Abstract
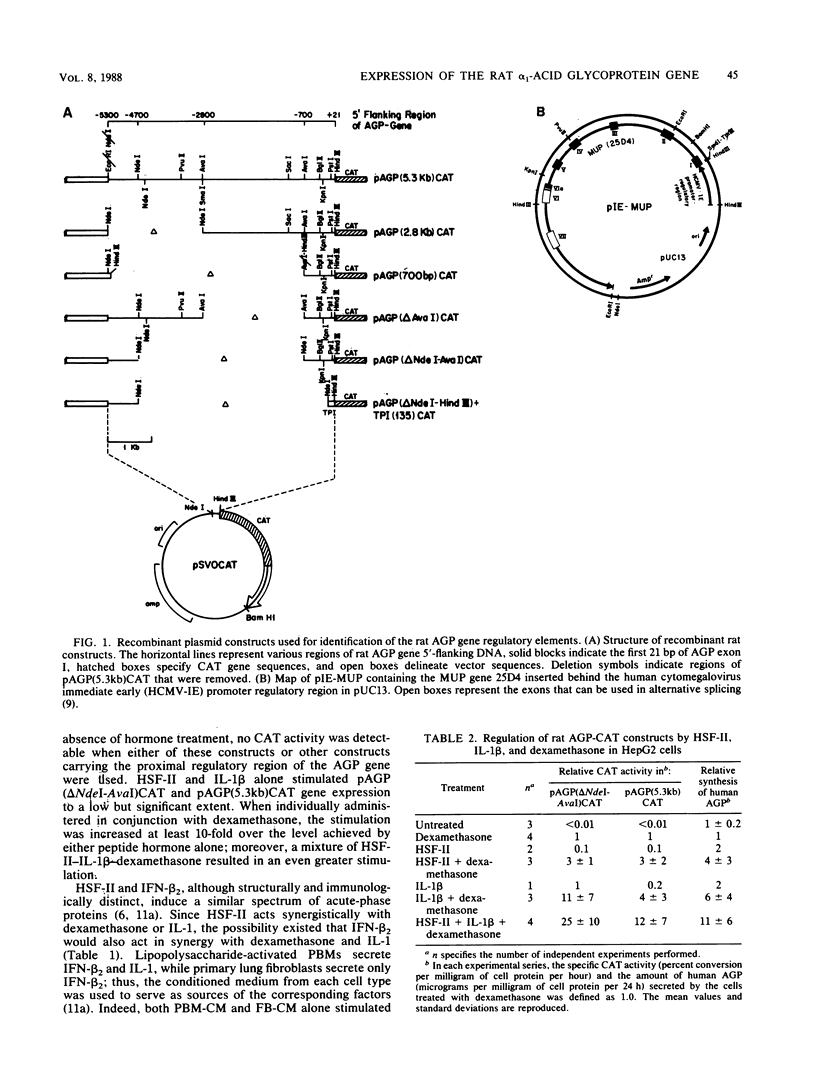
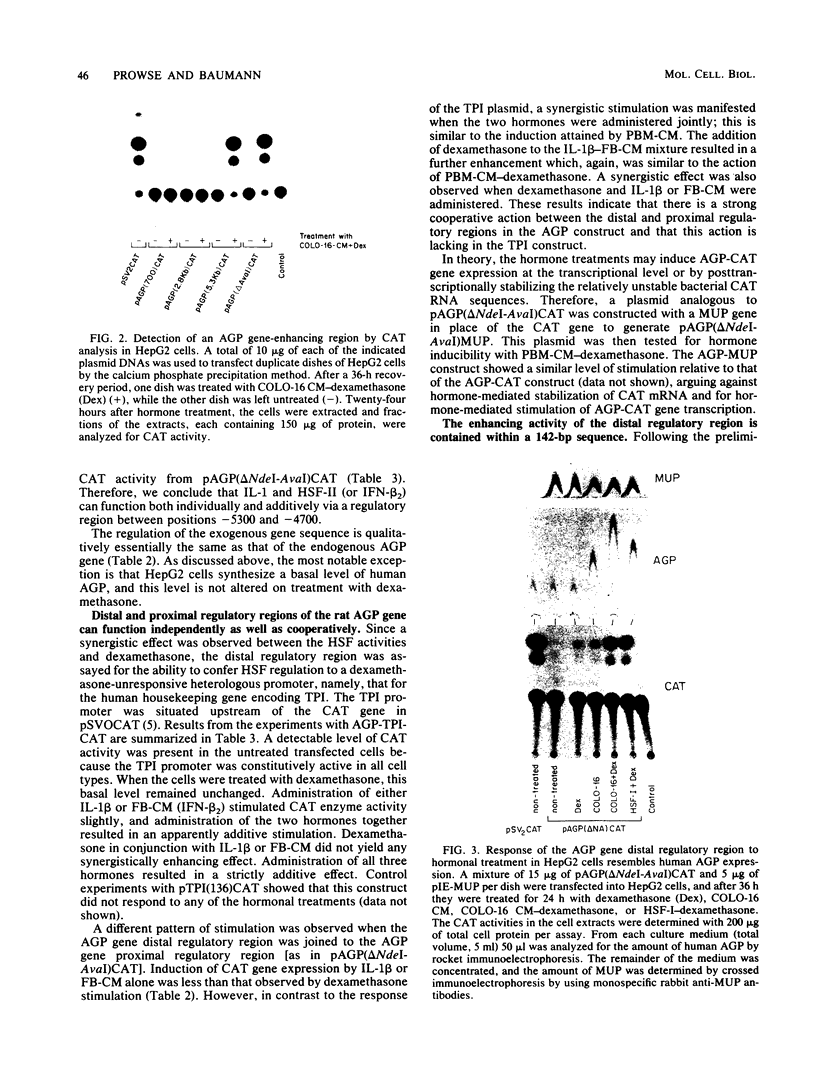
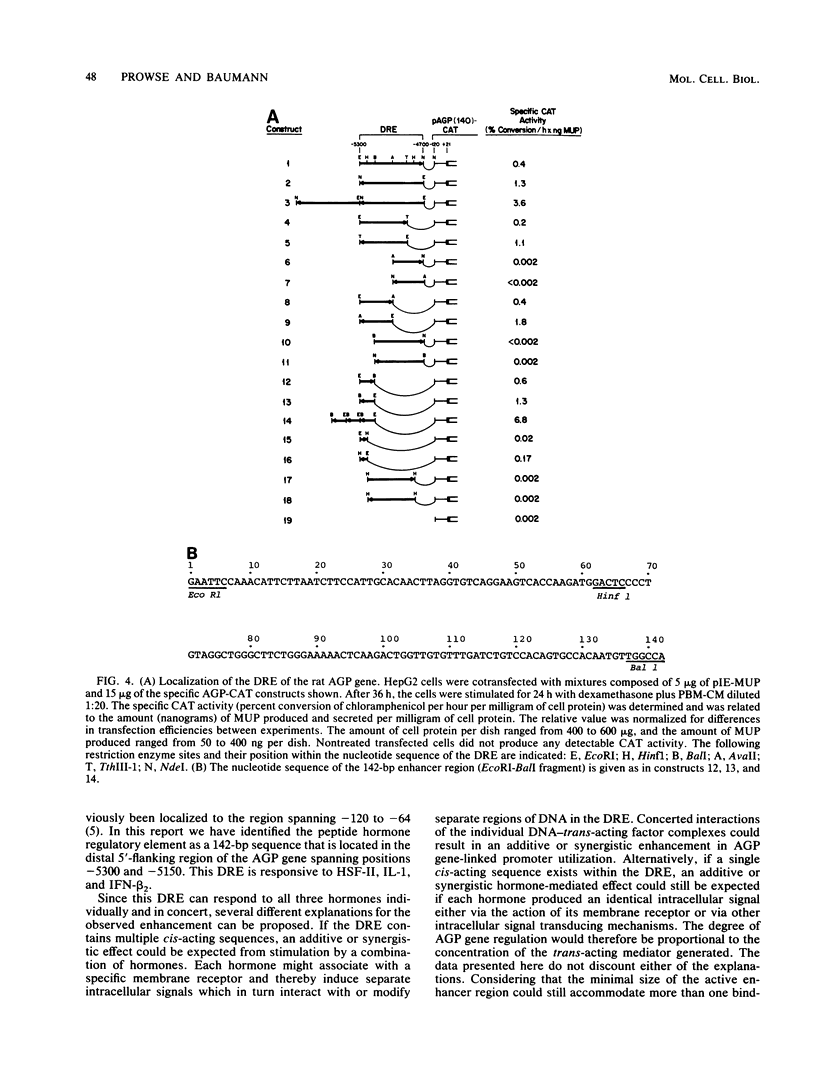
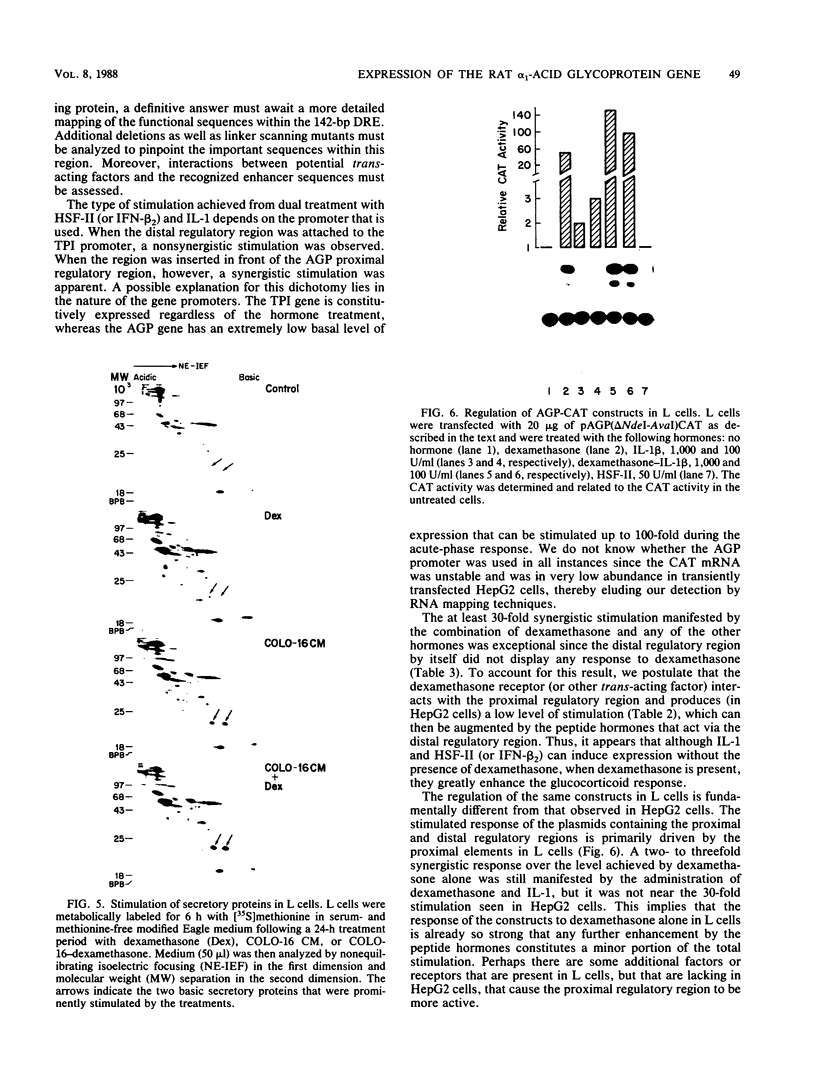
The rat alpha 1-acid glycoprotein (AGP) gene is transcriptionally regulated by dexamethasone, interleukin 1 (IL-1), hepatocyte-stimulating factor, and beta 2 interferon. The steroid and peptide hormones stimulate expression of the AGP gene synergistically as well as independently. The regulatory sequence responsible for dexamethasone-stimulated expression has been localized previously to a region that is 120 to 64 base pairs (bp) upstream of the transcription start site (H. Baumann and L. E. Maquat, Mol. Cell. Biol. 6:2551-2561, 1986). To identify the regulatory sequence that is responsive to the peptide hormones, different lengths of the AGP gene 5'-flanking DNA were linked to the chloramphenicol acetyltransferase gene and then assayed for hormone-inducible chloramphenicol acetyltransferase gene expression in transiently transfected HepG2 cells. We demonstrate that an enhancer region that is responsive to IL-1, hepatocyte-stimulating factor, and beta 2 interferon lies within a 142-bp sequence located 5,300 to 5,150 bp upstream of the transcription start site. This distal regulatory region can confer hormone inducibility to a heterologous promoter; exert its affect in either orientation; and function, to a lesser degree, in nonhepatic but IL-1-responsive cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Held W. A. Biosynthesis and hormone-regulated expression of secretory glycoproteins in rat liver and hepatoma cells. Effect of glucocorticoids and inflammation. J Biol Chem. 1981 Oct 10;256(19):10145–10155. [PubMed] [Google Scholar]
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Baumann H., Maquat L. E. Localization of DNA sequences involved in dexamethasone-dependent expression of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1986 Jul;6(7):2551–2561. doi: 10.1128/mcb.6.7.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Onorato V., Gauldie J., Jahreis G. P. Distinct sets of acute phase plasma proteins are stimulated by separate human hepatocyte-stimulating factors and monokines in rat hepatoma cells. J Biol Chem. 1987 Jul 15;262(20):9756–9768. [PubMed] [Google Scholar]
- Bennett M., Schmid K. Immunosuppression by human plasma alpha 1-acid glycoprotein: importance of the carbohydrate moiety. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6109–6113. doi: 10.1073/pnas.77.10.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch H. E., Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem. 1986 Jun 25;261(18):8077–8080. [PubMed] [Google Scholar]
- Clark A. J., Clissold P. M., Al Shawi R., Beattie P., Bishop J. Structure of mouse major urinary protein genes: different splicing configurations in the 3'-non-coding region. EMBO J. 1984 May;3(5):1045–1052. doi: 10.1002/j.1460-2075.1984.tb01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., Hopp T. P., Cantrell M., Deeley M., Gillis S., Henney C. S., Urdal D. L. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986 Nov 20;324(6094):266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gallagher J. F., Hohman C. M., Kuhn N. J., Sampsell B. M., Hughes R. G., Jr Identification and characterization of functional genes encoding the mouse major urinary proteins. Mol Cell Biol. 1987 Oct;7(10):3705–3712. doi: 10.1128/mcb.7.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Reinke R., Feigelson P. Acute phase mediators and glucocorticoids elevate alpha 1-acid glycoprotein gene transcription. J Biol Chem. 1985 Dec 15;260(29):15386–15389. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reinke R., Feigelson P. Rat alpha 1-acid glycoprotein. Gene sequence and regulation by glucocorticoids in transfected L-cells. J Biol Chem. 1985 Apr 10;260(7):4397–4403. [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Stinski M. F., Roehr T. J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985 Aug;55(2):431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Ringold G. M., McLean J. W., Taylor J. M. Induction of the acute-phase reactant, alpha 1-acid glycoprotein, by glucocorticoids in rat hepatoma cells. DNA. 1983;2(3):205–212. doi: 10.1089/dna.1983.2.205. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]







