Abstract
Background
The recently approved drugs, sofosbuvir and ledipasvir, for chronic hepatitis C virus (HCV) treatment are more efficacious and safer but are substantially more expensive than the old standard-of-care (oSOC). It remains unclear whether and in which patients their improved efficacy justifies their increased cost.
Objective
To evaluate the cost-effectiveness and budget impact of sofosbuvir- and ledipasvir-based therapies.
Design
Simulation model of the natural history of HCV.
Data Sources
Published literature.
Target population
Treatment-naive and treatment-experienced HCV population defined on the basis of HCV genotype, age and fibrosis distribution in the United States.
Time Horizon
Lifetime.
Perspective
Third-party payer.
Interventions
Simulation of sofosbuvir/ledipasvir-based therapies compared with the oSOC that consisted of interferon-based therapies.
Outcomes Measures
Quality-adjusted life years (QALYs), incremental cost-effectiveness ratios (ICERs), and 5-year spending on antiviral drugs.
Results of Base-Case Analysis
Sofosbuvir-based therapies added 0.56 QALY relative to the oSOC, at an incremental cost of $55 400 per additional QALY. The ICERs ranged from $9700 to $284 300 per QALY depending on the patient’s status with respect to prior treatment, HCV genotype, and the presence of cirrhosis. At $100 000 willingness-to-pay per QALY, sofosbuivr-based therapies were cost-effective in 83% of treatment-naive and 81% of treatment-experienced patients. Compared with the oSOC, new drugs would cost an additional $65 billion in the next 5 years to treat eligible HCV-infected people in the United States, whereas the resulting cost offsets would be $16 billion.
Results of Sensitivity Analysis
Results were sensitive to the drug price, drug efficacy and quality-of-life after a successful treatment.
Limitation
Data on real world effectiveness of new antivirals is lacking.
Conclusions
HCV treatment is cost-effective in the majority of patients, but additional resource and value-based patient prioritization are needed to manage HCV patients.
INTRODUCTION
More than 3 million people are chronically infected with hepatitis C virus (HCV) in the United States (US), and the majority of them are undiagnosed (1, 2). HCV infection is the leading cause of hepatocellular carcinoma (HCC) and is the most common indication for liver transplantation (3). In 2011, the annual economic burden associated with chronic HCV infection in the US was $6.5 billion (4).
The recent approval of three new drugs—sofosbuvir, the first-in-class once-daily HCV RNA polymerase inhibitor, and simeprevir, a once-daily protease inhibitor, and sofosbuvir with ledipasvir, the first oral combination therapy—by the Food and Drug Administration (FDA) marked the beginning of a new era for HCV treatment (5–7). Until then, the old standard-of-care (oSOC) was based on peginterferon and ribavirin, with or without boceprevir and telaprevir. With the advent of the new drugs, HCV treatment can for the first time be provided without interferon-based therapy, which is associated with considerable toxicity (8). As a result, many patients who were unable to tolerate previous therapies are now eligible for HCV treatment. These agents are superior, with sustained virologic response (SVR) rates above 95% in the majority of patients, with shorter duration of treatment and fewer adverse effects than the oSOC (9, 10).
In order to guide clinicians, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Disease Society of America (IDSA) jointly published a practice guideline with new recommendations for HCV treatment as a web document with plans for ongoing updates (11). These recommendations include FDA-approved as well as off-label drug combinations of sofosbuvir, with and without PEG, but have not yet taken into account the recent FDA approval of the combination of sofosbuvir and ledipasvir.
However, enthusiasm for the new drugs has been dampened by their high price—sofosbuvir is currently priced at $1000 per day, and sofosbuvir with ledipasvir at $1125 a day. The total cost of treatment can be as high as $150 000 per patient. The high price of sofosbuvir has drawn criticism from patient advocates (12), US lawmakers (13), the World Health Organization (14), and private payers (15), especially considering that the manufacturing cost of these drugs is $200 for 12-week treatment (16). Challenged with the budget needed to treat all HCV patients, at least 35 US states have restricted these treatments to advanced stage Medicaid patients (17). Similarly, private payers require prior authorization. With more than a million patients needing HCV treatment in the next 3–5 years in the US, the high price of these drugs will substantially impact the budget of private payers and government (18). Treatment cost may, therefore, become the primary barrier to HCV eradication (19, 20).
The manufacturer contends that sofosbuvir-based treatment provide a good value (21). However, it remains unclear whether and in which patients the improved benefits of new therapies justify the increased cost compared with the oSOC. In addition, total spending on new drugs to treat a large number of HCV patients is not known. Therefore, the objective of our study was to evaluate the cost-effectiveness and budget impact of sofosbuvir/ledipasvir-based treatments from a third-party payer’s perspective.
METHODS
We developed a Markov-based individual-level state-transition model, titled Markov-based Analyses of Treatments for Chronic Hepatitis C (MATCH), that simulated the clinical course of HCV patients who received antiviral treatment. We used a weekly cycle length to advance time in the model. The structure of the model was based on our previously published and validated Markov cohort model (22, 23).
Base Case Population
Our base case population represented HCV-infected patients in the US. We defined a total of 120 patient profiles based on patients’ treatment history (naive or experienced); interferon-tolerance (yes or no; for treatment-naive patients only); HCV genotype (G1, G2, G3, or G4), sex (male or female), and METAVIR fibrosis score (no fibrosis [F0], portal fibrosis without septa [F1], portal fibrosis with few septa [F2], numerous septa without fibrosis [F3], or cirrhosis [F4]) (24). We also assigned different baseline ages by fibrosis score using a validated simulation model of the HCV disease burden in the US (Appendix Table 1) (25).
Treatment
For each of the 120 patient profiles we simulated the following two scenarios: treatment using oSOC and treatment using sofosbuvir/ledipasvir-based therapies (Table 1) (11). We used efficacy data from the recent clinical trials of sofosbuvir and ledipasvir in treatment-naïve, treatment-experienced, interferon-intolerant patients: ION-1 (26), ION-2 (10), ION-3 (27), NEUTRINO (9), FISSION (9), VALENCE (28), POSITRON (29), FUSION (29), and the Egyptian Ancestry study (30). We defined treatment ineligibility due to interferon-intolerance as one or more of the following conditions: bipolar disorder, anemia (Hgb < 10 g/d), pregnancy and neutropenia (neutrophils <750 cells/mm3; 1.2%) (31). For the efficacy data of comparator arms, we either used the above clinical trials (when the study included the oSOC), or published studies of protease inhibitors and peginterferon/ribavirin (32–40). The duration of treatment in our model varied between 8 and 48 weeks depending on treatment arm, HCV genotype and prior treatment history. We also included the possibility of early treatment discontinuation because of adverse events or clinical futility rules (for oSOC only).
Table 1.
Treatment-Related Parameters for Sofosbuvir/Ledipasvir-Based Therapies and the Old Standard of Care for a Cost-Effectiveness Analysis
| HCV Geno type | HCV Treatment | Regimen and Duration (weeks) | SVR Rate Non-cirrhosis | SVR Rate-cirrhosis | Discontinuation Rate | Probability of Anemia | Duration of Anemia (6weeks) |
|---|---|---|---|---|---|---|---|
| Treatment-Naive Interferon-Tolerant Patients | |||||||
|
| |||||||
| G1 | SOF/LDV-based (26, 27) | LDV+SOF 8a | 97% | -- | 1% | 1% | 1 |
| LDV+SOF 12a | 96% | 97% | 1% | 1% | 2 | ||
|
| |||||||
| oSOC (32, 33) | BOC+PEG+RBV | 67% | 52% | 28–42% | 49% | 15–21 | |
| TEL+PEG+RBV | 75% | 62% | 21% | 37% | 12 | ||
|
| |||||||
| G2 | SOF-based (9) | SOF+RBV 12 | 97% | 83% | 1% | 8% | 4 |
|
| |||||||
| oSOC (9) | PEG+RBV 24 | 81% | 62% | 11% | 11% | 8 | |
|
| |||||||
| G3 | SOF-based (28) | SOF+RBV 24 | 94% | 92% | 2% | 11% | 7 |
|
| |||||||
| oSOC (37) | PEG + RBV 24 | 70% | 49% | 7% | 16% | 8 | |
|
| |||||||
| G4 | SOF-based (9) | SOF+PEG+RBV 12 | 99% | 85% | 2% | 21% | 4 |
|
| |||||||
| oSOC (36) | PEG+RBV 48 | 58% | 32% | 7% | 16% | 18 | |
|
| |||||||
| Treatment-Naive Interferon-Intolerant Patientsb | |||||||
|
| |||||||
| G1 | SOF/LDV-based (26, 27) | LDV+SOF 8a | 97% | -- | 1% | 1% | 1 |
| LDV+SOF 12a | 96% | 97% | 1% | 1% | 2 | ||
|
| |||||||
| G2 | SOF-based (29) | SOF+RBV 12 | 92% | 94% | 2% | 13% | 4 |
|
|
|||||||
| G3 | SOF-based (28) | SOF+RBV 24 | 93% | 92% | 2% | 11% | 7 |
|
|
|||||||
| G4 | SOF-based (30) | SOF+RBV 24 | 93% | 93% | 1% | 11% | 7 |
|
|
|||||||
| G1–4 | oSOC | No treatment | 0% | 0% | -- | -- | -- |
|
| |||||||
| Treatment-Experienced Patients | |||||||
|
| |||||||
| G1 | SOF/LDV-based (10) | LDV+SOF 12 | 95% | -- | 0% | 0% | -- |
| LDV+SOF 24 | -- | 99% | 0% | 1% | 4 | ||
|
| |||||||
| oSOC (38–40) | BOC+PEG+RBV | 58% | 52% | 27–33% | 41–47% | 16–19 | |
| TEL+PEG+RBV | 70% | 58% | 23–36% | 30% | 12 | ||
|
| |||||||
| G2 | SOF-based (29) | SOF+RBV 12 | 96% | 60% | 1% | 11% | 4 |
|
| |||||||
| oSOC (34) | PEG+RBV 24 | 65% | 51% | 7% | 16% | 8 | |
|
| |||||||
| G3 | SOF-based (28) | SOF+RBV 24 | 85% | 60% | 2% | 11% | 7 |
|
| |||||||
| oSOC (34) | PEG + RBV 24 | 60% | 47% | 7% | 16% | 8 | |
|
| |||||||
| G4 | SOF-basedc | SOF+PEG+RBV 12 | 69% | 69% | 10% | 21% | 4 |
|
| |||||||
| oSOC (34) | PEG+RBV 48 | 31% | 24% | 40% | 16% | 14 | |
|
| |||||||
Abbreviations: AASLD, American Association for the Study of Liver Diseases; IDSA, Infectious Diseases Society of America; G1–4, genotype 1–4; SOF, sofosbuvir; PEG, peginterferon; RBV, ribavirin; oSOC, old standard of care; BOC, boceprevir; TEL, telaprevir; LDV, ledipasvir. SVR = sustained virologic response
In non-cirrhotic treatment-naïve patients, the duration of LDV+SOF depends on patient’s baseline HCV RNA. Those with HCV RNA less than 6 million IL/mL are considered for 8 weeks of treatment, and 12 weeks otherwise. Among this patient group, 57% of patients were eligible for 8 weeks of treatment.
We defined treatment ineligibility due to interferon-intolerance as one or more of the following conditions: bipolar disorder, anemia (Hgb < 10 g/d), pregnancy and neutropenia (neutrophils <750 cells/mm3; 1.2%)
No clinical study evaluated the combination of sofosbuvir with peginterferon and ribavirin in genotype 4 patients. Therefore, we derived SVR rates of this combination using data from another study that used sofosbuvir and ribavirin for 24 weeks in genotype 4 patients.(30) We assumed that the addition of peginterferon would increase the SVR rates by another 10%, i.e. from 59% to 69%.
Natural History of HCV
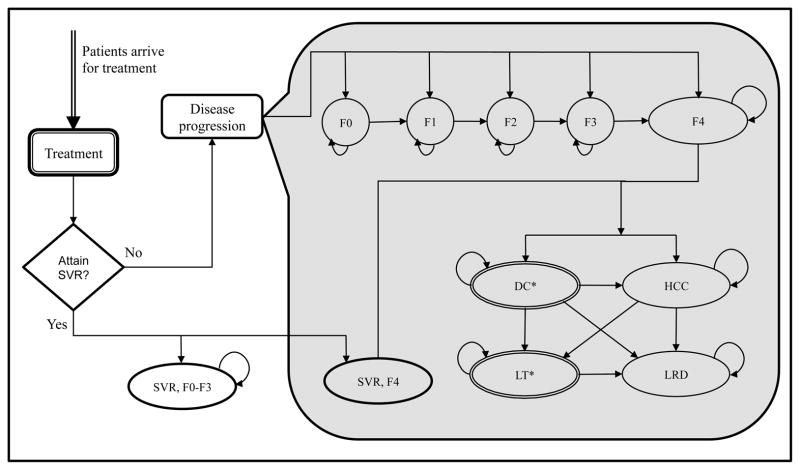
Patients who did not achieve SVR transitioned into the natural history phase of the model, which was defined using Markov health states. Patients could start in one of the F0–F4 Markov states defined on the basis of the degree of liver fibrosis (Figure 1). Patients could develop the adverse outcomes of decompensated cirrhosis and/or HCC, could receive a liver transplant, or experience a liver-related death. Patients who achieved SVR were assumed to transition into normal health status only if they did not have METAVIR stage F4 (cirrhosis). In cirrhotic patients, we assumed that disease would progress even after achieving SVR, though at a slower rate (41).
Figure 1.
State-Transition Diagram of Hepatitis C Treatment Model for a Cost-Effectiveness Analysis of Sofosbuvir and Ledipasvir. At any given time, a patient occupies one of the health states represented by circles/ovals. Arrows between states represent possible transitions based on annual probabilities. As time progresses, patients can transition to other states and acquire cost and health-utilities associated with that state. The model stops when all patients transition to death state. Note that a patient could transition to a death state from any of the above states because of background mortality (these transitions are not shown in the figure for clarity)
Abbreviations: SVR, sustained virologic response; F0–F4, METAVIR fibrosis score; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplant; LRD, liver-related death.
*DC and LT states were further divided into first-year and subsequent-year to account of different mortality rates and costs; however, they are collapsed into one state for presentation purpose only.
Data Sources for Transition Probabilities
We used a published meta-regression analysis to estimate fibrosis progression from F0 to F4 (Appendix Table 2) (42), which was dependent on patient’s baseline fibrosis score, HCV genotype, duration of HCV infection, sex, and age at HCV acquisition (42). We estimated disease progression in cirrhosis and decompensated cirrhosis from published observational studies (Appendix Table 3) (43, 44). Patients developing decompensated cirrhosis or HCC were eligible to receive a liver transplant (22, 45, 46); and had higher mortality (47). Patients who achieved SVR were at higher risk for non–liver-related deaths than the general population; therefore, we adjusted their all-cause mortality with sex-specific hazard ratios (2.58 for men and 1.97 for women) (48–50).
Medical Costs
The model was developed from a third-party payer perspective. All costs were converted to a 2014 baseline using Consumer Price Index. The weekly costs of sofosbuvir and ledipasvir were $7000 and $875, respectively (51). The weekly costs of peginterferon, ribavirin, boceprevir, and telaprevir were $587, $309, $1100 and $4100, respectively (51). Because the majority of payers get discounts, we applied the average discount of 11% on all drugs (Appendix S1.6). We used our previously published study to estimate health-state specific annual costs (22, 52).
Quality of Life Weights
We assigned lower quality-of-life (QOL) weight while on treatment with interferon-based therapies in comparison with all-oral therapies (Table 2). Patients who developed anemia had a further decrement in QOL for the duration of anemia (53). We assigned health-state specific QOL weights from a previously published study using the EuroQol-5D instrument (54, 55), and adjusted these weights to the US population norm (Appendix Table 4) (56). We assumed the QOL of patients who achieved SVR to be equivalent to that of the general population (54).
Table 2.
Lifetime Cost-effectiveness of Sofosbuvir/Ledipasvir-Based Therapies to Treat Hepatitis C in Comparison with the Old Standard of Care in 120 Patient Profiles
| QALY: oSOC | QALY: SOF-based | Cost: oSOC ($) | Cost: SOF-based ($) | ICER ($/QALY) | pCE at $50Kb | pCE at $100Kc | |
|---|---|---|---|---|---|---|---|
| Treatment-Naive Patients | |||||||
| Genotype 1a | |||||||
| Non-cirrhoticb | 10.605 | 11.056 | 54 052 | 68 228 | 31 452 | 0.77 | 0.99 |
| Cirrhotic | 8.279 | 9.447 | 85 170 | 96 498 | 9703 | 0.88 | 0.95 |
| Genotype 2 | |||||||
| Non-cirrhotic | 10.669 | 11.041 | 22 736 | 78 230 | 149 463 | 0.02 | 0.23 |
| Cirrhotic | 8.378 | 9.161 | 46 336 | 95 208 | 62 428 | 0.36 | 0.74 |
| Genotype 3 | |||||||
| Non-cirrhotic | 10.559 | 11.015 | 25 134 | 154 649 | 284 327 | <0.01 | 0.01 |
| Cirrhotic | 8.119 | 9.354 | 50 235 | 167 634 | 95 083 | 0.10 | 0.43 |
| Genotype 4 | |||||||
| Non-cirrhotic | 10.404 | 11.065 | 41 742 | 95 798 | 81 802 | 0.09 | 0.51 |
| Cirrhotic | 7.810 | 9.204 | 69 357 | 112 553 | 30 986 | 0.62 | 0.85 |
| Genotypes 1–4 | |||||||
| Non-cirrhotic | 10.608 | 11.051 | 48 023 | 75 276 | 61 517 | 0.34 | 0.88 |
| Cirrhotic | 8.277 | 9.401 | 77 747 | 100 989 | 20 673 | 0.79 | 0.92 |
| All treatment-naïve | 10.035 | 10.646 | 55 326 | 81 593 | 43 034 | 0.45 | 0.89 |
| Treatment-Experienced Patients | |||||||
| Genotype 1a | |||||||
| Non-cirrhotic | 10.668 | 11.035 | 71 605 | 84 744 | 35 853 | 0.72 | 0.97 |
| Cirrhotic | 8.500 | 9.508 | 98 456 | 178 295 | 79 238 | 0.18 | 0.55 |
| Genotype 2 | |||||||
| Non-cirrhotic | 10.648 | 11.048 | 26 650 | 78 161 | 128 770 | 0.01 | 0.20 |
| Cirrhotic | 8.380 | 8.580 | 48 868 | 105 046 | 281 317 | 0.08 | 0.08 |
| Genotype 3 | |||||||
| Non-cirrhotic | 10.603 | 10.917 | 27 555 | 156 443 | 410 548 | <0.01 | <0.01 |
| Cirrhotic | 8.285 | 8.624 | 50 300 | 180 083 | 382 819 | 0.03 | 0.03 |
| Genotype 4 | |||||||
| Non-cirrhotic | 10.215 | 10.732 | 45 496 | 87 245 | 80 793 | 0.09 | 0.56 |
| Cirrhotic | 7.796 | 8.790 | 69 938 | 104 102 | 34 349 | 0.61 | 0.85 |
| Genotypes 1–4 | |||||||
| Non-cirrhotic | 10.657 | 11.026 | 62 699 | 88 433 | 69 707 | 0.25 | 0.76 |
| Cirrhotic | 8.463 | 9.324 | 88 662 | 168 069 | 92 302 | 0.12 | 0.45 |
| All treatment-experienced | 10.118 | 10.608 | 69 078 | 107 999 | 79 457 | 0.22 | 0.69 |
| Treatment-Naive and Experienced Patients | |||||||
| All patientsd | 10.067 | 10.631 | 60 686 | 91 886 | 55 378 | 0.35 | 0.83 |
Abbreviations: QALY, quality-adjusted life years; SOF, sofosbuvir; oSOC, old standard of care; LDV, ledipasvir; ICER, incremental cost-effectiveness ratio; pCE, probability of cost-effectiveness.
Treatment for genotype 1 patients was based on a combination of sofosbuvir and ledipasvir
Non-cirrhotic patients are defined as those having fibrosis scores F0–F3, and cirrhotic patients are defined as those having a fibrosis score F4
Probability of cost-effectiveness at a $50 000 willingness-to-pay threshold using probabilistic sensitivity analysis
Probability of cost-effectiveness at a $100 000 willingness-to-pay threshold using probabilistic sensitivity analysis
Overall results were estimated using the weighted average by representative proportion of the HCV population in the United States.
Cost-Effectiveness Analysis
We validated our natural history model with a recently published multicenter follow-up study of patients with advanced fibrosis, and with previously published cost-effectiveness studies (Supplementary Appendix 2) (22, 55, 57, 58). In patients who failed to achieved SVR, the predicted 10-year cumulative incidence of decompensated cirrhosis, HCC, and liver-related death plus liver transplant were within the range of reported values (57). In cirrhotic patients who achieved SVR and continued to progress, the predicted cumulative incidence of HCC was within the reported range; however, the cumulative incidence of decompensated cirrhosis and liver-related death plus liver transplant were overestimated, thereby making model underestimate the benefits of new therapies.
For both scenarios, we projected the expected quality-adjusted life years (QALYs), total lifetime costs, and cost of antiviral drugs. We estimated the incremental cost-effectiveness ratio (ICER) of sofosbuvir/ledipasvir-based treatments in comparison with the oSOC. We used a lifetime horizon and discounted all future costs and QALYs at 3% per year. In addition, we projected the cumulative incidence of advanced liver-related complications (decompensated cirrhosis and HCC), liver transplants, and liver-related deaths.
Budget Impact Analysis
Cost-effectiveness analysis does not provide the impact of new therapies on payers’ budget; therefore, we also estimated the budget needed to treat all eligible patients in the US. Using a validated prediction model of HCV disease burden in the US (25), we estimated the number of people who will be eligible for treatment in the next 5 years and resources needed to treat them.
Sensitivity Analysis
We performed 1-way sensitivity analysis to estimate the effects of transition probabilities, QOL weights and cost inputs on ICERs. To account for lower SVR rates in practice in contrast to clinical trials, we applied a decrement in SVR of 0%–20% to oSOC and 0%–15% to sofosbuvir/ledipasvir-based therapies (59). We also performed probabilistic sensitivity analysis using 5000 second-order samples of the parameters defined in Appendix Table 3.
Scenario and Subgroup Analysis
Since HCV progresses slowly, payers might not achieve the full benefits of treating HCV patients with expensive drugs if patients transition to a different insurance payer after treatment. Therefore, we conducted cost-effectiveness analyses for shorter time horizons—10-year, 20-year and 30-year. We also evaluated the cost-effectiveness of sofosbuvir/ledipasvir by patients’ fibrosis scores (F0–F4), sex, and three age categories (40, 55 and 70 years).
Role of Funding Source
This study was supported by the National Institutes of Health under award number KL2TR000146. The content is solely the responsibility of the authors and does not represent the views of the NIH.
RESULTS
The average per person QALYs using the oSOC and sofosbuvir/ledipasvir-based therapies were 10.07 and 10.63 (increment = 0.56), respectively (Table 2). The increment in QALYs gained from the use of sofosbuvir/ledipasvir substantially differed by prior treatment history and presence of cirrhosis: 0.44 in non-cirrhotic versus 1.12 in cirrhotic treatment-naive patients, and 0.37 in non-cirrhotic versus 0.86 in cirrhotic treatment-experienced patients. Compared with the oSOC, treating 10 000 patients using sofosbuvir/ledipasvir-based therapies could prevent 600 decompensated cirrhosis, 310 HCC, 60 liver transplants and 550 liver-related deaths. The reduction of these adverse endpoints was greater in cirrhotic patients than in non-cirrhotic patients (Appendix Figure 1A–B). The average per patient cost of oSOC ranged from $15 000 to $71 600 depending on HCV genotype and prior treatment status, whereas the cost of sofosbuvir/ledipasvir-based therapies ranged from $66 000 to $154 000 (Appendix Figure 2).
Cost-effectiveness of sofosbuvir/ledipasvir-based Therapies
The ICER of sofosbuvir/ledipasvir-based therapies in comparison with the oSOC was $55 400 per additional QALY gained (Table 2). Depending on HCV genotype, treatment history and cirrhosis status, the ICERs ranged from $9700 to $284 300 per QALY. In treatment-naive patients, the ICER was $43 000 per QALY; whereas in treatment-experienced patients the ICER was $79 500 per QALY (Table 2). The ICERs were lower in patients who were interferon-intolerant ($34 900) versus interferon-tolerant ($48 300) (Appendix Table 6). At the $50 000 willingness-to-pay (WTP) threshold, sofosbuvir/ledipasvir-based therapies were cost-effective in 82% of treatment-naive and in 60% of treatment-experienced patients. The corresponding percentages at $100 000 WTP were 83% and 81%, respectively.
Budget Impact for HCV Treatment
In 2014, 1.32 million treatment-naive and 0.45 million treatment-experienced people would be aware of their HCV disease (25). In addition, 0.51 million people would be diagnosed in the next 5 years because of risk-based and birth-cohort HCV screening (25). Assuming that 63% of treatment-naive and 100% of treatment-experienced patients have insurance coverage (60), we estimated that 1.60 million people would be eligible for treatment during the next 5 years.
Payers would need $136 billion to cover drug costs of all treatment-eligible HCV patients during the next 5 years, $61 billion of which would need to be paid by the government (Figure 2). Compared with the oSOC, new drugs would cost an additional $65 billion; whereas, the cost-offsets because of the use of sofosbuvir/ledipasvir would be $16 billion (24% of the additional spending on drugs).
Figure 2.
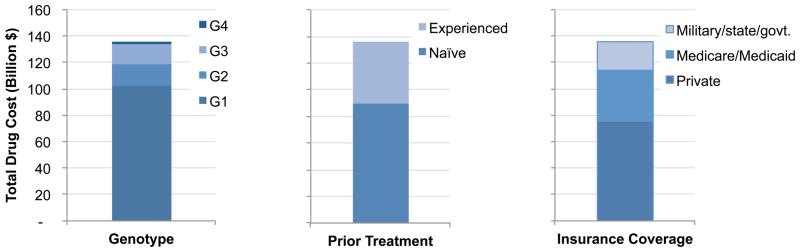
Total drug spending on sofosbuvir (SOF)- and ledipasvir (LDV)-based therapies to treat all HCV-infected patients in the United States in the next 5 years; A. By HCV genotype (G1–G4), prior treatment history (naïve or experienced), and insurance coverage (military/state/government [govt.], Medicaid/Medicare, and private);
Sensitivity Analysis
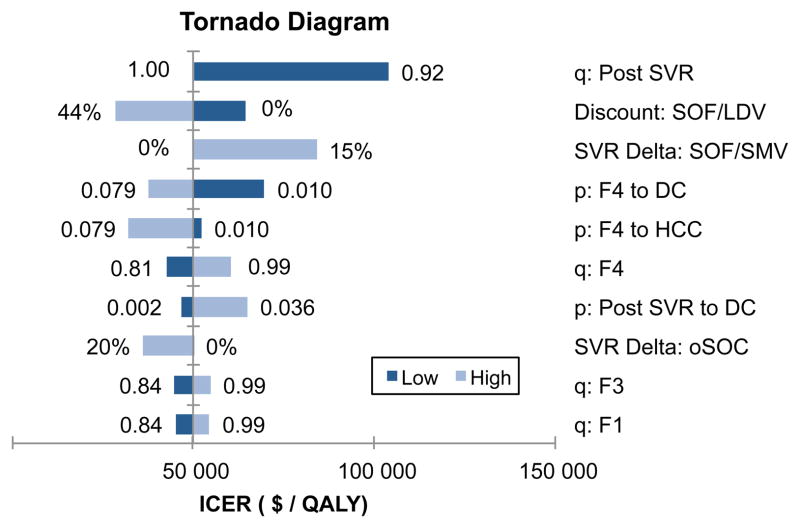
Using one-way sensitivity analyses, we identified the top 10 variables that had the biggest effect on ICERs (Figure 3). The ICERs were most sensitive to the QOL post SVR, discounts on sofosbuvir/ledipasvir, decrease in SVR rates of sofosbuvir/ledipasvir, probability of decompensated cirrhosis or HCC in cirrhotic patients, probability of decompensated cirrhosis after achieving SVR, and QOL associated with fibrosis stages, F0–F4. Similar trends were observed in treatment naive- and experienced-patients (Appendix Figure 3A–B).
Figure 3.
One-way Sensitivity Analysis Showing Top 10 Most Sensitive Parameters.
Abbreviations: q: Post SVR, quality of life after achieving sustained virologic response (SVR); SVR Detla: SOF/LDV, Reduction in SVR in sofosbuvir (SOF)- and ledipasvir (LDV)-based therapies; p: F4 to DC, probability of developing decompensated cirrhosis (DC) from fibrosis score F4; q: F4, quality-of-life (QOL) weight associated with F4; p: F4 to HCC, probability of developing hepatocellular carcinoma (HCC) from F4; SVR Delta: oSOC, Reduction in SVR in the old standard of care (oSOC); p: Post SVR to DC, probability of developing DC in F4 patients who achieved SVR; q: F3, QOL weight associated with fibrosis score F3; q: F2, QOL weight associated with fibrosis score F2; q: F1, QOL weight associated with fibrosis score F1.
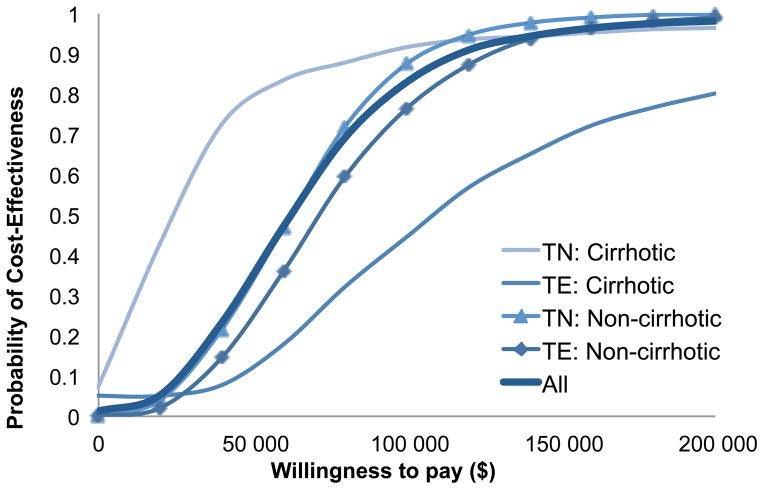
Using probabilistic sensitivity analysis, we estimated that sofosbuvir/ledipasvir-based therapies were cost-effective with 35% probability at $50 000 WTP threshold and with 83% probability at $100 000 threshold (Figure 4). The probabilities of cost-effectiveness were 34% and 79% in non-cirrhotic and cirrhotic treatment-naive patients, respectively at $50 000 WTP. In treatment-experienced patients, the corresponding probabilities were 25% and 12%, respectively. The probability of cost-effectiveness of each of the 12 scenarios is provided in Appendix Figures 4–6.
Figure 4.
Probabilistic Sensitivity Analysis of Sofosbuvir- and Ledipasvir-Based Therapies showing the Cost-Effectiveness Probability by Willingness-to-pay Thresholds.
Abbreviations: TN, treatment-naïve, TE, treatment-experienced; ICER, incremental-cost-effectiveness ratio.
Scenario and Subgroup Analysis
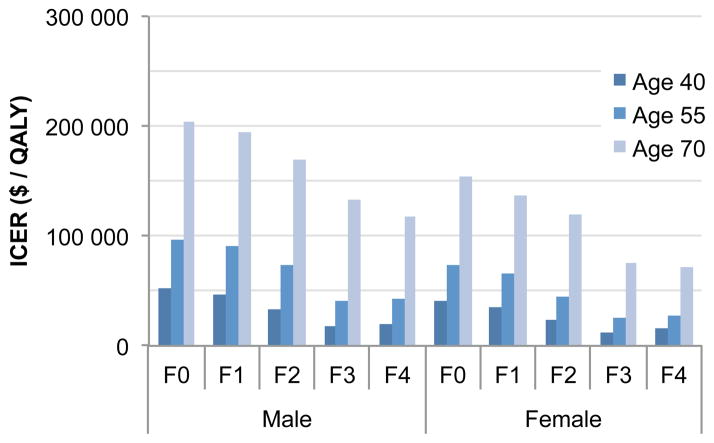
The ICERs of sofosbuvir/ledipasvir-based therapies with 10-year, 20-year, and 30-year time horizons were $148 500, $82 100 and $66 800, respectively (Appendix Tables 7–9). Therefore the value of sofosbuvir/ledipasvir decreased with shortening of the time horizon. In addition, age and fibrosis scores had substantial effects on the ICERs—ranging from cost saving to $939 200 (Figure 5 and Appendix Figures 7–9). The ICERs in 40-year versus 70-year patients were $25 000 and $125 900, respectively. In addition, the ICERs in males were higher than those of females.
Figure 5.
Incremental Cost-Effectiveness Ratios (ICERs) of Sofosbuvir- and Ledipasvir-Based Therapies by fibrosis score (F0–F4), sex, and age.
Abbreviations: F0–F4, METAVIR fibrosis scores. Note that ICERs of males were higher than those of females because of higher background mortality of males than females
DISCUSSION
The use of sofosbuvir- and ledipasvir-based therapies would substantially reduce the clinical burden of HCV. At their current price, these therapies are cost-effective in selected patient groups using a threshold of $50 000 WTP per additional QALY. However, at $100 000 WTP, these therapies are cost-effective in the majority of patients. The new therapies provide a better value for money in patients who have HCV genotype 1, are in advanced stages of disease, or are younger. Though the reported ICERs are within the range of therapies for other medical conditions in the US (61–63), resources needed to treat a large number of eligible HCV patients could be immense and unsustainable. Compared with the oSOC, the downstream cost-offsets because of using sofosbuvir/ledipasvir-based therapies would only be 24% of the additional $65 billion spent on these new drugs. Therefore, our analysis does not support the assertion that new drugs as currently priced will lead to overall reduction in the cost of HCV disease.
To our knowledge, this is the first study to fully evaluate the cost-effectiveness of sofosbuvir/ledipasvir-based regimens. Earlier cost-effectiveness studies of oral HCV therapies either did not evaluate the current recommendations or made conclusions based on drug prices that were significantly lower than the listed drug prices (64–67). Another report assessed the value of sofosbuvir/simeprevir but did not use modeling to simulate downstream events (68). In contrast, we present a comprehensive and up-to-date analysis of the value of HCV treatment by including four major genotypes, interferon-tolerance, and prior treatment history. In addition, we conducted a budget-impact analysis, which is especially important given the high price of new antivirals.
The large number of HCV-infected persons needing treatment could put a huge burden on health expenditures, reaching an average of $27 billion per year, which is 10% of US prescription drug spending in 2012 (69). A large portion of the treatment cost will fall on the shoulders of the government. The Affordable Care Act is expected to increase the number of HCV patients on Medicaid (70). In addition, with widespread implementation birth-cohort HCV screening, many new diagnoses are expected in people covered under Medicare. Though manufacturers generally provide discounts to most purchasers, current law prohibits Medicare from negotiating drug prices (71). Therefore, treating all HCV patients with currently priced sofosbuvir/ledipasvir would dramatically affect the financial resources of Medicare and Medicaid.
The cost-effectiveness of HCV treatment depends on society’s willingness to pay for improvements in health. Unlike most of the other developed countries, the US has not adopted any official threshold to determine if a new intervention is cost-effective or not (72). The commonly used $50 000 threshold is questionable and the more appropriate threshold could be between $100 000 and $200 000 (73, 74). However, despite the HCV treatment being cost-effective, our analysis shows that it is unaffordable at the current price. This raises a question if the threshold should depend on the budget available and disease prevalence; i.e. lower thresholds for the treatment of disease like HCV, and higher for the treatment of a rare disease.
The cost-effectiveness of HCV treatment is also dependent on the insurance type. For private payers, where the median length of patient enrollment is less than 10 years, sofosbuvir/ledipasvir-based therapies may not be cost-effective. Therefore, lower drug price may provide a better value to private payers. Whereas for Medicaid/Medicare and the Veterans Administration, where length of enrollment is longer, these therapies may be cost-effective. Therefore, providing additional resources to these public programs for HCV treatment could provide a good value for money.
Our results were highly sensitive to the quality of life after achieving SVR. Therefore, further research is needed in patients who achieved SVR with new therapies. The results were also sensitive to discounts given to sofosbuvir/ledipasvir; therefore, giving higher discounts will improve the value of treatment. In addition, results were sensitive to the following baseline patient demographic—HCV genotype, presence of cirrhosis, treatment history, and age.
Our study has several limitations. First, several clinical studies included in our analysis were not randomized and did not directly compare the efficacy of new drugs; therefore, our study only used best available evidence on treatment efficacy, which might have high uncertainty owing to the low number of patients. We used efficacy data from phase II clinical trials when data were not available from either phase III trials or meta-analyses, but performed sensitivity analyses. The use of data from international clinical trials for US population could have resulted in overestimation of benefits of new therapies. Our analysis assumed that quality of life after achieving SVR is equivalent to that of a normal person. This could have overestimated the benefits of new therapies. We also did not model the future possibility of retreatment with next generation antivirals because of lack of these data at the time of our study. Finally, we did not consider changes in the insurance pool as a result of the Affordable Care Act, which may impact the budget impact of HCV treatment.
Conclusion
The use of sofosbuvir- and ledipasvir-based therapies will substantially reduce HCV-related complications and are cost-effective in the majority of patients. However, treating all treatment-eligible HCV patients in US would have an immense budgetary impact on both private and government providers. Additional resources as well as value-based patient prioritization are needed to manage HCV patients with these regimens.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000146. Dr. Kanwal’s effort was supported in part by the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413).
We thank Elamin Elbasha, PhD and Scott Cantor, PhD for constructive comments that improved the quality of the manuscript, and Mina Kabiri, MS and Qiushi Chen for help with data analysis. We also thank Jill Delsigne, PhD and Diane Hackett for editing the manuscript.
Footnotes
Author contribution:
Study concept and design: Chhatwal, Kanwal, Roberts, Dunn.
Drafting of manuscript: Chhatwal.
Critical revision of the manuscript for important intellectual content: Chhatwal, Kanwal, Roberts, Dunn.
Statistical analysis: Chhatwal.
Interpretation of data: Chhatwal, Kanwal, Roberts, Dunn.
Conflict of interest disclosures: Chhatwal received a consulting fee from Merck and Gilead for unrelated projects, and none to report for Kanwal, Roberts and Dunn.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic Hepatitis C Virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of Internal Medicine. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen HR. Chronic Hepatitis C Infection. New England Journal of Medicine. 2011;364(25):2429–38. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 4.Razavi H, El Khoury A, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–70. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA Press Release. [Accessed March 18, 2014];FDA approves Sovaldi for chronic hepatitis C. 2013 Dec 3; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm377888.htm.
- 6.FDA News Release. [Accessed March 18, 2014];FDA approves new treatment for hepatitis C virus. 2013 Nov; http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm376449.htm.
- 7.FDA Press Release. FDA approves first combination pill to treat hepatitis C. 2014 Oct 10;2014 Retrieved from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm418365.htm. [Google Scholar]
- 8.Drenth JP. HCV Treatment—No More Room for Interferonologists? N Engl J Med. 2013;368(20):1931–2. doi: 10.1056/NEJMe1303818. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New England Journal of Medicine. 2013;368(20):1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 10.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 11. [Accessed November 4, 2014];American Association for the Study of Liver Diseases and Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C. 2014 doi: 10.1002/hep.31060. Available at: http://www.hcvguidelines.org/full-report-view. [DOI] [PMC free article] [PubMed]
- 12.Knox R. NPR. Vol. 2013 NPR: NPR; Dec 30, 2014. [Accessed March 21, 2014]. $1,000 pill for hepatitis C spurs debate over drug prices. http://www.npr.org/blogs/health/2013/12/30/256885858/-1-000-pill-for-hepatitis-c-spurs-debate-over-drug-prices. [Google Scholar]
- 13.Terhune C. U.S. lawmakers ask Gilead to justify hepatitis C drug’s $84,000 price. [Accessed: November 4, 2014]; [Accessed April 12; March 21, 2014];The Los Angeles Times. http://www.latimes.com/business/money/la-fi-mo-hepatitis-c-gilead-pricing-20140321,0,3617096.story-axzz2ygvTgaWZ.
- 14.Hepatitis C Fact Sheet. World Health Organization; [Accessed April 11, 2014]. 2014. http://www.who.int/mediacentre/factsheets/fs164/en/ [Google Scholar]
- 15.Silverman E. ‘Unsustainable for Our Country’: Express Scripts Calls Out Pricey Meds. [Accessed on April 11, 2014];The Wall Street Journal. 2014 http://blogs.wsj.com/corporate-intelligence/2014/04/08/unsustainable-for-our-country-express-scripts-calls-out-pricey-meds/
- 16.Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing Hepatitis C Direct Acting Antivirals, for use in large-scale treatment access programs in developing countries. Clinical Infectious Diseases. 2014 doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japsen B. As Pricey Hepatitis Pill Harvoni Joins Sovaldi, States Erect Medicaid Hurdles. Forbes; 2014. Retrieved from: http://www.forbes.com/sites/brucejapsen/2014/10/10/as-hepatitis-pill-harvoni-joins-sovaldi-states-erect-medicaid-hurdles/ [Google Scholar]
- 18.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–70. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sussman NL, Remien CH, Kanwal F. The end of hepatitis C. Clin Gastroenterol Hepatol. 2014;12(4):533–6. doi: 10.1016/j.cgh.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle JH, Sherker AH. Therapy for hepatitis C—The costs of success. N Engl J Med. 2014;370:1552–3. doi: 10.1056/NEJMe1401508. [DOI] [PubMed] [Google Scholar]
- 21.Terhune C, Brown E. Prices of new hepatitis C drugs are tough to swallow for insurers. [Accessed: November 4, 2014];Los Angeles Times. 2014 Mar 9;2014 http://www.latimes.com/business/la-fi-hepatitis-c-drug-costs-20140310,0,5308461.story-ixzz2vZtBo3PQ. [Google Scholar]
- 22.Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, et al. Cost-Effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 Infection in the United States. Value in Health. 2013;16(6):973–86. doi: 10.1016/j.jval.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrante SA, Chhatwal J, Brass CA, El Khoury AC, Poordad F, Bronowicki J-P, et al. Boceprevir for previously untreated patients with chronic hepatitis C Genotype 1 infection: a US-based cost-effectiveness modeling study. BMC infectious diseases. 2013;13(1):190. doi: 10.1186/1471-2334-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24(2):289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 25.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C in the United States: Model-based predictions. Annals of Internal Medicine. 2014;161(3):170–80. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 27.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 28.Zeuzem S, Dusheiko G, Salupere R, Mangia A, Flisiak R, Hyland R. Sofosbuvir+ ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE Trial. Hepatology. 2013;58(Supp 1):733A. [Google Scholar]
- 29.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New England Journal of Medicine. 2013;368(20):1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 30.Ruane P, Ain D, Riad J. Sofosbuvir plus ribavirin in the treatment of chronic HCV genotype 4 infection in patients of Egyptian ancestry [abstract no. 1090] Hepatology. 2013;58(4 Suppl):736A. doi: 10.1016/j.jhep.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Talal A, LaFleur J, Hoop R, Pandya P, Martin P, Jacobson I, et al. Absolute and relative contraindications to pegylated-interferon or ribavirin in the US general patient population with chronic hepatitis C: results from a US database of over 45 000 HCV-infected, evaluated patients. Alimentary pharmacology & therapeutics. 2013;37(4):473–81. doi: 10.1111/apt.12200. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine. 2011;364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 33.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. New England Journal of Medicine. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136(5):1618–28. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 35.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011 doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khuroo MS, Khuroo MS, Dahab ST. Meta-analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Alimentary Pharmacology & Therapeutics. 2004;20(9):931–8. doi: 10.1111/j.1365-2036.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Solá R, et al. Peginterferon Alfa-2a and Ribavirin for 16 or 24 Weeks in HCV Genotype 2 or 3. New England Journal of Medicine. 2007;357(2):124–34. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 38.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. New England Journal of Medicine. 2011;364(13):1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. New England Journal of Medicine. 2011;364(25):2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 40.Bronowicki J, Davis M, Flamm S, Gordon S, Lawitz E, Yoshida E, et al. Sustained virologic response (SVR) in prior pegInterferon/ribavirin (PR) treatment failures after retreatment with boceprevir (BOC)+ PR: PROVIDE study interim results. J Hepatol. 2012;56:S6. [Google Scholar]
- 41.Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. Journal of Hepatology. 2010;52(5):652–7. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Thein H, Yi Q, Dore G, Krahn M. Estimation of stage specific fibrosis progression rates in chronic hepatitis C virus infection: A meta analysis and meta regression. Hepatology. 2008;48(2):418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 43.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 44.Planas R, Ballesté B, Antonio Álvarez M, Rivera M, Montoliu S, Anton Galeras J, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. Journal of Hepatology. 2004;40(5):823–30. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Thuluvath P, Guidinger M, Fung J, Johnson L, Rayhill S, Pelletier S. Liver transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10(4p2):1003–19. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 46.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. Journal of Hepatology. 2009;50(1):89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe R, Roys E, Merion R. Trends in Organ Donation and Transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10(4p2):961–72. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong G, Wasley A, Simard E, McQuillan G, Kuhnert W, Alter M. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144(10):705. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C Infection in the United States. PLoS ONE. 2013;8(3):e58975. doi: 10.1371/journal.pone.0058975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arias E. United states life tables, 2006. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;58(21):1. [PubMed] [Google Scholar]
- 51.First DataBank, Inc. [Accessed March 6, 2014];Drug databases. http://www.firstdatabank.com/Support/drug-pricing-policy.aspx.
- 52.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: A managed care perspective. J Manag Care Pharm. 2011;17(7):531–46. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson J, Yao G, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technology Assessment. 2007;11(13):1–202. doi: 10.3310/hta11130. [DOI] [PubMed] [Google Scholar]
- 54.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. The American Journal of Gastroenterology. 2003;98(3):630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 55.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth B, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52(3):425. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Medical Decision Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 57.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 58.Bennett W, Inoue Y, Beck J, Wong J, Pauker S, Davis G. Estimates of the cost-effectiveness of a single course of interferon-2b in patients with histologically mild chronic hepatitis C. Annals of Internal Medicine. 1997;127(10):855. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 59.Kanwal F, El-Serag HB. HCV treatment: The unyielding chasm between efficacy and effectiveness. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Stepanova M, Kanwal F, El-Serag HB, Younossi ZM. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011;53(3):737–45. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 61.Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum pretium—The just price. Journal of Clinical Oncology. 2013;31(28):3600–4. doi: 10.1200/JCO.2013.49.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly RJ, Hillner BE, Smith TJ. Cost effectiveness of crizotinib for anaplastic lymphoma kinase-positive, non-small-cell lung cancer: who is going to blink at the cost? J Clin Oncol. 2014;32(10):983–5. doi: 10.1200/JCO.2013.54.6002. [DOI] [PubMed] [Google Scholar]
- 63.Chambers JD, Neumann PJ, Buxton MJ. Does Medicare have an implicit cost-effectiveness threshold? Medical Decision Making. 2010;30(4):E14–E27. doi: 10.1177/0272989X10371134. [DOI] [PubMed] [Google Scholar]
- 64.Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. Journal of Hepatology. 2014;60(3):530–7. doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Hagan L, Yang Z, Ehteshami M, Schinazi R. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. Journal of Viral Hepatitis. 2013;20(12):847–57. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 66.Petta S, Cabibbo G, Enea M, Macaluso FS, Plaia A, Bruno R, et al. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59(5):1692–705. doi: 10.1002/hep.27010. [DOI] [PubMed] [Google Scholar]
- 67.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 HCV in interferon ineligible/intolerant individuals. Hepatology. 2014;60(1):37–45. doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tice JA, Ollendorf DA, Pearson SD. Institute for Clinical and Economic Review. 2014. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic hepatitis C infection: A technology assessment. [Google Scholar]
- 69.Martin AB, Hartman M, Whittle L, Catlin A Team tNHEA. National health spending in 2012: Rate of health spending growth remained low for the fourth consecutive year. Health Affairs. 2014;33(1):67–77. doi: 10.1377/hlthaff.2013.1254. [DOI] [PubMed] [Google Scholar]
- 70.Ngo-Metzger Q, Ward JW, Valdiserri RO. Expanded hepatitis C virus screening recommendations promote opportunities for care and cure. Annals of Internal Medicine. 2013;159(5):364–5. doi: 10.7326/0003-4819-159-5-201309030-00675. [DOI] [PubMed] [Google Scholar]
- 71.Frakt AB, Pizer SD, Feldman R. Should Medicare adopt the Veterans health administration formulary? Health Economics. 2012;21(5):485–95. doi: 10.1002/hec.1733. [DOI] [PubMed] [Google Scholar]
- 72.Neumann PJ. Using cost-effectiveness analysis to improve health care: opportunities and barriers. New York: Oxford University Press; 2005. [Google Scholar]
- 73.Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. New England Journal of Medicine. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 74.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.