Abstract
After a decade of work to address cellular uptake, the principal obstacle to RNAi-based therapeutics, there is now well-deserved, renewed optimism about RNAi-based drugs. Phase I and II studies have shown safe, strong, and durable-gene knockdown (80–90 %, lasting for a month after a single injection) and/or clinical benefit in treating several liver pathologies. Although promising, these studies have also highlighted the need for robust delivery techniques to develop RNAi therapeutics for treating other organ systems and diseases. Conjugation of siRNAs to cell-specific, synthetic RNA ligands (aptamers) is being proposed as a viable solution to this problem. While encouraging, the extended use of RNA aptamers as a delivery tool for siRNAs awaits the identification of RNA aptamer sequences capable of targeting and entering the cytoplasm of many different cell types. We describe a cell-based selection process for the rapid identification and characterization of RNA aptamers suited for delivering siRNA drugs into the cytoplasm of target cells. This process, termed “cell-internalization SELEX (Systematic Evolution of Ligands by Exponential Enrichment),” entails the combination of multiple sophisticated technologies, including cell culture-based SELEX procedures, next-generation sequencing (NGS), and novel bioinformatics tools.
Keywords: RNA aptamers, Systematic evolution of ligands by exponential enrichment (SELEX), Cell-internalization SELEX, Cell-targeted aptamers, Next-generation sequencing (NGS), Bioinformatics, Quantitative PCR
1 Introduction
Aptamers are synthetic, structural oligonucleotides that bind particular target molecules with high affinity and specificity [1]. Aptamers have several advantages as therapeutic reagents, including that they (1) can be chemically synthesized, (2) can be chemically conjugated to secondary reagents using well-defined and site-specific covalent coupling mechanisms, (3) have low immunogenicity (due to chemical modifications), (4) bind their targets (usually proteins) with affinities comparable to antibody/antigen interactions, and (5) often impair the function of their protein target (e.g., function as receptor antagonists) [2]. The clinical potential of aptamers is highlighted by the FDA approval of an aptamer-based drug for macular degeneration [3, 4] and by clinical trials that demonstrate the safety and efficacy of systemically administered RNA aptamers [5]. Aptamers that bind the extracellular domains of cell surface receptors have previously been used to deliver secondary reagents to cells that express the targeted receptor (i.e., cell type-specific delivery [6–9]). Various aptamer conjugates have been explored with this basic approach, including aptamer-drug [5, 7, 10] and aptamer-nanoparticle conjugates [11]. We previously pioneered the cell type-specific delivery of siRNAs with RNA aptamers using a simple all-RNA reagent [12]. This approach leverages the power of both the aptamer and RNAi platforms to yield a simple means of downregulating the expression of virtually any gene in the genome in a cell type-specific manner. The single-component makeup (all RNA) of aptamer-siRNA chimeras (AsiCs) also reduces the future regulatory hurdles in the drug approval process and the likelihood that the reagents will exhibit toxicity. The promise of this technology for clinical applicability is further highlighted by the demonstrations by several groups of low-dose efficacy in diverse animal disease models [13–17].
1.1 Aptamer Identification via SELEX
The SELEX (Systematic Evolution of Ligands by EXponential enrichment) process by which aptamers for a chosen target are identified is distinct from other ligand development platforms and provides important advantages for the aptamer platform [18, 19]. SELEX is a powerful ligand identification process capable of screening combinatorial nucleic acid sequence libraries with vast complexities (typically 1012–1014 distinct sequences are screened). SELEX does not necessitate expression of aptamers or their derivatives in cells or organisms (e.g., as with antibody development), which can lead to loss of potential “winning” sequences due to expression difficulties. Furthermore, the essential information needed to identify and regenerate each aptamer is encoded in the aptamer (through PCR amplification) itself. This property is at the heart of the SELEX process and it enables the easy adaptation of SELEX to a wide variety of contexts such as cells in culture and even entire organisms. It also enables the construction of selection schemes that favor isolation of aptamers that exhibit particular properties (e.g., cell internalization) beyond basic target binding.
1.2 Cell-Internalization SELEX
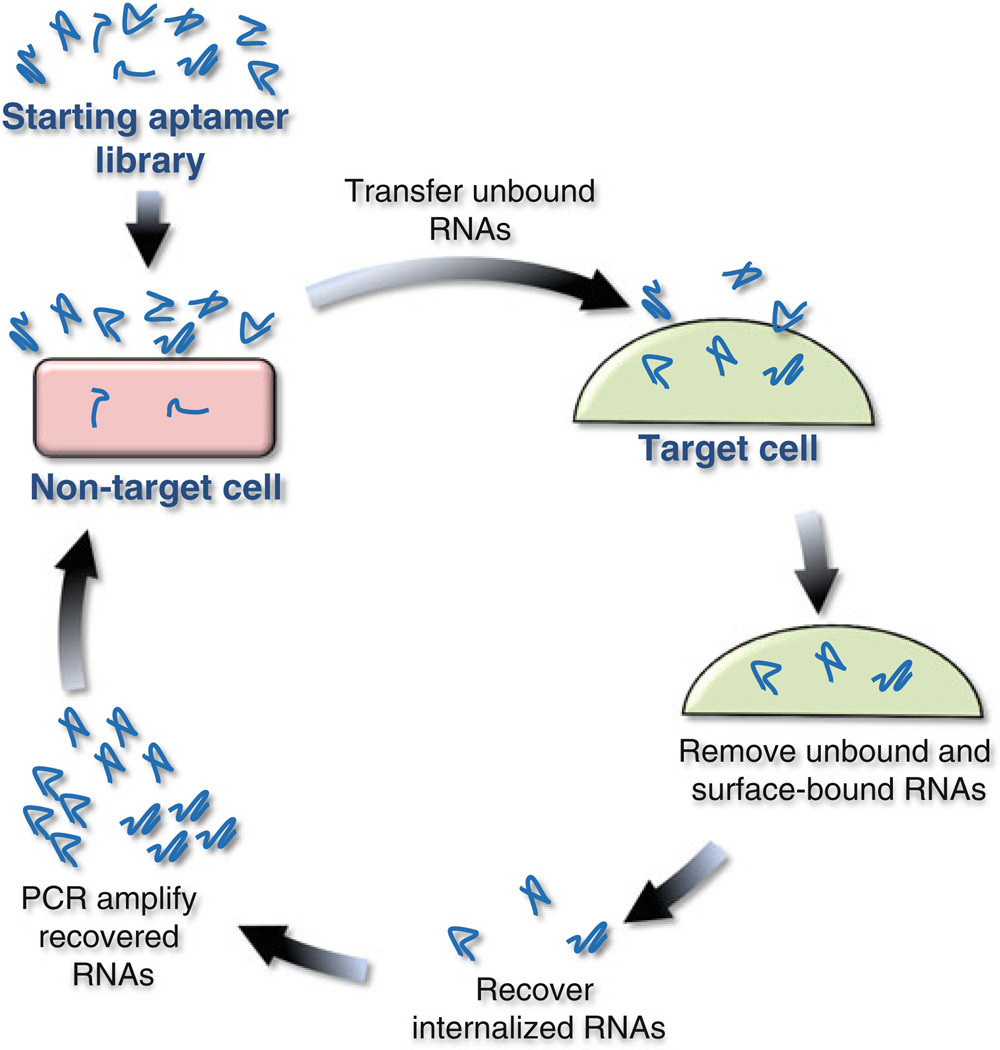
Internalization into a cell is an essential property of RNA aptamers that are to be used to deliver secondary reagents, such as siRNAs. Many ligands of cell surface proteins are efficiently internalized after binding their protein targets on the cell surface. We recently used the versatility of SELEX to selectively identify RNA aptamers that not only bind a particular target protein on the surface of cells, but are also subsequently internalized into the cell [20–22]. The process we developed, termed “cell-internalization SELEX” (Fig. 1), involves addition of a combinatorial sequence library to the culture media of live cells that express a targeted cell surface receptor and incubation at 37 °C for a period of time. Unbound aptamers and aptamers that remain on the surface of the cells are removed with a stringent wash and the internalized sequences are recovered and amplified with RT-PCR for generation of the subsequent selection round. Importantly, an initial “pre-clearing” step is used to remove sequences that are internalized by cell surface components other than the target receptor. This step is ideally carried out with control cells that are identical to the target receptor-expressing cells, except that they lack the target receptor. Using this approach, together with next-generation sequencing of resulting sequence pools, we identified cell-internalizing RNA aptamers specific for two distinct receptor tyrosine kinases [20, 22].
Fig. 1.
Cell-internalization SELEX (Systematic Evolution of Ligands by EXponential enrichment). The starting RNA aptamer library is pre-cleared by incubating the library with nontarget cells. Those RNAs that do not bind or internalize into the nontarget cells are then transferred to the target cells. Unbound and surface-bound RNAs are discarded and internalized RNAs recovered. The recovered RNAs are amplified using PCR and the resulting amplified dsDNA in vitro transcribed for the subsequent round of selection. The selection is terminated when the library has reached ~80–90 % sequence convergence (~6–10 rounds of selection) as measured by NGS technologies
1.3 SELEX with Next-Generation Sequencing (NGS) and Bioinformatics Analysis
Identification of the sequences that emerge after several rounds of SELEX was previously accomplished by cloning and sequencing <100 individual sequences from the PCR amplification step. While this often provided a sufficient number of winning sequences to proceed with aptamer characterization, the number of sequences that were analyzed was quite small in comparison with the sequence complexity of evolved oligonucleotide pools. We and others recently sought to evaluate more sequences within evolved SELEX sequence pools in order to generate a more complete picture of the winning sequences that emerge from a selection [20–28]. For this, we used NGS technology to determine the sequences of thousands of individual aptamers recovered at various stages of several SELEX protocols [20–23]. Importantly, these data, when coupled with appropriate bioinformatics analyses, enabled us to identify winning aptamer sequences at earlier selection rounds and thereby reduced the time and resources needed to complete aptamer identification. Here we provide detailed methods for performing the cell-internalization SELEX protocol and for identifying winning sequences with NGS and bioinformatics analysis.
2 Materials
2.1 Primers
Primers should be resuspended at 100 µM in PCR-grade H2O and aliquoted.
Sel2 5′-primer: 5′-TAATACGACTCACTATAGGGAGGACGATGCGG-3′.
Sel2 3′-primer: 5′-TCGGGCGAGTCGTCTG-3′.
Sel1 5′-primer: 5′-GGGGGAATTCTAATACGACTCACTATA GGGAGAGAGGAAGAGGGATGGG-3′.
Sel1 3′-primer: 5′-GGGGGGATCCAGTACTATCGACCTCTGGGTTATG-3′.
2.2 Sel2 N20 Library ssDNA Template Oligo
The ssDNA template oligo should be PAGE purified for better results.
Sel2 N20 ssDNA template oligo: 5′-TCGGGCGAGTCGTCTG-N20-CCGCATCGTCCTCCC-3′.
2.3 Sel1 Processing Control Aptamer (M12–23) [29]
M12-23: 5′-GGGAGAGAGGAAGAGGGAUGGGCGACCGAACGUGCCCUUCAAAGCCGUUCACUAACCAGUGGCAUAACCCAGAGGUCGAUAGUACUGGAUCCCCCC-3′.
2.4 SELEX Reagents, Solutions, and Supplies
1.0 M Tris–HCl pH 8.0 (Sigma SLBF9645).
Taq DNA polymerase (Denville Scientific Choice Taq 5 units/µL).
10× buffer Taq DNA polymerase buffer (Denville Scientific).
10 mM dNTP mix (Invitrogen 4893).
DNA miniprep kit (Qiagen 27106).
T7 RNA polymerase 5× buffer: 20 % w/v PEG-8000 (Sigma P5413), 200 mM Tris–HCl pH 8.0 (Life Technologies 15568-025), 60 mM MgCl2 (Sigma M8266), 5 mM spermidine HCl (Sigma 233994), 25 mM DTT (Sigma 646563).
10× rNTP mix: 30 mM 2′F-C/U (2′F-CTP: TriLink N-1008-013008; 2′F-UTP: TriLink N1010-T1MH01A); 10 mM 2′OH-G/A (2′OH-ATP Roche 14470220; 2′OH-GTP Roche 14611221).
IPPI (Roche 13529722).
Y639F T7 RNA polymerase [30].
DNAse I 10 units/µL (Roche 04716728001).
Chloroform (Mallinckrodt Chemicals 4440-04).
2× Formamide RNA loading dye: 0.01 g Xylene Cyanol (Sigma Aldrich X4126-10G), 0.01 g Bromophenol Blue (Sigma Aldrich B5525-10G), 500 µL 10× TBE (rpi, T32024-4000), 10 mL Formamide (Amresco 0464-500 mL).
Acrylamide gel solution: 115 g Urea (rpi U20200-1000), 62.5 mL 40 % acrylamide:bis 29:1 (Bio-Rad 161-0146), 12.5 mL 10× TBE (rpi, T32024-4000) and bring to 250 mL with dH2O. Filter and store at 4 °C.
0.5× TBE (rpi, T32024-4000).
10 % APS (Sigma 248614).
TEMED (Sigma Aldrich T9281).
20 × 20 cm Vertical Electrophoresis System (Fisher Biotech FB-VE20-1).
Fluor-coated TLC plate (Ambion 10110).
0.5 M EDTA (Ambion AM9260G).
0.1 mM EDTA 10 mM TE pH 7.5 (Affymetrix 75793).
0.2 µm cellulose acetate Centrex MF filter (Whatman 10467013).
10 kDa MWCO regenerated cellulose centrifugal filter (Millipore UFC801024).
Linear acrylamide 5 mg/mL (Ambion AM9520).
10 M ammonium acetate: 770 g ammonium acetate (Amresco 01023-2.5KG) in 1 L dH2O. Solution should be filter-sterilized.
Cell culture medium (e.g., Opti-MEM, DMEM).
tRNA block: Resuspend yeast tRNA (Invitrogen 15401-018) to 10 mg/mL with PCR-grade H2O.
Ice-cold PBS (GIBCO 14040).
Ice-cold 0.5 M NaCl PBS: Add 50 mL 5 M NaCl to 450 mL PBS (GIBCO 14040).
TRIzol (Invitrogen 15596-018).
Superscript III and supplied 10× FS buffer (Life Technologies 18080-044).
RNase A 10 mg/mL (Fermentas EN0532).
Phenol/chloroform/isoamyl alcohol (Roche 03117979001).
iQ SYBR green 2× supermix (Bio-Rad 170-8882).
RNase/DNase-free 1.5 mL microfuge tubes.
15 and 50 mL conical tubes.
Cell culture dishes (15 cm) and plates (6-well).
Cryo vials.
2.5 Equipment
Swinging bucket centrifuge.
Microfuge tube centrifuge.
PCR machine.
Real-time PCR machine.
Handheld 254 nm UV lamp.
Heat block.
Cell culture incubator.
UV spectrometer or NanoDrop.
3 Methods
3.1 Making the Sel2 N20 dsDNA Library
Annealing step: Combine 9 µL 100 mM Tris–HCl pH 8.0, 2 nmol of Sel2 5′-primer, and 1 nmol Sel2 N20 ssDNA template oligo. Bring these to 90 µL total volume with H2O and aliquot into five PCR tubes at 18 µL/tube. Run an annealing program on a PCR machine using the following protocol: (1) 95 °C for 5 min; (2) 25 °C for 20 min; and (3) 4 °C hold.
Extension step: Combine 50 µL 10× Taq DNA polymerase buffer (with MgCl2), 10 µL 10 mM dNTP mix, and 20 units of Taq DNA polymerase to a total volume of 410 µL with PCR-grade H2O. Aliquot 82 µL per PCR tube containing the annealed template oligo and Sel2 5′-primer. Run an extension program on a PCR machine using the following protocol: (1) 72 °C for 30 min; (2) 25 °C for 10 min; and (3) 4 °C hold.
Purification step: The dsDNA library should be purified using DNA miniprep columns or PCR purification columns as directed in the supplier’s manual. To confirm the presence and quality of the Sel2 N20 DNA library, load 5–10 µL of the purified material on a 2–3 % agarose gel. Determine the concentration of the Sel2 N20 dsDNA library by UV spectrometry. The molecular weight of the Sel2 N20 dsDNA library is approximated to be MW 41,600.
3.2 Making the Sel2 N20 RNA Library
In vitro transcription step (125 µL reaction): Combine 25 µL T7 RNA polymerase 5× buffer, 12.5 µL 5× rNTP mix, 1.25 units IPPI, 62.5 pmol Sel2 N20 dsDNA template, and 1–5 µL Y639F T7 RNA polymerase [30] (see Note 1) and bring up to a total volume of 125 µL with PCR-grade H2O. Incubate at 37 °C overnight. Add 1 µL DNase and incubate at 37 °C for 20 min. Chloroform-extract RNA by adding 500 µL chloroform to the 125 µL in vitro RNA transcription reaction, vortex, and centrifuge at maximum rpm for 10 min at room temperature. Carefully pipette top aqueous phase only into a fresh microfuge tube. Add one volume of 2× formamide RNA loading dye. Heat the RNA-dye solution to 65 °C for 10 min prior to loading onto the acrylamide gel.
Gel purification step: Pour 10 % acrylamide gel by combining 25 mL acrylamide gel solution with 75 µL 10 % APS and 25 µL TEMED. Assemble gel apparatus and fill with 0.5× TBE. Preheat polymerized gel at 24 W for 20–30 min. Wash each well with 0.5× TBS to remove urea. Load RNA into the pre-washed wells and run at 24 W for approximately 30–40 min. Carefully remove the gel from the plates and place on a sheet of Saran Wrap. Detect the RNA aptamer library by UV shadow using a handheld UV lamp with fluor-coated TLC plates. Excise the RNA band using clean razor blades. Elute RNA from the excised acrylamide band in 4 mL 0.1 mM EDTA 10 mM TE pH 7.5 for >1 h at 37 °C while rotating. Transfer eluted RNA to 0.2 µm cellulose acetate Centrex MF filter and centrifuge at ~2,000 ×g to remove any gel fragments. Transfer flow-through to 10 kDa MWCO regenerated cellulose centrifugal filter, centrifuge at ~4,000 ×g for 10–15 min, and discard flow-through. Repeat the elution from the gel a second time using the same Centrex MF filter and 10 kDa MWCO filter. Wash RNA by adding 4 mL 0.1 mM EDTA 10 mM TE pH 7.5 to the 10 kDa MWCO filter and centrifuge at ~4,000 ×g for 10–15 min. The concentration of the Sel2 N20 RNA library should be determined by UV spectrometry.
RNA folding step: The RNA library should be heated and refolded at a concentration of 1–3 µM in cell culture medium without supplements or FBS: (1) 95 °C for 5–10 min; (2) 65 °C for 10–15 min; and (3) 37 °C for 20 min. Folded RNA can be stored at −20 °C and then thawed at 37 °C for 20 min.
3.3 Cell-Internalization SELEX
The Cell-Internalization SELEX protocol is intended for adherent cells. Cells that are non-adherent or detach easily will need to have the protocol adjusted accordingly. The cell-internalization SELEX protocol is entirely dependent on the quality/viability of the cells used. Thus, proper culture conditions are paramount to the success of the selection. This protocol has been used to identify aptamers that internalize into specific cell types (e.g., vascular smooth muscle cells vs. endothelial cells) [21] or cells that express specific cell surface receptors (e.g., HER2 or TrkB) [20, 22]. A well-characterized target cell line (positive selection step) and at least one nontarget cell line (negative selection step) need to be available prior to beginning the selection. Throughout the selection process, selection conditions (e.g., temperature, salt concentration, ratio of RNA to cells) can be altered in order to increase or decrease the selection pressure. Selection conditions need to be determined empirically for any given selection. The cell passage number should be kept consistent (within 1 or 4 passages) throughout the selection process to minimize variability due to passage number. This is particularly important for those selections performed on primary cells. Prior to starting a selection, cells should be tested for the desired phenotype/genotype (e.g., HER2 positivity) [20]. In addition, cells should be screened for mycoplasma contamination as this bacterium has been shown to secrete nucleases capable of degrading 2′-fluro-modified RNA aptamers [31].
Prepare nontarget and target cells: On day 1, seed both target and nontarget cells on 15 cm plates. For the selection, cells should be plated at >90 % confluence to minimize contact of RNA aptamers with plastic. All incubations are done at 37 °C and at 5 % CO2, unless otherwise specified.
Pre-clear aptamer library against nontarget cells: On day 2, dilute RNA aptamer to working concentration (~150 nM) in 12 mL of serum-free cell culture medium or Opti-MEM and add tRNA to 100 µg/mL. Wash nontarget cells twice with 15 mL of cell culture medium. This step will remove unattached/dead cells, which could lead to nonspecific binding of the RNA library. Block the nontarget cells with 15 mL of 100 µg/mL tRNA in cell culture media at 37 °C for 15 min. Discard the tRNA block, add the folded Sel2 N20 RNA library to the nontarget cells, and incubate for 15 min at 37 °C. Collect the pre-cleared Sel2 N20 RNA library containing media from the nontarget cells and centrifuge at 2,000 ×g for 5 min to pellet cell debris.
Incubate pre-cleared aptamer library with target cells: Wash target cells 2× with cell culture medium. Proceed to block the target cells with 15 mL tRNA for 15 min at 37 °C. Remove tRNA block and add the pre-cleared Sel2 N20 RNA library containing media to the target cells. Incubate at 37 °C for 30 min. Swirl media gently at least one time during the 30-min incubation.
Remove unbound and surface-bound RNAs: Wash the target cells 1× with 15 mL ice-cold PBS. Wash one time with 15 mL cold 0.5 M NaCl PBS (short wash), followed by one time 5-min wash with 15 mL cold 0.5 M NaCl at 4 °C. Wash one time with 15 mL ice-cold PBS. Tilt plates for ~30 s and aspirate to minimize residual PBS.
Recover internalized RNAs: Lyse cells with 4 mL TRIzol. Allow TRIzol to lyse cells completely by incubating at room temperature for 3–5 min. Collect as much TRIzol as possible using a cell scraper and pipette TRIzol-lysed samples into clean microfuge tubes (~4 tubes). Samples can be stored frozen at −80 °C at this step.
TRIzol extraction step: If necessary, thaw samples and proceed to shear DNA by vortexing samples for ~1 min. Add 200 µL chloroform per 1 mL TRIzol and vortex for 30 s. Centrifuge at room temperature at 15,000 ×g for 15 min. Transfer as much of the aqueous phase (400 µL) to a clean 1.5 mL microfuge tube being careful not to transfer any of the organic phase. Add 8–10 µL RNase A, mix carefully, and incubate at 37 °C for 30 min. The RNase treatment will degrade endogenous RNA but not the 2′-fluoro-modified RNA aptamers, thereby eliminating amplification of non-aptamer sequences during the PCR step. Add 1× volume (~600 µL) phenol/chloroform/isoamyl alcohol and vortex. Centrifuge at room temperature at 15,000 ×g for 10 min. Transfer as much of the aqueous phase (400 µL) to a clean microfuge tube. Add 1× volume (~600 µL) chloroform and vortex. Centrifuge at room temperature at 15,000 ×g for 10 min. Transfer as much of the aqueous phase (400 µL) to a clean microfuge tube. Divide the aqueous phase into four microfuge tubes. For each microfuge tube, ethanol precipitate the RNA aptamer by adding 5 µL linear acrylamide as a carrier, 1/10 volume 10 M ammonium acetate (30 µL), and 2× volume 100 % ice-cold ethanol (600 µL). Incubate at −80 °C overnight to precipitate RNA. Centrifuge at 4 °C for 30 min at 15,000 ×g to pellet precipitated RNA. Discard supernatant and wash pellet with 300 µL of 95 % ice-cold ethanol. Vortex and centrifuge at room temperature at 15,000 ×g for 10 min. Discard supernatant and air-dry pellet at 65 °C for 30 min or until no ethanol is present. Resuspend RNA pellet in 25 µL PCR-grade H2O. Incubate at 65 °C for 10–20 min to ensure that the RNA is completely dissolved. Pool samples and store at −80 °C. This RNA sample represents a single round of selection. Proceed with the RT and PCR steps to generate the RNA aptamer library for the next round of selection.
Generate the RNA aptamer library for the next round of selection: RT-PCR is performed to amplify the recovered RNA. We routinely perform a two-step RT-PCR protocol as follows: Anneal the Sel2 3′ primer to the recovered RNA. Combine: 10 µL 5× FS buffer, 1 µL 0.1 M DTT, 1 µL 100 µM Sel2 3′-primer, 31 µL PCR-grade H2O, and 5 µL recovered aptamer RNA. Incubate samples at (1) 65 °C for 5 min; (2) 22 °C for 5 min; and (3) 25 °C hold. Initiate reverse transcription by adding 1 µL 10 mM dNTP mix and 1 µL Superscript III reverse transcriptase. Run a Superscript III reverse transcriptase protocol: (1) 55 °C for 60 min; (2) 72 °C for 15 min; and (3) 4 °C hold. Use the RT in a PCR reaction. Combine 50 µL 10× Taq polymerase buffer; 20 µL 10 mM dNTP mix; 5 µL 100 µM Sel2 5′-primer; 5 µL 100 µM Sel2 3′-primer; 25 units of Taq polymerase; and 25 µL of the RT reaction; bring to 500 µL with PCR-grade H2O. Aliquot 100 µL of the PCR mix per PCR tube. Run a PCR amplification protocol: (1) 95 °C for 2 min; (2) 95 °C for 30 s; (3) 55 °C for 30 s; (4) 72 °C for 5 s; (5) repeat steps 2–4 for 25 cycles; (6) 72 °C for 5 min; and (7) hold at 4 °C. Clean the PCR product as outlined in Subheading 3.1, step 3. To generate the RNA for the next round of selection follow the in vitro RNA transcription protocol outlined in Subheading 3.2.
3.4 Assess the Progress of the Cell-Internalization Selection
Tracking the progress of the cell-internalization SELEX process is crucial for the success of the selection and for identifying cell-specific internalizing RNA aptamers. This information is necessary to appropriately adjust the selection pressure (e.g., increase no. of washes, increase RNA:cell ratio during selection, introduce an additional pre-clear step, and reduce incubation time of RNA with target cells), and determine when to stop a selection.
DNA melt assay: The DNA melt assay allows for a rapid assessment of the progress of the SELEX process. This assay is used to assess the overall sequence complexity in a given round. A decrease in sequence complexity is indicative of library convergence. To 33 µL of 0.5 µM dsDNA (from a selection round) add 33 µL of SYBR green supermix. Pipette 20 µL of dsDNA/SYBR green mix into a qPCR plate in triplicate wells. Run a reverse DNA melt program on a real-time PCR machine using the following protocol: (1) 95 °C for 15 min; (2) 95 °C for 15 s; (3) 95–25 °C ramp over 20 min; (4) 25 °C for 15 s; and (5) 4 °C hold. Average triplicate data for each melt curve and plot temperature (65–95 °C) on the X-axis against fluorescence on the Y-axis. A shift to the right in the melt curve (higher temperatures) from round to round is indicative of a decrease in sequence complexity of the RNA pool.
Cell-internalization assay: All incubations are done at 37 °C and at 5 % CO2, unless otherwise specified. On day 1, target and nontarget cells should be seeded in 6-well plates. Cells should be >90 % confluent after 24 h. The following day, dilute the RNA aptamer pool or individual aptamer sequence to the working concentration (1 nM to 1 µM) in serum-free medium and add tRNA to 100 µg/mL. Wash cells 2× with 2–3 mL cell culture media. Block the cells with 1 mL of 100 µg/mL tRNA in cell culture media at 37 °C for 15 min. Discard tRNA block and add folded RNA aptamer to each well for 30 min at 37 °C. Swirl media every 15 min. Discard unbound RNA aptamer. Wash cells as outlined in Subheading 3.3, step 4. Lyse cells with 1 mL TRIzol that contains 5 × 10−3 pmol/mL of control Sel1 RNA. Incubate the TRIzol solution on cells for 3–5 min. Pipette TRIzol-lysed samples into clean microfuge tubes. Samples can be frozen and stored at −80 °C at this step. TRIzol extraction should be performed as outlined in Subheading 3.3, step 6 (see Note 2). The recovered RNA is quantified by RT-qPCR using SYBR green supermix as appropriate during the PCR detailed in Subheading 3.3, step 7. For each RNA sample perform two independent RT and PCR reactions: one with the Sel2 primers (Sel2 PCR) and one with the Sel1 primers (Sel1 PCR). The Sel1 PCR is used as a normalization control for sample processing. Proper processing should result in similar Sel1 PCR values (CT values) for each sample. Any samples that are excessively low or high should be flagged as possibly problematic due to issues with the TRIzol extraction. Average the triplicates of the Sel2 samples and normalize these data to the corresponding Sel1 samples. Data analysis from this point forward should be done as appropriate for the experiment, including calculation of experimental error and statistical tests.
3.5 Next-Generation Sequencing (NGS) of RNA Aptamer Pools
The following protocol is based on Illumina Sequencing technology, although other NGS methods can also be used.
The DNA or RNA from any given round of selection can be used for NGS. If using RNA, first perform an RT reaction using superscript III, but substitute the Sel2 3′-primer with the appropriate NGS primer (e.g., reverse or 3′-primer). The reverse-transcribed RNA or dsDNA from a given selection round may then be used for PCR amplification as done during the SELEX process with Taq DNA polymerase using the appropriate NGS primers instead of the Sel2 primers. A water control should be run with each sample as a control. If rounds of selection are going to be multiplexed for NGS the forward or 5′-NGS primer should contain a unique barcode.
The resulting PCR reaction should be run on a 1 % agarose gel along with the corresponding water control. The appropriate band should be excised and gel purified and an accurate sample concentration determined. Samples can be combined at an equal molar ratio if multiplexing.
The sample can be submitted for NGS and will undergo a separate quality/size analysis prior to processing. Sequencing from both ends of the PCR product should be chosen if the option is available.
3.6 Processing RNA Aptamer Library High-Throughput Sequencing Data
The Galaxy web server (https://usegalaxy.org/) provides all of the online tools necessary to do the following initial bioinformatics analysis of next-generation sequencing data. A brief outline of the steps is shown below:
Raw reads need to be parsed for quality (eng. intact 5′ and 3′ constant regions, “N” sequencing read errors).
Multiplexed data needs to be separated by barcode.
Constant regions can be filtered to give only the variable region.
Unique reads need to be determined and the number of each unique reads calculated by collapsing the data. The number of unique reads and the number of total reads can be used to determine the sequence enrichment of a selection round using the following equation: % sequence enrichment = 1 − (unique/total).
For an in-depth bioinformatics analysis several different strategies exist for examining RNA aptamer selection data including fold enrichment, percent enrichment, sequence relatedness, and predicted structure similarity.
Footnotes
The volume of the Y639F T7 RNA polymerase used for in vitro transcription is determined experimentally by testing the efficiency of transcription using different dilutions of the enzyme.
The remaining TRIzol organic phase may be used to isolate total protein or total DNA.
References
- 1.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiel KW, Giangrande PH. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides. 2009;19:209–222. doi: 10.1089/oli.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 4.Apte RS. Pegaptanib sodium for the treatment of age-related macular degeneration. Expert Opin Pharmacother. 2008;9:499–508. doi: 10.1517/14656566.9.3.499. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram P, Kurniawan H, Byrne ME, Wower J. Therapeutic RNA aptamers in clinical trials. Eur J Pharm Sci. 2013;48:259–271. doi: 10.1016/j.ejps.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Thiel KW, Giangrande PH. Intracellular delivery of rna-based therapeutics using aptamers. Ther Deliv. 2010;1:849–861. doi: 10.4155/tde.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerchia L, Giangrande PH, McNamara JO, de Franciscis V. Cell-specific aptamers for targeted therapies. Methods Mol Biol. 2009;535:59–78. doi: 10.1007/978-1-59745-557-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Rossi JJ. Cell-specific aptamer-mediated targeted drug delivery. Oligonucleotides. 2011;21:1–10. doi: 10.1089/oli.2010.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Bobbin ML, Burnett JC, Rossi JJ. Current progress of RNA aptamer-based therapeutics. Front Genet. 2012;3:234. doi: 10.3389/fgene.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanwar JR, Roy K, Kanwar RK. Chimeric aptamers in cancer cell-targeted drug delivery. Crit Rev Biochem Mol Biol. 2011;46:459–477. doi: 10.3109/10409238.2011.614592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundaram P, Wower J, Byrne ME. A nanoscale drug delivery carrier using nucleic acid aptamers for extended release of therapeutic. Nanomedicine. 2012;8:1143–1151. doi: 10.1016/j.nano.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.McNamara JO, II, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 13.Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, II, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27:839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler LA, Vrbanac V, Trifonova R, Brehm MA, Gilboa-Geffen A, Tanno S, Greiner DL, Luster AD, Tager AM, Lieberman J. Durable knockdown and protection from hiv transmission in humanized mice treated with gel-formulated cd4 aptamer-siRNA chimeras. Mol Ther. 2013;21:1378–1389. doi: 10.1038/mt.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, Smith DD, Swiderski P, Rossi JJ, Akkina R. An aptamer-siRNA chimera suppresses hiv-1 viral loads and protects from helper cd4(+) t cell decline in humanized mice. Sci Transl Med. 2011;3:66ra66. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni X, Zhang Y, Ribas J, Chowdhury WH, Castanares M, Zhang Z, Laiho M, DeWeese TL, Lupold SE. Prostate-targeted radio-sensitization via aptamer-shRNA chimeras in human tumor xenografts. J Clin Invest. 2011;121:2383–2390. doi: 10.1172/JCI45109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 19.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: Rna ligands to bacteriophage t4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 20.Thiel KW, Hernandez LI, Dassie JP, Thiel WH, Liu X, Stockdale KR, Rothman AM, Hernandez FJ, McNamara JO, II, Giangrande PH. Delivery of chemo-sensitizing siRNAs to her2+-breast cancer cells using RNA aptamers. Nucleic Acids Res. 2012;40:6319–6337. doi: 10.1093/nar/gks294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiel WH, Bair T, Peek AS, Liu X, Dassie J, Stockdale KR, Behlke MA, Miller FJ, Jr, Giangrande PH. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS One. 2012;7:e43836. doi: 10.1371/journal.pone.0043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YZ, Hernandez FJ, Gu B, Stockdale KR, Nanapaneni K, Scheetz TE, Behlke MA, Peek AS, Bair T, Giangrande PH, McNamara JO., II RNA aptamer-based functional ligands of the neurotrophin receptor, trkb. Mol Pharmacol. 2012;82:623–635. doi: 10.1124/mol.112.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiel WH, Bair T, Thiel WK, Dassie JP, Rockey WM, Howell CA, Liu XY, Dupuy AJ, Huang L, Owczarzy R, Behlke MA, McNamara JO, Giangrande PH. Nucleotide bias observed with a short selex RNA aptamer library. Nucleic Acid Ther. 2011;21:253–263. doi: 10.1089/nat.2011.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer S, Maufort JP, Nie J, Stewart R, McIntosh BE, Conti LR, Ahmad KM, Soh HT, Thomson JA. Development of an efficient targeted cell-selex procedure for DNA aptamer reagents. PLoS One. 2013;8:e71798. doi: 10.1371/journal.pone.0071798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiss DJ, Howard FM, Mobley HL. A novel approach for transcription factor analysis using selex with high-throughput sequencing (tfast) PLoS One. 2012;7:e42761. doi: 10.1371/journal.pone.0042761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann B, Gesell T, Chen D, Lorenz C, Schroeder R. Monitoring genomic sequences during selex using high-throughput sequencing: neutral selex. PLoS One. 2010;5:e9169. doi: 10.1371/journal.pone.0009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagannathan V, Roulet E, Delorenzi M, Bucher P. Htpselex–a database of high-throughput selex libraries for transcription factor binding sites. Nucleic Acids Res. 2006;34:D90–D94. doi: 10.1093/nar/gkj049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roulet E, Busso S, Camargo AA, Simpson AJ, Mermod N, Bucher P. High-throughput selex sage method for quantitative modeling of transcription-factor binding sites. Nat Biotechnol. 2002;20:831–835. doi: 10.1038/nbt718. [DOI] [PubMed] [Google Scholar]
- 29.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1bb binding aptamers costimulate cd8+ t cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa R, Padilla R. A mutant t7 RNA polymerase as a DNA polymerase. EMBO J. 1995;14:4609–4621. doi: 10.1002/j.1460-2075.1995.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez FJ, Stockdale KR, Huang L, Horswill AR, Behlke MA, McNamara JO., II Degradation of nuclease-stabilized RNA oligonucleotides in mycoplasma-contaminated cell culture media. Nucleic Acid Ther. 2012;22:58–68. doi: 10.1089/nat.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]