Abstract
Background
Emotional symptoms (ES) emerge forme fruste in adolescence, prior to manifesting as fully-fledged emotional disorders (ED). Studies indicate that subsyndromal ES precede the onset of ED. We hypothesised that adolescents showing subsyndromal ES will show perturbations in the emotion regulatory fronto-limbic network (FLN) during emotion-processing.
Methods
Fifty-eight female adolescents underwent functional magnetic resonance imaging (fMRI) whilst viewing an image-based emotion-processing task. Within this sample 33 (56.9%) displayed emotional symptoms and 25 (43.1%) did not. Clinical measures including assessments of mood and anxiety were administered and participants were allocated to one of two groups based on the presence (ES+) or absence (ES−) of subsyndromal ES. Group comparisons were used to identify differential patterns of neural engagement and their relationship to clinical variables.
Results
Groups displayed emotion-specific differences in FLN activity with increased frontal activity in ES+ girls during positive emotion-processing and decreased frontal and limbic activity during negative emotion-processing. Trait anxiety was the strongest clinical predictor of group membership (ES+ versus ES−) and displayed a significant negative correlation with hippocampal neural activity during negative emotion-processing. In addition, between the groups the hippocampus displayed a pattern of reverse coupling with the amygdala and insula that was also significantly correlated with trait anxiety.
Conclusions
There is divergence in the pattern of FLN neural processing in adolescent females determined by emotional symptoms. Future research is needed to corroborate these findings and to underline their implications longitudinally.
Keywords: fMRI, depression, anxiety, adolescents, emotional network, emotional disorders
Introduction
Depression and anxiety are two of the most commonly occurring psychiatric disorders in adulthood (1) and are characterised by emotion dysregulation (2, 3). Clinically, they are usually coterminous and collectively, their overlapping presentations are often referred to as emotional disorders (ED). Adolescents are commonly diagnosed with ED (4) and are at considerable risk for the symptoms to become recurrent and chronic (5, 6). Therefore identifying vulnerability to ED in adolescents is critical for shaping effective early interventions that may avert the progression and emergence of ED (7–9).
During adolescence, teenagers individuate, form dyadic relationships and cultivate peer networks, all of which involve potentially stressful interpersonal interactions (8, 10). Consequently, the adolescent brain undergoes substantial neurobiological maturation, particularly within emotion related networks, which involves shaping affective regulation in response to stressful life experiences (7). Therefore to understand the pathophysiology of ED, it is necessary to examine the relationship between early clinical symptoms and the neurobiolgical changes that occur in the emotion regulatory network before the onset of illness.
Functional magnetic resonance imaging (fMRI) is a safe and non-invasive technique for investigating in vivo brain function. Extant neuroimaging research implicates the fronto-limbic network (FLN) that encompasses the medial and lateral prefrontal cortex (PFC), including in particular the anterior cingulate cortex (ACC) and inferior frontal gyrus (IFG), amygdala (Ag), hippocampus (Hipp), and insula (Ins) in emotion regulation (11–14). Studies investigating anxiety and depression in adults have identified functional changes in the FLN and interestingly, findings from studies employing a variety of neuroimaging techniques in paediatric and adolescent ED populations, have implicated functional deficits in this same emotion regulatory network and its interconnections (15, 16).
It is therefore likely that adolescents vulnerable to ED will initially have subtle functional activity and connectivity changes involving the FLN, and it may be possible to identify these neural perturbations as emotional symptoms (ES) gradually emerge.
To date, no studies have investigated the neurobiological basis of this putative vulnerability to ED, and therefore in this study we set out to elucidate the functional antecedents to ED by investigating the neural activity and functional connectivity in the emotion regulatory network (FLN) during emotion-processing in adolescents displaying subclinical ES. To identify subclinical symptoms of ED we used a recognised assessment tool (see Methods and Materials) and on this basis separated our sample into a vulnerable group with emotional symptoms (ES+) and a less vulnerable group, without emotional symptoms (ES−). It was hypothesised that ES+ girls would show a different pattern of FLN activity and connectivity as compared to ES− girls, and that the differences will be significantly associated with clinical variables.
Methods and Materials
Participants
We targeted females in middle adolescence, because they are twice as likely to suffer from ED as males (17, 18). We examined girls in middle adolescence so as to avoid the potential confound of puberty at a younger age and yet still manage to sample the time period when most ED are taking form. Sixty-three adolescent girls (average age 15 years) were recruited from a girls’ school in Sydney. Participants with a history of significant neurological illness (eg., traumatic head injury, loss of consciousness, epilepsy or other neurological events), developmental disability, or any medical/physical condition that precluded them from neuroimaging were excluded. Written informed consent was obtained from the parents/guardians. The study was approved by the hospital Human Research Ethics Committee and the principal of the school. All participants underwent neuroimaging and on the same day they completed a series of self-report questionnaires in the CADE clinic (www.cadeclinic.com). Participants qualified as ES+ if they surpassed the cut-off score for any mental disorder according to The Child and Adolescent PsychProfiler (CAPP) (19), or had a history of a mental health issue that received psychological treatment.
Questionnaires
Participants completed a series of questionnaires measuring mood and anxiety, personality, self-concept, coping styles, and emotion regulation. These included: 1) the Children’s Depression Inventory (CDI) (20), 2) Mood Disorders Questionnaire - Adolescent Version (MDQ-A) (21), 3) Goldberg Mania Questionnaire (GMQ) (22), 4) State-Trait Anxiety Inventory (STAI) (23), 5) The Big Five Inventory (BFI) (24), 6) Piers-Harris Children’s Self Concept Scale Second Edition (25), 7) Ways of Coping Questionnaire (WCQ) (26), and 8) Difficulties in Emotional Regulation Scale (DERS) (27). The CAPP (19) screening tool was also used to measure subsyndromal symptoms of mental health problems. This instrument screens for 20 common psychiatric and psychological disorders comprising DSM-IV-TR criteria, with a positive result indicating a possible disorder that requires further diagnostic assessment.
Imaging Data Acquisition
Imaging datasets (structural and functional) were acquired on a 3T Siemens Magnetom Trio Scanner (Erlangen, Germany) at the Advanced Research and Clinical High-field Imaging (ARCHI) facility of the University of Sydney. A T2*-weighted gradient echo echo planner imaging (EPI) sequence (29 axial slices, slice thickness 4mm with 1mm gap, TR=2000ms, TE=35ms, flip=70°, 64 × 64 matrix) was used to acquire functional data. A high resolution T1-weighted structural image was also acquired for precise localisation of brain activity using a magnetisation prepared rapid gradient echo (MPRAGE) sequence (TR=1570ms, TE=3.22ms, Flip=15°, matrix 256 × 256, 192 slices).
fMRI Task
An explicit emotion-processing task (28) that reliably activates emotion regulatory regions was employed. It was programmed in Presentation software (http://www.neurobs.com). Participants viewed a series of 60 IAPS images of positive, negative, and neutral affect. Based on the IAPS norms for females (29), the positive and negative picture sets differed significantly with respect to valence (t(38) = −38.36, p <0.001). IAPS normative ratings for positive, negative, and neutral images were 8.0, 2.4, and 5.0, respectively. The level of arousal was comparable across positive (mean = 5.5) and negative images (mean = 5.6), whereas neutral pictures had lower standard emotional arousal ratings (mean = 3.4).
Images were displayed in 20-second blocks (5 images per block, and 2 blocks of each valence per run), alternating with 20-second baseline blocks of blank, greyscale images (6 blocks per run), over two functional runs of four minutes each (See Figure S1). Images were not repeated, and the order of blocks was counterbalanced. Before acquiring data, participants were introduced to the task using a separate practice set of IAPS images within the scanner. During the fMRI experiment participants were instructed to nominate quickly and accurately the valence of emotional images (i.e., negative, positive or neutral) and grey intensity of greyscale images (light, medium, and dark) by pressing one of three buttons. Immediately following the experiment participants completed pleasure ratings for each image on a 9-point scale (1 = most unpleasant; 9 = most pleasant).
Data Analysis
For an overview of the steps taken in the data analysis please see Approaches undertaken in the analysis in the Supplemental Information.
Behavioural Data Analysis
The behavioural data was analysed using a 2 (emotional symptoms: ES+, ES−) × 3 (image valence: positive, neutral, negative) mixed Analysis of Variance (ANOVA), with valence as a within-subjects factor and ES as a between-subjects factor.
Clinical Data Analysis
Clinical data was analysed using PASW Statistics Version 18. A series of One-Way Multivariate Analyses of Variance (MANOVA) were computed to compare ES+ and ES− on the measures of mood and anxiety, personality, self-concept, ways of coping, and difficulties in emotion regulation. All clinical measures that significantly differentiated ES+ and ES− were subsequently explored in a forward entry binary logistic regression model to determine which of these most strongly discriminated ES+ and ES−. The Bonferroni adjustment was applied for multiple contrasts to control for Type I errors.
fMRI Data Analysis
A detailed description of the pre-processing procedures is provided in the Supplemental Information.
First level model
Using the general linear model framework (31), each experimental condition (positive, negative, and neutral) was modelled with a box-car function convolved with a canonical hemodynamic response function. In order to remove low-frequency confounds, data were high-pass filtered (128sec). Temporal correlations were estimated using restricted maximum likelihood estimates of variance components using a first-order autoregressive model (AR1), and the resulting nonsphericity were used to form maximum likelihood estimates of the activations. For each subject, voxelwise statistical parametric maps (SPM) were calculated to identify brain regions implicated in processing positive and negative emotions using the experimental contrasts positive-neutral and negative-neutral, respectively.
Based on the extant literature, analyses were performed within a priori anatomical regions of interest (ROIs) namely, the Ag, Hipp, Ins, IFG and the ACC. Within the IFG the pars triangularis region was selected because of its key role in mood disorders as identified by a recent quantitative meta-analysis (32). Regional masks were created using the Automated Anatomical Labelling (AAL) software (33).
Second level model
In order to determine areas of activation during processing of an emotion, SPMs from all participants related to that particular emotion were entered into a second level random effects analysis (one-sample t-test). Activity was considered significant using a thresholded of p < 0.05 (corrected for multiple comparisons using false discovery rate, FDR) and a cluster size of 10voxels. To investigate differences between groups in regional brain activity, SPMs were entered into a two-sample t-test and masked by the regions that had shown significant activity in all subjects. Because of a priori hypotheses an uncorrected threshold of p < 0.05 and a cluster size of 10 voxels were used to detect subtle changes in adolescents with subsyndromal ES.
Correlation Between BOLD and Clinical Outcome Measures
Percentage BOLD signal change during the presentation of emotional images (compared to neutral images) was calculated from the clusters of voxels showing brain activity differences between groups during processing of emotion using the MarsBaR Toolbox (http://marsbar.sourceforge.net). These changes were then correlated with the clinical outcome measure that best differentiated the groups.
Post-hoc Functional Connectivity Analysis
To further understand the link between Hipp activity and trait anxiety a psychophysiological interaction (PPI) and correlation analyses were carried out. In PPI analyses the activity of a brain region (target region) is regressed onto the activity of another brain region (source region) to quantify the degree to which an experimental manipulation changes the coupling between these two regions (34). Since only Hipp activity (during processing of negative images) displayed a significant correlation with the measure of trait anxiety it was used as the seed region and other nodes of FLN as the target regions in the analysis.
The first eigenvariate time series from the Hipp cluster (x = 21, y =36, z =9) (See Table S2) showing the strongest correlation with the trait anxiety was extracted. The contrast depicting interaction between the source region and the experimental manipulation in the first level was taken to the second level to perform a random effect analysis to test for whether negative emotion modulates functional coupling of the Hipp with other nodes of the FLN differentially according to ES+ and ES− status. The statistical threshold was set to p < 0.01 (uncorrected) consistent with earlier studies (34–37). Finally, trait anxiety was correlated with the strength of coupling to investigate whether impaired functional connectivity in FLN underlies anxiety.
Results
Participants
Five participants were excluded from the analyses because of excessive motion during MRI acquisition, and final analyses were therefore conducted on 58 adolescents (Mean Age = 15.1 years, SD = 0.34). Within this sample 33 (56.9%) were assigned to the ES+ group, and 25 (43.1%) were assigned to the ES− group. There was no significant difference in age between the ES+ and ES− groups. Clinical measures that differed significantly between groups are reported in Table 1. As can be seen from the analyses there were overall significant differences between the groups on each clinical domain. The ES+ group had significantly greater levels of psychopathology with greater mood and anxiety symptoms, higher neuroticism, lower extraversion and conscientiousness, poorer self-concept and coping, and lower emotion regulation, as compared to the ES− group. Further, of the clinical measures that significantly differed between the groups trait anxiety was found to be the strongest predictor of group membership (χ2 = 31.09, df = 1, p < 0.0001, Nagelkerke R2 = 0.56, classification rate = 79.3%, Exp(B) = 1.272), and no other clinical measure contributed significantly over and above trait anxiety.
Table 1.
Comparisons between adolescents with (ES+) and without (ES−) subsyndromal emotional symptoms on clinical outcome measures.
| Clinical Domain / Measure | MES− | MES+ | SDES− | SDES− | F | p |
|---|---|---|---|---|---|---|
| Mood and Anxiety (MANOVA: Wilks =0.59; F(5,52)=7.26, p<0.0001) | ||||||
| Chidren’s Depression Inventory | 5.68 | 12.52 | 4.14 | 6.88 | 19.325 | <0.0001 |
| State Anxiety (STAI) | 19.76 | 26.61 | 5.70 | 11.27 | 7.703 | 0.007 |
| (CDI) Trait Anxiety (STAI) |
26.60 | 40.15 | 7.05 | 9.33 | 36.789 | <0.0001 |
| Personality (Big Five Inventory) (MANOVA: Wilks =0.66; F(5,50)=5.17, p=0.001) | ||||||
| Neuroticism | 16.80 | 22.52 | 5.50 | 4.76 | 16.488 | <0.0001 |
| Extraversion | 27.88 | 25.03 | 4.63 | 5.38 | 4.233 | 0.044 |
| C onscientiousness | 29.08 | 24.94 | 5.02 | 4.42 | 10.772 | 0.002 |
| Piers-Harris Self-Concept Scale (MANOVA: Wilks =0.68; F(6,51)=5.17, p=0.002) | ||||||
| Behavioural Adjustment | 13.20 | 11.55 | 1.08 | 2.08 | 13.122 | 0.001 |
| Intellectual and School Status | 13.96 | 10.73 | 2.11 | 3.16 | 19.472 | <0.0001 |
| Physical Appearance and | 8.72 | 6.64 | 2.15 | 2.53 | 10.920 | 0.002 |
| Freedom from Anxiety | 10.48 | 7.58 | 2.43 | 3.23 | 14.106 | <0.0001 |
| Happiness and Satisfaction | 8.84 | 7.42 | 1.40 | 1.94 | 9.536 | 0.003 |
| Ways of Coping Questionnaire (MANOVA: Wilks =0.72; F(8,46)=2.23, p=0.042) | ||||||
| Self-Controlling | 8.72 | 11.48 | 4.06 | 3.33 | 8.461 | 0.005 |
| Escape Avoidance | 5.36 | 9.58 | 3.70 | 4.68 | 15.066 | <0.0001 |
| Difficulties in Emotion Regulation Scale (MANOVA: Wilks =0.59; F(6,51)=5.81, p<0.0001) | ||||||
| Total score | 68.44 | 94.82 | 13.32 | 22.43 | 27.23 | <0.0001 |
Behavioural Results
Mauchly's Test of Sphericity was significant, indicating that sphericity is violated, thus Greenhouse-Geisser was used to correct for this. A significant multivariate main effect for image valence emerged, Wilks’ λ = 0.06, F(1.14,63.85) = 715.62, p < 0.05, partial η2= .93. Pairwise comparisons revealed that all three valences are rated significantly different, positive (M = 7.48, SD = .12), neutral images (M = 4.10, SD = 0.36), and negative images (M = 2.17, SD = 0.92). There was no significant main effect of emotional symptoms, and similarly, the interaction between image valence and emotional symptoms did not reach significance, p > 0.05.
Group fMRI Results
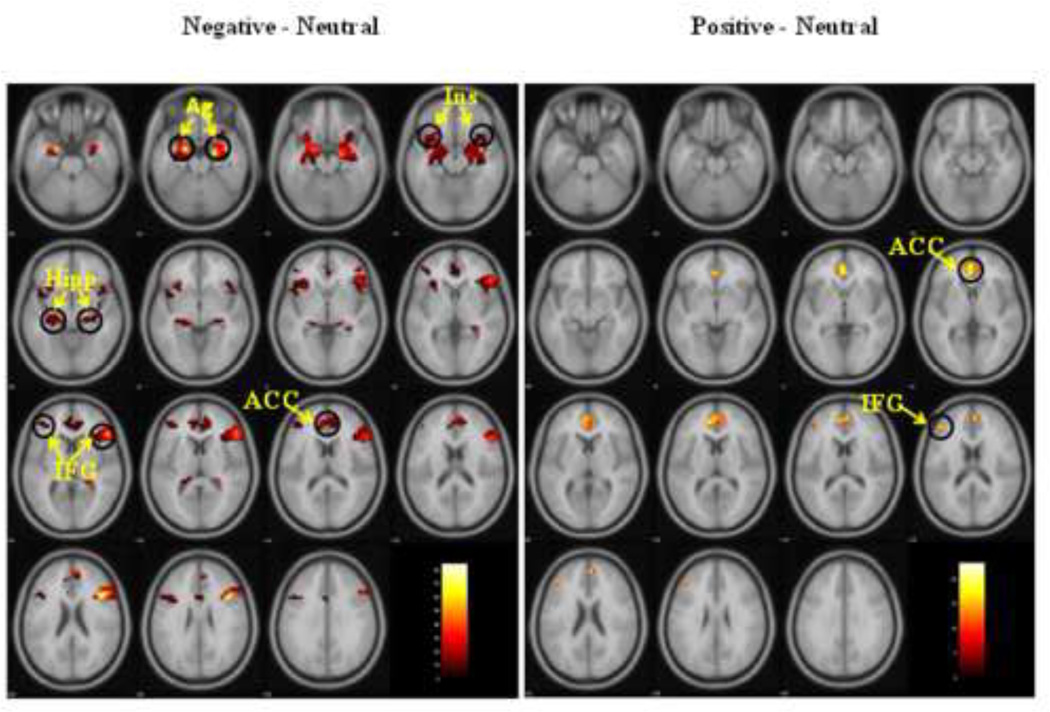
In all subjects the processing of negative emotions (compared to neutral) produced increased activity in both frontal and limbic regions, whereas the processing of positive emotion produced increased activity only in the frontal regions (See Table S3, Figure 1).
Figure 1.
In all subjects, compared to neutral images, negative images produced increased neural activity in the frontal (IFG and ACC) and limbic (Ag, Hipp, and Ins) regions. Positive images produced increased neural activity only in the frontal regions (IFG and ACC).
Legend: Ag = amygdala; Ins = insula; Hipp = hippocampus; ACC = anterior cingulate cortex; IFG = inferior frontal gyrus.
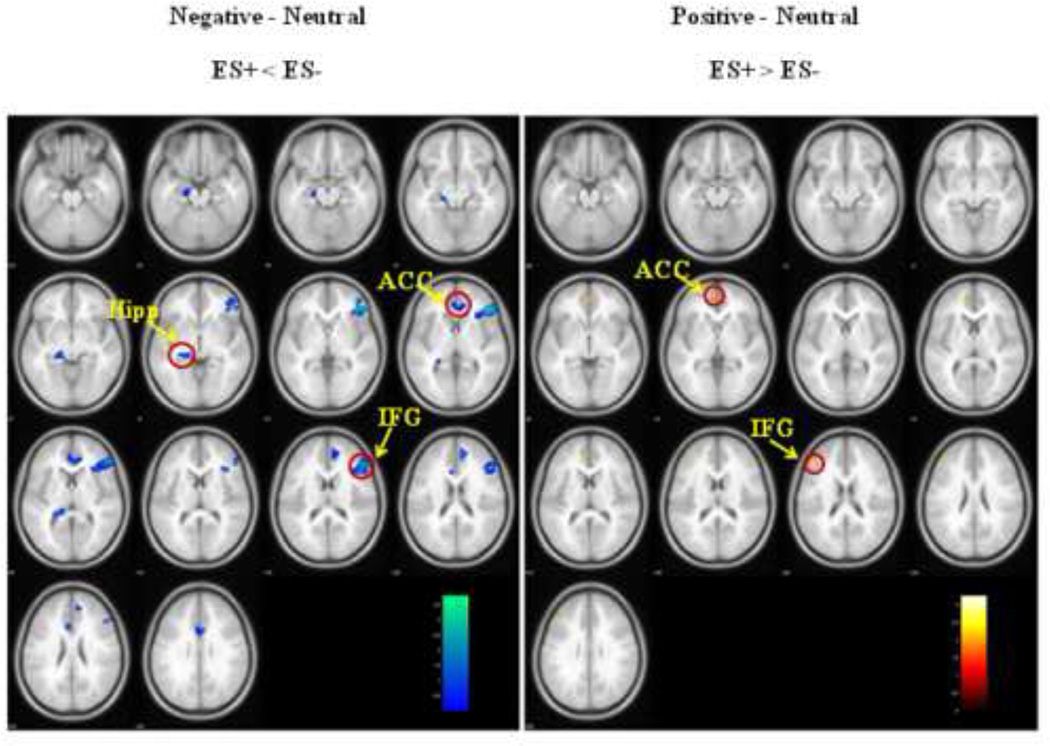
Between-group analyses revealed significant differences during both negative and positive emotion-processing. Relative to neutral images the processing of negative images produced less activity in ES+ adolescents in comparison to ES− adolescents. Specifically, activity was significantly reduced both in the frontal (right IFG, ACC) and limbic (left Hipp) regions (See Table S2, Figure 2). Conversely, during positive image processing ES+ adolescents showed relatively greater activity in frontal regions such as the IFG and ACC (See Table S2, Figure 2).
Figure 2.
Between-group analyses revealed that activity was significantly reduced both in the frontal (IFG, ACC) and limbic (Hipp) regions in adolescents with subsyndromal emotional symptoms (ES+) during the processing of negative emotion, whereas it was increased only in the frontal regions (IFG and ACC) during positive image processing.
Legend: Ins = insula; Ag = amygdala; IFG = inferior frontal gyrus.
Correlation between BOLD and Clinical Outcome Measures Results
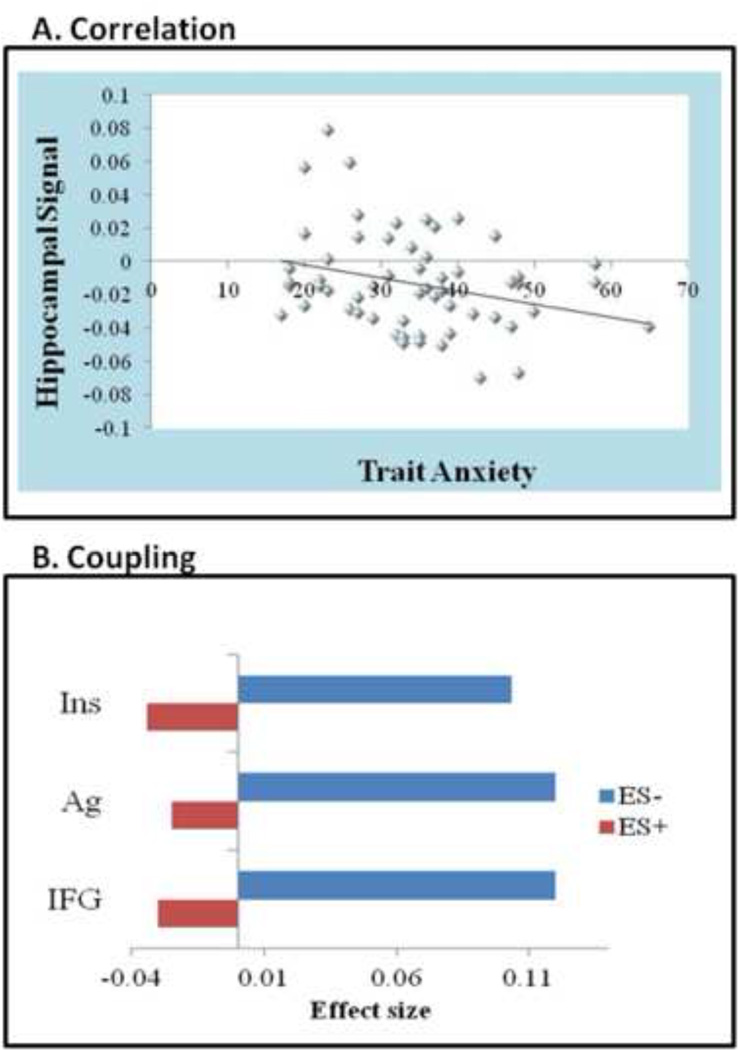
Percent BOLD signal changes that were estimated from the clusters of brain regions showing differences between groups during emotion-processing (See Table S2) were correlated with the measure of trait anxiety that significantly discriminated the two groups. Among these regions, only signal change in the Hipp during negative emotion-processing correlated with trait anxiety (r = −0.384, p = 0.003) (See Figure 3a).
Figure 3.
a. Hippocampal correlation: During negative emotion-processing the left hippocampus was the only ROI that significantly correlated with trait anxiety (the strongest predictor of group membership). As trait anxiety increased, the hippocampus signal decreased (negative correlation).
b. Hippocampal coupling: Results from psychophysiological interaction analysis showing regions where adolescents with (ES+) and without (ES−) subsyndromal emotional symptoms displayed a significant difference in hippocampus coupling during negative emotion-processing. Specifically, these included the insula (MNI coordinates −33, −18, −21, cluster size 25 voxels, z-score 3.10), amygdala (MNI coordinates 30, 9, 27, cluster size 13 voxels, z-score 3.01), and inferior frontal gyrus (MNI coordinates −54, 03, 3, cluster size 14 voxels, z-score 2.67). This is indicated by the figure which shows the direction of coupling and effect size.
Post-hoc Functional Connectivity Results
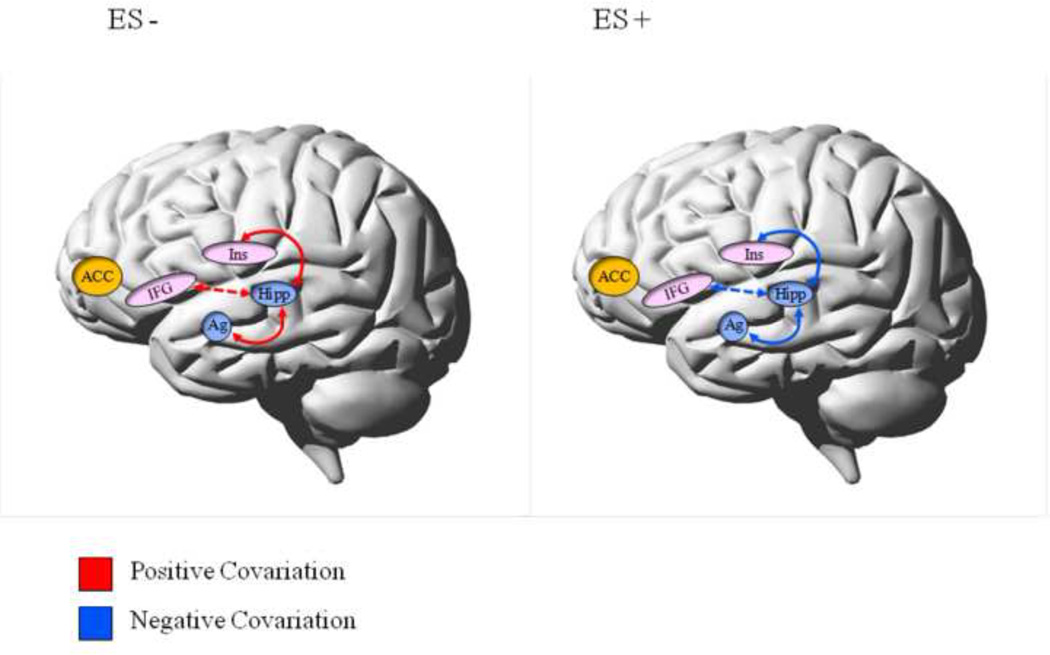
Significant differences between groups were observed in coupling of the Hipp with the left Ins, right Ag, and left IFG (See Figure 3b). This difference was such that the nature of coupling was positive in ES− and negative in ES+ (presented schematically in Figure 4). Moreover, coupling of the Hipp with the Ag (r = −0.358, p = 0.003) and Ins (r = - 0.318, p = 0.015) displayed significant negative correlations with trait anxiety (r = −0.358, p = 0.006 and r = −0.318, p = 0.015, respectively). Trait anxiety was high when the Hipp coupled negatively with the Ag and Ins and low when it coupled positively.
Figure 4.
Schematic summary of the differences between adolescents with (ES+) and without (ES−) subsyndromal emotional symptoms in the coupling of the hippocampus with frontal and limbic ROIs.
Legend: Trait anxiety significantly correlated with the coupling strength between the hippocampus and both the amygdala and the insula, respectively. Trait anxiety was high when hippocampus coupled negatively with the amygdala and insula but low when it coupled positively.
Solid line = significant correlation; dashed line = trend level correlation; ACC = anterior cingulate cortex; IFG = inferior frontal gyrus; Ins = insula; Ag = amygdala; Hipp = hippocampus.
Discussion
Subthreshold ES often precede the onset of ED (9, 38, 39) and therefore the present study examined the relationship between FLN activity during emotion-processing and subsyndromal symptoms in order to characterise the functional changes that may indicate a vulnerability to ED in adolescence. Two notable findings emerged from this preliminary investigation that may help better understand the neural basis of emotion dysfunction at its inception. First, ES+ girls displayed impairment in FLN functioning during both positive and negative emotion-processing, as compared to emotionally healthy girls. Second, in all subjects, Hipp activity and connectivity with Ag and Ins correlated robustly with trait anxiety. Finally, ES+ girls displayed reduced Hipp activity during negative emotion-processing and also a reverse pattern of Hipp connectivity. Together, these findings suggest that in adolescents, impaired functional connectivity within emotion regulatory FLN nodes is associated with ES and this subtle dysfunction may be a neural antecedent to the emergence of ED.
Depending upon their valence, emotional images produced differential patterns of FLN engagement in all subjects. Specifically, both positive and negative emotion produced increased frontal activity involving the IFG and ACC. Notably, IFG activity was greater during the processing of negative emotion than positive emotion. In addition, limbic regions (Ag, Ins, Hipp) were activated only during negative emotion-processing. These findings are consistent with findings from earlier research in both healthy adults and those with depression, which have shown that both negative and positive emotions reliably elicit FLN activity in the Ag, Hipp and PFC, but differ to the extent that particular nodes within the network are activated (40, 41).
Comparing the neural activity in ES+ and ES− participants, valence-contingent differences emerged with respect to activation of the FLN nodes. In particular, ES+ girls displayed increased frontal activity during positive emotion-processing but decreased frontal activity during negative emotion-processing. In addition to frontal changes, ES+ girls also displayed reduced limbic (Hipp) activity during negative emotion-processing. This is noteworthy because Hipp activity during negative emotion-processing was found to correlate with trait anxiety across the whole group (ES+ and ES−). Specifically, as trait anxiety increased, Hipp neural activity diminished. Interestingly, a recent study in depressed adults reported frontal and limbic hypoactivation and suggested that anxiety modulates this activity in response to different emotional stimuli (42). This could explain why in our study trait anxiety correlated with Hipp activity and why in ES+ girls this was diminished as compared to those without ES.
Regional activations involving individual nodes of the emotional network form only part of the picture. The associations and linkages between various nodes are also important, as impaired functional connectivity in this network has been suggested to underlie both affective dysregulation and anxiety (43). In this regard, our study found that the nature of coupling between the Hipp and other FLN nodes was dependent upon whether girls had subsyndromal psychopathology. Specifically, Hipp activity coupled positively with activity in other nodes of the network such as the Ag, Ins and IFG in ES− participants, but negatively in ES+ participants. Our finding of significant associations between trait anxiety and the nature of the coupling of Hipp with the Ag and Ins supports the view that dysfunction in this network underpins anxiety (44). Indeed, a recent study (45) found that emotion dysregulation increases the risk for a wide range of psychopathology in adolescence. This longitudinal study that assessed adolescents aged 11–18 years over a four-year period, found that emotion dysregulation predicts the likelihood of anxiety symptoms. This finding concords with earlier reports of emotional dysregulation in youths with anxiety disorders (46, 47). However, it has been proposed that emotion dysregulation per se is a transdiagnostic factor that is relevant to many types of psychopathology (48–50) and, as such, deficits in emotional regulation are a broad risk factor for adolescent psychopathology (51, 52). This is understandable in the context of adolescence being a critical developmental period, during which many emotion-laden situations have to be negotiated successfully in order to achieve social competence (53). Indeed, our finding of increased prefrontal activity in response to positive images in ES+ girls compared to ES− girls, may be an early indication of emergent dysfunction in mood circuitry that is also necessary for social functioning. Of note, impairment of the latter in adolescence predicts mood disorders in adulthood (60). Therefore, a key skill that adolescents normally acquire is the ability to adaptively regulate their emotions and those that do not learn how to effectively manage their affect are at greater risk for the development of psychiatric disorders (53, 54).
In adults with depression, the Hipp has emerged as a key structure because of its strong anatomical links to emotion-processing brain regions that form part of the FLN (61). It is thought to be important to the pathophysiology of mood because of its role in regulating the hypothalamic-pituitary adrenal (HPA) axis, which is often disturbed in depression (62) and because patients with depression often have hippocampal-dependent learning and memory deficits (63). A recent study that examined healthy adolescent girls (aged 9 to 15 years) found that in those at risk of developing depression because of maternal major depressive disorder, the Hipp was of smaller volume (64). In adults, abnormal functional relationships between the Hipp and cortical brain regions have been identified in individuals with major depression (65) and functional disconnection between limbic and frontal brain regions is thought to reflect an emotion-processing bias (66). This is consistent with our findings of Hipp hypofunction in ES+ girls in response to negative emotion and its correlation with trait anxiety, along with reciprocal coupling (as compared to ES− girls) between Hipp activity and other limbic structures. This suggests that Hipp dysfunction, especially in the context of trait anxiety, may be a potential precursor to ED. Interestingly, previous research in adults that has identified links between structural and functional changes in limbic structures such as the Hipp, Ag, and Ins, has not identified trait anxiety as a key influence (67).
Importantly, in validating the use of picture sets from the IAPS norms for females (29) we found that in our sample of adolescents, ES+ girls did not rate the images differently to those without such symptoms.
Although this study provides new insights toward understanding the emergence of ED, it has several limitations. First, we acknowledge that those who are classified as vulnerable (ES+) in this study may not go on to develop an ED, as emotional and social circuitry maturation processes continue into late adolescence and early adulthood (68). Further, the CAPP instrument that was used to classify participants into vulnerable and control groups is necessarily broad, with a positive result indicating that a participant meets screening criteria for one or more of a range of possible disorders comprising DSM-IV-TR. This broad grouping was employed because ED are typically preceded by non-specific ES and can manifest as a range of disorders until they crystallise into syndromal ED in early adulthood (69). Therefore, while use of this grouping measure provided a sensitive measure of vulnerability to ED, it may have diluted specific findings. Nevertheless, in the absence of a clear prodrome for depressive and anxiety disorders, the CAPP instrument provided an adequate cross-sectional indication of vulnerability status, as indicated by findings of significant brain activity differences between groups.
It is also noteworthy that an emerging body of research has indicated that hormonal changes during the menstrual cycle modulate behavioural and neural responses (70–72). Similarly, neuroimaging studies have revealed that otherwise healthy women with primary dysmenorrhea (PMD) (menstrual pain) compared to women without it, show structural and functional brain alterations (73, 74), with one study (73) also finding an association between PMD onset and state anxiety. In our study the menstrual cycle phase and associated menstrual pain where not assessed and therefore it is possible that changes related to menstruation may have contributed to both the neuroimaging and/or behavioural results.
Additionally, in this study our consideration of regions of the FLN is not exhaustive and there are, of course, other regions that are potentially equally important to the pathophysiology of ED. Further, it is important to note that though the changes we have identified have been conceptualised as causal they may in fact be compensational. Finally, our findings cannot be generalised to male adolescents, however the investigation of females in this study has the advantage of minimising the potential confound of gender differences and may be generalisable to adolescent females in the prodromal phase of EDs. Future research should adopt a longitudinal approach and be cognisant of the possible influence of menstrual cycle and menstrual pain and control for these. Balancing these limitations to some extent, it is perhaps noteworthy that our sample is homogenous with respect to gender and age.
Conclusion
Most psychiatric disorders have their origins in adolescence and childhood, often years before the emergence of symptoms and well before the diagnosis of disorders. This study identified key emotion-processing differences between groups of girls with and without subsyndromal ES. In this context trait anxiety appears to modulate functioning of the emotion regulatory network. This early divergence in the pattern of neural processing within the FLN may provide an important lead to the aetiopathogenesis of ED. However, to fully understand the evolution of ED, longitudinal evaluation of the emergent deficits is necessary.
Supplementary Material
Acknowledgements
The authors thank all of the students and their families who participated in the study and made it possible, as well as the school faculty for assisting with logistical aspects of the study. We also thank Mrs. Vicki Campbell, Mrs. Karen Searle, and Mrs. Amanda D’Mello for their assistance with administrative and logistical aspects of the study. The authors also acknowledge the NHMRC Program Grant (510135) for essential financial support. PD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
GSM has received grant or research support from NHMRC, NSW Health, AstraZeneca, Eli Lilly & Co., Organon, Pfizer, Servier, and Wyeth; has been a speaker for AstraZeneca, Eli Lilly & Co., Janssen Cilag, Lundbeck, Pfizer, Ranbaxy, Servier, and Wyeth; and has been a consultant for AstraZeneca, Eli Lilly & Co., Janssen Cilag, Lundbeck, and Servier. VC has received research support from the National Institutes of Health, National Science Foundation, Department of Energy, has done some legal consultation, has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in various scientific venues; and has generated books or book chapters for publishers of various texts.
Footnotes
Financial Disclosures
PD, CC, DMB, MT, and KLP have no conflicts of interest to report.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Merikangas KR, He J-p, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Avenevoli S, Merikangas KR. Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 6.Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: A 14-year follow-up during childhood, adolescence, and young adulthood. Am J Psychiatry. 2003;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- 7.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Larson RW, Moneta G, Richards MH, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Dev. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- 9.Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Collins WA, Laursen B. Changing relationships, changing youth: Interpersonal contexts of adolescent development. Journal of Early Adolescence. 2004;24:55–62. [Google Scholar]
- 11.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 13.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Organisation WH. The global burden of disease: 2004 update. World Health Organisation; 2008. http://www.searo.who.int/LinkFiles/Reports_GBD_report_2004update_full.pdf, editor. WHO Library Cataloguing-in-Publication Data. [Google Scholar]
- 18.Collishaw S, Maughan B, Natarajan L, Pickles A. Trends in adolescent emotional problems in England: a comparison of two national cohorts twenty years apart. Journal of Child Psychology and Psychiatry. 2010;51:885–894. doi: 10.1111/j.1469-7610.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 19.Langsford S, Houghton S, Douglas G. The Child and Adolescent PsychProfiler User Manual. Psychological and Educational Consultancy Services, Subiaco; WA. 2004 [Google Scholar]
- 20.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 21.Hirschfeld RMA, Williams JBW, Spitzer RL, Calabrese JR, Flynn L, Keck PE, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The mood disorder questionnaire. Am J Psychiatry. 2000;157:1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg I Goldberg Mania Questionnaire [online] [Google Scholar]
- 23.Spielberger C. Palo Alto, CA: Consultant Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 24.Goldberg LR. An alternative “description of personality”: The Big-Five factor structure. J Pers Soc Psychol. 1990;59:1216–1229. doi: 10.1037//0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- 25.Piers EVH, David S, Harris, Dale B. Piers-Harris Children’s Self-Concept Scale. Second Edition. Los Angeles: Western Psychological Services; 1984. [Google Scholar]
- 26.Folkman S, Lazarus RS. If it changes it must be a process - study of emotion and coping during 3 stages of a college-examination. J Pers Soc Psychol. 1985;48:150–170. doi: 10.1037//0022-3514.48.1.150. [DOI] [PubMed] [Google Scholar]
- 27.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 2004;26:41–54. [Google Scholar]
- 28.Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 29.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- 30.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SCR, Frackowiak RSJ, et al. Analysis of fmri time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 32.Chen C-H, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 35.Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, et al. Pathways for fear perception: Modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 36.Das P, Kemp AH, Flynn G, Harris AWF, Liddell BJ, Whitford TJ, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Williams LM. An integrative neuroscience model of “significance” processing. J Integr Neurosci. 2006;5:1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- 38.Williams LM. An integrative neuroscience model of “significance” processing. J Integr Neurosci. 2006;5:1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- 39.Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Pelletier M, Bouthillier A, Levesque J, Carrier S, Breault C, Paquette V, et al. Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport. 2003;14:1111–1116. doi: 10.1097/00001756-200306110-00003. [DOI] [PubMed] [Google Scholar]
- 41.Tao RR, Alley CS, Hart J, Mayes TL, Nakonezny PA, Lu HZ, et al. Brain activity in adollescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlund MW, Verduzco G, Cataldo MF, Hoehn-Saric R. Generalized anxiety modulates frontal and limbic activation in major depression. Behav Brain Funct. 2012:8. doi: 10.1186/1744-9081-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 44.Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin KA, Hatzenbuehler ML, Mennin DS, Nolen-Hoeksema S. Emotion dysregulation and adolescent psychopathology: A prospective study. Behav Res Ther. 2011;49:544–554. doi: 10.1016/j.brat.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suveg C, Zeman J. Emotion regulation in children with anxiety disorders (vol 33, pg 750, 2004) J Clin Child Adolesc Psychol. 2005;34:2–2. doi: 10.1207/s15374424jccp3304_10. [DOI] [PubMed] [Google Scholar]
- 47.Southam-Gerow MA, Kendall PC. A preliminary study of the emotion understanding of youths referred for treatment of anxiety disorders. J Clin Child Psychol. 2000;29:319–327. doi: 10.1207/S15374424JCCP2903_3. [DOI] [PubMed] [Google Scholar]
- 48.Kring AM, Sloan DM. Emotion regulation in psychopathology: A transdiagnostic approach to eitiology and treatment. New York: Guilford; 2010. [Google Scholar]
- 49.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehring T, Watkins ER. Repetitive negative thinking as a transdiagnostic process. Int J Cogn Ther. 2008;1:192–205. [Google Scholar]
- 51.Aldao A, Nolen-Hoeksema S. Specificity of cognitive emotion regulation strategies: A transdiagnostic examination. Behav Res Ther. 2010;48:974–983. doi: 10.1016/j.brat.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg L, Dahl RE, Kupfer DJ, Masten A, Pine D. The study of developmental psychopathology in adolescence: integtrating affective neuroscience with the study of context. In: Cicchetti D, Cohen D, editors. Developmental Pscyhopathology. New York: Wiley; 2006. pp. 710–741. [Google Scholar]
- 54.Silk JS, Vanderbilt-Adriance E, Shaw DS, Forbes EE, Whalen DJ, Ryan ND, et al. Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Dev Psychopathol. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- 55.Barbour T, Pruitt P, Diwadkar VA. fMRI responses to emotional faces in children and adolescents at genetic risk for psychiatric illness share some of the features of depression. J Affect Disord. 2012;136:276–285. doi: 10.1016/j.jad.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Soc Cogn Affect Neurosci. 2012;7:521–534. doi: 10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: A functional MRI study. Schizophr Res. 2012;134:158–164. doi: 10.1016/j.schres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Das P, Calhoun V, Malhi GS. Mentalizing in male schizophrenia patients is compromised by virtue of dysfunctional connectivity between task-positive and task-negative networks. Schizophr Res. 2012;140:51–58. doi: 10.1016/j.schres.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malhi GS, Lagopoulos J, Das P, Moss K, Berk M, Coulston CM. A functional MRI study of Theory of Mind in euthymic bipolar disorder patients. Bipolar Disord. 2008;10:943–956. doi: 10.1111/j.1399-5618.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 60.Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, et al. Premorbid social functioning in schizophrenia and bipolar disorder: Similarity and differences. Am J Psychiatry. 1997;154:1544–1550. doi: 10.1176/ajp.154.11.1544. [DOI] [PubMed] [Google Scholar]
- 61.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 62.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007;164:516–U516. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- 64.Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao X, Liu Z, Xu C, Li J, Gao Q, Sun N, et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naive patients with major depressive disorder. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Moller H-J, et al. Functional connectivity of emotional processing in depression. J Affect Disord. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 68.Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 69.Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, Altman SE. Subthreshold conditions as precursors for full syndrome disorders: a 15-year longitudinal study of multiple diagnostic classes. Journal of Child Psychology and Psychiatry. 2009;50:1485–1494. doi: 10.1111/j.1469-7610.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-Exner I, Gur RC, et al. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33:1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang RJ, Chen LF, Yeh TC, Tu PC, Tu CH, Hsieh JC. The resting frontal alpha asymmetry across the menstrual cycle: A magnetoencephalographic study. Horm Behav. 2008;54:28–33. doi: 10.1016/j.yhbeh.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Hwang RJ, Wu CH, Chen LF, Yeh TC, Hsieh JC. Female menstrual phases modulate human prefrontal asymmetry: A magnetoencephalographic study. Horm Behav. 2009;55:203–209. doi: 10.1016/j.yhbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Tu CH, Niddam DM, Chao HT, Liu RS, Hwang RJ, Yeh TC, et al. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage. 2009;47:28–35. doi: 10.1016/j.neuroimage.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 74.Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.