascular wall. Ultimately, a better understanding of the arrangement of the cellular connections to extracellular matrix components and their plasticity in the in situ configuration should provide better means to elucidate the process of mechanotransduction and remodeling within the arteriolar wall.
The Adventitia: more than a structural scaffold
The adventitial layer consists of some fibroblasts embedded in an extracellular matrix made predominantly of thick bundles of collagen fibers oriented along the longitudinal axis of the vessel [1, 65]. In comparison to the vascular smooth muscle cells in the media, fibroblasts in the adventitia are not surrounded by a basement membrane [1], and there is evidence that some of the cells considered adventitial fibroblasts may be stem mesenchymal progenitor cells [66]. Nonmyelinated nerve endings are also present in the adventitia of arterioles at a distance of approximately 5 µm from the outermost layer of vascular smooth muscle [1, 43]. Recent findings also indicate that elastic fibers arranged with a longitudinal pitch are also present in the adventitial layer of arterioles from a number of vascular beds. These elastic fibers appear to serve the purpose of allowing resistance arteries and arterioles to elongate and recoil longitudinally in expandable tissues such as skeletal muscle and mesentery, where resistance arterioles possess an additional external elastic lamina that is absent in non-expandable tissues such as the brain [67].
Historically, the role of the adventitial layer on vascular function has been restricted to a structural support for the vessel and a scaffold for the anchoring of nerve endings. Recently, however, the role of the adventitial fibroblast in vascular control has been greatly expanded. In particular, adventitial fibroblasts have been associated with the production of ROS that in turn modulate the activity of smooth muscle cells in the vascular media and partake in the initiation of vascular remodeling [68]. Adventitial fibroblasts also produce a number of cellular growth factors and vasoactive compounds such as transforming growth factor beta, basic fibroblast growth factor, and endothelin-1, all of which have important effects on medial cell proliferation and the control of vascular tone [69]. In addition, adventitial fibroblasts play a preponderant role in vascular repair. In response to injury, fibroblasts are transformed into myofibroblasts. This transformation allows them to increase their contractile capabilities via the expression of alpha actin, and to produce extracellular matrix proteins such as collagen. It has also been proposed that myofibroblasts can transdifferentiate into vascular smooth muscle cells, at least the synthetic phenotype, but this hypothesis remains to be fully corroborated [70]. The aforementioned findings make evident that the adventitial fibroblast provides an additional level of plasticity to the arteriolar wall via the modulation of vascular smooth muscle activity, the production and remodeling of extracellular matrix compounds, the generation of inflammatory signals, and even potentially the generation of tensile force.
Conclusion
The wall of arterioles is composed of cells and extracellular matrix components that interact with each other in such intimate fashions of architectural and functional interdependence that the anatomical boundaries separating the traditional layered subdivisions become blurred. This is particularly evident in arterioles undergoing remodeling, as is becoming increasingly clear that cellular and extracellular components change their position and structure across layers more rapidly than previously envisioned. In general cells are the main components that actively participate in the acute control of vascular tone and permeability, while the extracellular components play more of a structural role with additional capabilities as reservoirs of soluble and insoluble signals for the cells. Although abundant information exists on the gross and ultrastructural characteristics and composition of arterioles, many microarchitectural, biomechanical, and dynamic features remain to be elucidated.
References
- 1.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 2.Rhodin JAG. Architecture of the vessel wall. In: Bohr DF, Somlyo AP, Sparks HV, editors. Handbook of Physiology. Washington, D. C.: American Physiological Soc; 1980. p. Ch 1. -31. [Google Scholar]
- 3.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 4.Meininger GA, Harris PD, Joshua IG. Distributions of microvascular pressure in skeletal muscle of one- kidney, one clip, two-kidney, one clip, and deoxycorticosterone-salt hypertensive rats. Hypertension. 1984;6:27–34. doi: 10.1161/01.hyp.6.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Sun D, Huang A, Kaley G. Mechanical compression elicits NO-dependent increases in coronary flow. American journal of physiology Heart and circulatory physiology. 2004;287:H2454–H2460. doi: 10.1152/ajpheart.00364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun D, Huang A, Sharma S, Koller A, Kaley G. Endothelial microtubule disruption blocks flow-dependent dilation of arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2087–H2093. doi: 10.1152/ajpheart.2001.280.5.H2087. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Medical & biological engineering & computing. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Y, Edwards-Bennett S, Bubb MR, Block ER. Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. American journal of physiology Cell physiology. 2003;284:C1542–C1549. doi: 10.1152/ajpcell.00248.2002. [DOI] [PubMed] [Google Scholar]
- 9.Su Y, Zharikov SI, Block ER. Microtubule-active agents modify nitric oxide production in pulmonary artery endothelial cells. American journal of physiology Lung cellular and molecular physiology. 2002;282:L1183–L1189. doi: 10.1152/ajplung.00388.2001. [DOI] [PubMed] [Google Scholar]
- 10.Brum Cde A, Duarte ID, Webb RC, Leite R. Disruption of microtubular network attenuates histamine-induced dilation in rat mesenteric vessels. American journal of physiology Cell physiology. 2005;288:C443–C449. doi: 10.1152/ajpcell.00130.2004. [DOI] [PubMed] [Google Scholar]
- 11.Yu PK, Yu D, Alder VA, Seydel U, Su E, Cringle SJ. Heterogeneous endothelial cell structure along the porcine retinal microvasculature. Experimental eye research. 1997;65:379–389. doi: 10.1006/exer.1997.0340. [DOI] [PubMed] [Google Scholar]
- 12.Thurston G, Baldwin AL. Endothelial actin cytoskeleton in rat mesentery microvasculature. The American journal of physiology. 1994;266:H1896–H1909. doi: 10.1152/ajpheart.1994.266.5.H1896. [DOI] [PubMed] [Google Scholar]
- 13.McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res. 2006;98:939–946. doi: 10.1161/01.RES.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 14.Ueki Y, Uda Y, Sakamoto N, Sato M. Measurements of strain on single stress fibers in living endothelial cells induced by fluid shear stress. Biochemical and biophysical research communications. 2010;395:441–446. doi: 10.1016/j.bbrc.2010.04.051. [DOI] [PubMed] [Google Scholar]
- 15.Schnittler HJ, Schneider SW, Raifer H, Luo F, Dieterich P, Just I, et al. Role of actin filaments in endothelial cell-cell adhesion and membrane stability under fluid shear stress. Pflugers Archiv : European journal of physiology. 2001;442:675–687. doi: 10.1007/s004240100589. [DOI] [PubMed] [Google Scholar]
- 16.Loufrani L, Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Medical & biological engineering & computing. 2008;46:451–460. doi: 10.1007/s11517-008-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. Journal of cellular physiology. 1995;163:179–193. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- 18.Reneman RS, Hoeks AP. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Medical & biological engineering & computing. 2008;46:499–507. doi: 10.1007/s11517-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thurston G, Baldwin AL, Wilson LM. Changes in endothelial actin cytoskeleton at leakage sites in the rat mesenteric microvasculature. The American journal of physiology. 1995;268:H316–H329. doi: 10.1152/ajpheart.1995.268.1.H316. [DOI] [PubMed] [Google Scholar]
- 20.Nag S, Robertson DM, Dinsdale HB. Intracerebral arteriolar permeability to lanthanum. The American journal of pathology. 1982;107:336–341. [PMC free article] [PubMed] [Google Scholar]
- 21.Ochoa CD, Wu S, Stevens T. New developments in lung endothelial heterogeneity: Von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost. 2010;36:301–308. doi: 10.1055/s-0030-1253452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto K, Yoshida K, Fujii S, Takemasa T, Sago H, Yamashita K. Heterogeneous responsiveness of the in situ rat vascular endothelial cells to mechanical stretching in vitro. European journal of cell biology. 1995;68:70–77. [PubMed] [Google Scholar]
- 23.Kobayashi N, Sakai T. Heterogeneity in the distribution of actin filaments in the endothelial cells of arteries and arterioles in the rat kidney. European journal of cell biology. 1993;60:57–66. [PubMed] [Google Scholar]
- 24.Imamura H, Ohta T, Tsunetoshi K, Doi K, Nozaki K, Takagi Y, et al. Transdifferentiation of bone marrow-derived endothelial progenitor cells into the smooth muscle cell lineage mediated by tansforming growth factor-beta1. Atherosclerosis. 2010;211:114–121. doi: 10.1016/j.atherosclerosis.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 25.Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovascular research. 2010;86:506–515. doi: 10.1093/cvr/cvq012. [DOI] [PubMed] [Google Scholar]
- 26.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circulation research. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 27.Lindblom A, Paulsson M. Basement Membranes. In: Comper WD, editor. Extracellular Matrix. Amsterdam: Harwood Academic Publisher GmbH; 1996. pp. 132–174. [Google Scholar]
- 28.Das A, Frank RN, Zhang NL, Turczyn TJ. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch's membrane. Arch Ophthalmol. 1990;108:421–429. doi: 10.1001/archopht.1990.01070050119045. [DOI] [PubMed] [Google Scholar]
- 29.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wight TN. Arterial Wall. In: Comper WD, editor. Extracellular Matrix. Amsterdam: Harwood Academic Publishers GmbH; 1996. pp. 175–202. [Google Scholar]
- 31.Sleek GE, Duling BR. Coordination of mural elements and myofilaments during arteriolar constriction. Circulation research. 1986;59:620–627. doi: 10.1161/01.res.59.6.620. [DOI] [PubMed] [Google Scholar]
- 32.Arribas SM, Briones AM, Bellingham C, Gonzalez MC, Salaices M, Liu K, et al. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am J Physiol Heart Circ Physiol. 2008;295:H2299–H2307. doi: 10.1152/ajpheart.00155.2008. [DOI] [PubMed] [Google Scholar]
- 33.Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries: effect of blood flow. Circulation research. 1996;78:799–805. doi: 10.1161/01.res.78.5.799. [DOI] [PubMed] [Google Scholar]
- 34.Guo ZY, Yan ZQ, Bai L, Zhang ML, Jiang ZL. Flow shear stress affects macromolecular accumulation through modulation of internal elastic lamina fenestrae. Clin Biomech (Bristol, Avon) 2008;23(Suppl 1):S104–S111. doi: 10.1016/j.clinbiomech.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? Journal of anatomy. 2009;214:258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, et al. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. The Journal of physiology. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BG, Gattone VHd, Overhage JM, Bohlen HG, Evan AP. Morphological evaluation of vascular smooth muscle cell: length and width from a single scanning electron micrograph of microvessels. Anat Rec. 1986;216:95–103. doi: 10.1002/ar.1092160116. [DOI] [PubMed] [Google Scholar]
- 38.Evan AP, Connors BA. Morphometric analysis of vascular smooth muscle cells by scanning electron microscopy. Int Rev Exp Pathol. 1996;36:31–52. [PubMed] [Google Scholar]
- 39.Ikebe T, Shimada T, Ina K, Kitamura H, Nakatsuka K. The three-dimensional architecture of retinal blood vessels in KK mice, with special reference to the smooth muscle cells and pericytes. J Electron Microsc (Tokyo) 2001;50:125–132. doi: 10.1093/jmicro/50.2.125. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. Faseb J. 2004;18:708–710. doi: 10.1096/fj.03-0634fje. [DOI] [PubMed] [Google Scholar]
- 41.Krizmanich WJ, Lee RM. Correlation of vascular smooth muscle cell morphology observed by scanning electron microscopy with transmission electron microscopy. Exp Mol Pathol. 1997;64:157–172. doi: 10.1006/exmp.1997.2217. [DOI] [PubMed] [Google Scholar]
- 42.Sosa-Melgarejo JA, Berry CL, Robinson NA. Effects of hypertension on the intercellular contacts between smooth muscle cells in the rat thoracic aorta. Journal of hypertension. 1991;9:475–480. doi: 10.1097/00004872-199105000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Higuchi K, Hashizume H, Aizawa Y, Ushiki T. Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Arch Histol Cytol. 2000;63:115–126. doi: 10.1679/aohc.63.115. [DOI] [PubMed] [Google Scholar]
- 44.Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, et al. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90:473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- 45.O'Connell MK, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, et al. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix biology : journal of the International Society for Matrix Biology. 2008;27:171–181. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Lemus LA, Sun Z, Trache A, Trzciakowski JP, Meininger GA. Integrins and regulation of the microcirculation: from arterioles to molecular studies using atomic force microscopy. Microcirculation. 2005;12:99–112. doi: 10.1080/10739680590896054. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, et al. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- 48.Jackson TY, Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. N-Cadherin and Integrin Blockade Inhibit Arteriolar Myogenic Reactivity but not Pressure-Induced Increases in Intracellular Ca. Front Physiol. 2010;1:165. doi: 10.3389/fphys.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. {alpha}v{beta}3- and {alpha}5{beta}1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 50.Gabella G. Asymmetric distribution of dense bands in muscle cells of mammalian arterioles. J Ultrastruct Res. 1983;84:24–33. doi: 10.1016/s0022-5320(83)90083-7. [DOI] [PubMed] [Google Scholar]
- 51.McGuffee LJ, Little SA. Tunica media remodeling in mesenteric arteries of hypertensive rats. The Anatomical record. 1996;246:279–292. doi: 10.1002/(SICI)1097-0185(199610)246:2<279::AID-AR14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 52.Hansen-Smith F, Greene AS, Cowley AW, Jr., Lougee L, Lombard JH. Structural alterations of microvascular smooth muscle cells in reduced renal mass hypertension. Hypertension. 1991;17:902–908. doi: 10.1161/01.hyp.17.6.902. [DOI] [PubMed] [Google Scholar]
- 53.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats : effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- 54.Heerkens EH, Shaw L, Ryding A, Brooker G, Mullins JJ, Austin C, et al. alphaV integrins are necessary for eutrophic inward remodeling of small arteries in hypertension. Hypertension. 2006;47:281–287. doi: 10.1161/01.HYP.0000198428.45132.02. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 56.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 57.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, et al. MMP-2 Mediates Angiotensin II-Induced Hypertension Under the Transcriptional Control of MMP-7 and TACE. Hypertension. 2011;57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Lemus LA, Zhao G, Galinanes EL, Boone M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. American journal of physiology Heart and circulatory physiology. 2011;300:H2005–H2015. doi: 10.1152/ajpheart.01066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol. 2006;26:819–825. doi: 10.1161/01.ATV.0000204344.90301.7c. [DOI] [PubMed] [Google Scholar]
- 60.Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling, and hypertension. Hypertension. 2007;49:1–4. doi: 10.1161/01.HYP.0000252753.63224.3b. [DOI] [PubMed] [Google Scholar]
- 61.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 62.Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- 63.Belkin AM, Zemskov EA, Hang J, Akimov SS, Sikora S, Strongin AY. Cell-surface-associated tissue transglutaminase is a target of MMP-2 proteolysis. Biochemistry. 2004;43:11760–11769. doi: 10.1021/bi049266z. [DOI] [PubMed] [Google Scholar]
- 64.Chen SH, Lin CY, Lee LT, Chang GD, Lee PP, Hung CC, et al. Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Res. 2010;30:4177–4186. [PubMed] [Google Scholar]
- 65.Sangiorgi S, Manelli A, Dell'Orbo C, Congiu T. A new method for the joint visualization of vascular structures and connective tissues: corrosion casting and 1 N NaOH maceration. Microscopy research and technique. 2006;69:919–923. doi: 10.1002/jemt.20366. [DOI] [PubMed] [Google Scholar]
- 66.Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochemical and biophysical research communications. 2008;368:305–310. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- 67.Lee RM. Morphology of cerebral arteries. Pharmacology & therapeutics. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 68.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 69.Di Wang H, Ratsep MT, Chapman A, Boyd R. Adventitial fibroblasts in vascular structure and function: the role of oxidative stress and beyond. Canadian journal of physiology and pharmacology. 2010;88:177–186. doi: 10.1139/Y10-015. [DOI] [PubMed] [Google Scholar]
- 70.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovascular research. 2010;88:395–405. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]