Abstract
Glutamate receptors are the primary excitatory neurotransmitter receptors in vertebrate brain and are of critical importance to a wide variety of neurological processes. Recent reports suggest that ionotropic glutamate receptors may have a unique transmembrane topology not shared by other ligand-gated ion channels. We report here the cloning of cDNAs from goldfish brain encoding two homologous kainate receptors with protein molecular masses of 41 kDa. Using a cell-free translation/translocation system, we show that (i) a portion of these receptors previously thought to be a large intracellular loop is actually located extracellularly and (ii) the putative second transmembrane region of the receptor thought to line the ion channel may not be a true membrane-spanning domain. An alternative model for the transmembrane topology of kainate receptors is proposed that could potentially serve as a framework for future detailed study of the structure of this important class of neurotransmitter receptors.
Full text
PDF

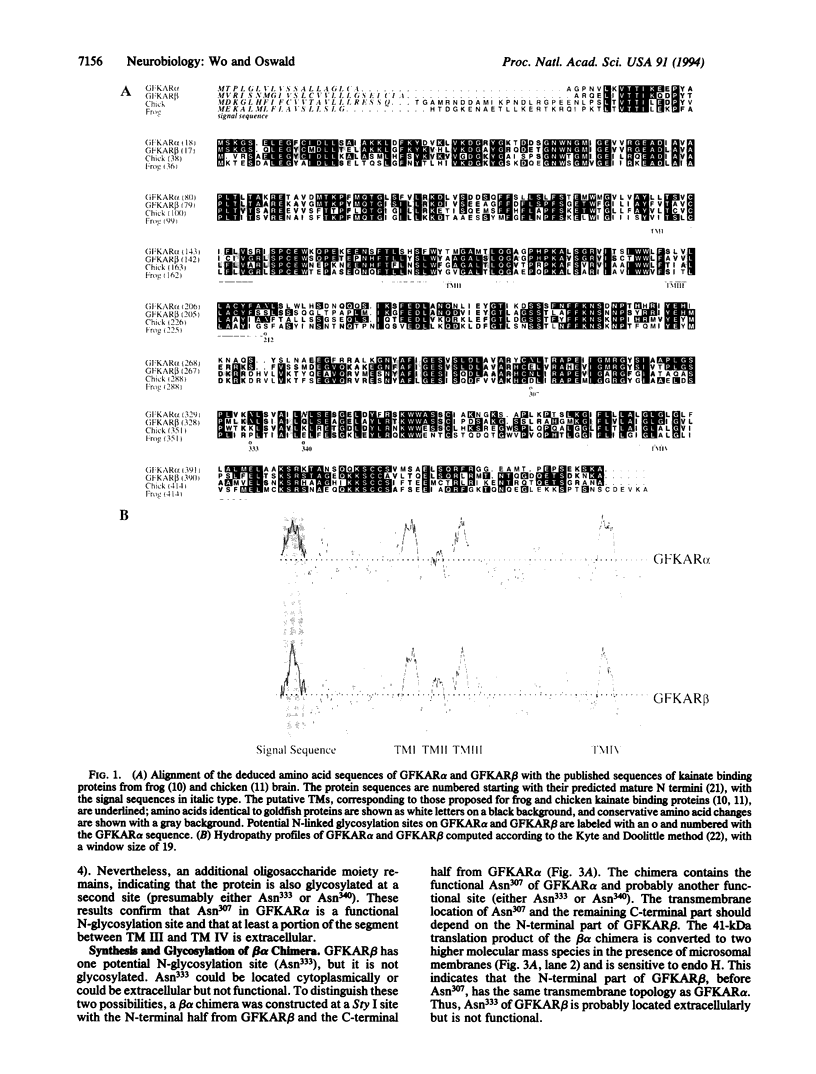
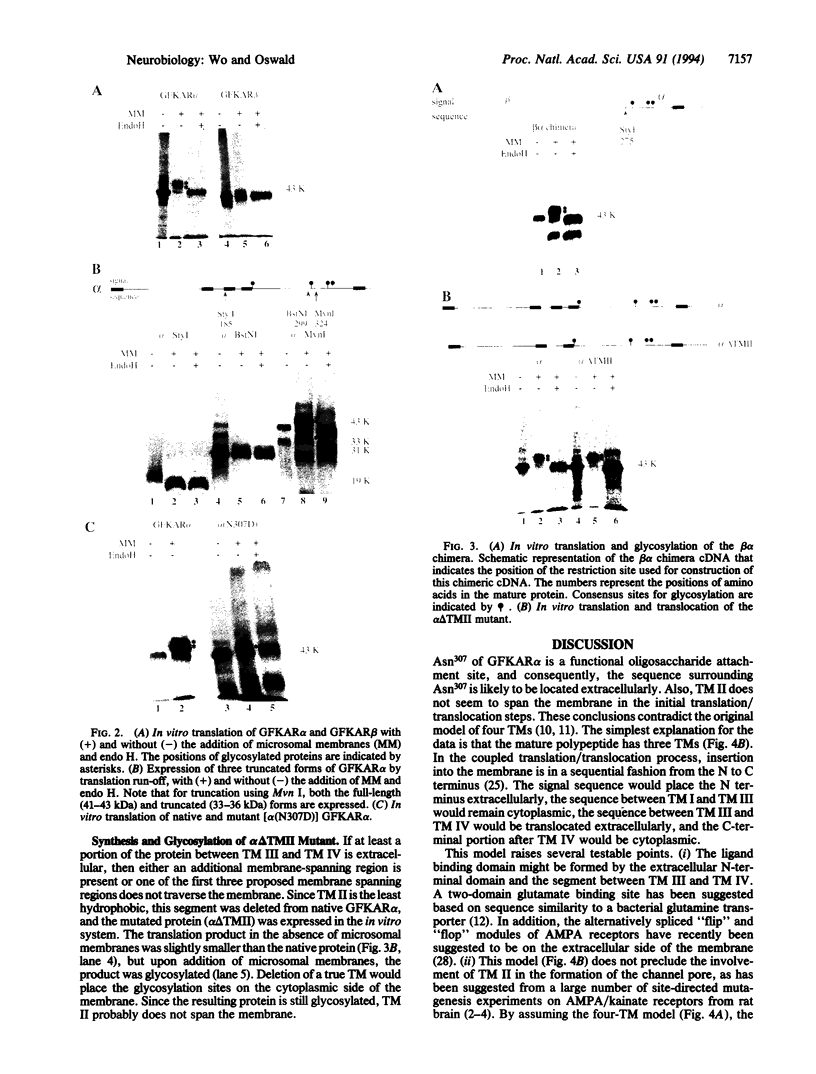

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Schoepfer R., Monyer H., Ruppersberg J. P., Günther W., Seeburg P. H., Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992 Sep 4;257(5075):1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Chavez R. A., Hall Z. W. The transmembrane topology of the amino terminus of the alpha subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1991 Aug 15;266(23):15532–15538. [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Gregor P., Mano I., Maoz I., McKeown M., Teichberg V. I. Molecular structure of the chick cerebellar kainate-binding subunit of a putative glutamate receptor. Nature. 1989 Dec 7;342(6250):689–692. doi: 10.1038/342689a0. [DOI] [PubMed] [Google Scholar]
- Gregor P., Yang X., Mano I., Takemura M., Teichberg V. I., Uhl G. R. Organization and expression of the gene encoding chick kainate binding protein, a member of the glutamate receptor family. Brain Res Mol Brain Res. 1992 Dec;16(3-4):179–186. doi: 10.1016/0169-328x(92)90223-x. [DOI] [PubMed] [Google Scholar]
- Hampson D. R., Wenthold R. J. A kainic acid receptor from frog brain purified using domoic acid affinity chromatography. J Biol Chem. 1988 Feb 15;263(5):2500–2505. [PubMed] [Google Scholar]
- Hart G. W., Brew K., Grant G. A., Bradshaw R. A., Lennarz W. J. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with natural and synthetic peptides. J Biol Chem. 1979 Oct 10;254(19):9747–9753. [PubMed] [Google Scholar]
- Henley J. M., Oswald R. E. Solubilization and characterization of kainate receptors from goldfish brain. Biochim Biophys Acta. 1988 Jan 13;937(1):103–111. doi: 10.1016/0005-2736(88)90232-5. [DOI] [PubMed] [Google Scholar]
- Imoto K. Ion channels: molecular basis of ion selectivity. FEBS Lett. 1993 Jun 28;325(1-2):100–103. doi: 10.1016/0014-5793(93)81422-v. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kimura N., Kurosawa N., Kondo K., Tsukada Y. Molecular cloning of the kainate-binding protein and calmodulin genes which are induced by an imprinting stimulus in ducklings. Brain Res Mol Brain Res. 1993 Mar;17(3-4):351–355. doi: 10.1016/0169-328x(93)90022-h. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Mori H., Yamakura T., Masaki H., Mishina M. Involvement of the carboxyl-terminal region in modulation by TPA of the NMDA receptor channel. Neuroreport. 1993 May;4(5):519–522. doi: 10.1097/00001756-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A., Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990 Nov;5(5):569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J. Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell. 1992 Oct 2;71(1):87–96. doi: 10.1016/0092-8674(92)90268-h. [DOI] [PubMed] [Google Scholar]
- Ortega A., Teichberg V. I. Phosphorylation of the 49-kDa putative subunit of the chick cerebellar kainate receptor and its regulation by kainatergic ligands. J Biol Chem. 1990 Dec 15;265(35):21404–21406. [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pongs O. Structural basis of voltage-gated K+ channel pharmacology. Trends Pharmacol Sci. 1992 Sep;13(9):359–365. doi: 10.1016/0165-6147(92)90109-j. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Schuster C. M., Ultsch A., Schloss P., Cox J. A., Schmitt B., Betz H. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991 Oct 4;254(5028):112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993 Sep;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Tingley W. G., Roche K. W., Thompson A. K., Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993 Jul 1;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Wada K., Dechesne C. J., Shimasaki S., King R. G., Kusano K., Buonanno A., Hampson D. R., Banner C., Wenthold R. J., Nakatani Y. Sequence and expression of a frog brain complementary DNA encoding a kainate-binding protein. Nature. 1989 Dec 7;342(6250):684–689. doi: 10.1038/342684a0. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Willard J. M., Ziegra C. J., Oswald R. E. The interaction of a kainate receptor from goldfish brain with a pertussis toxin-sensitive GTP-binding protein. J Biol Chem. 1991 Jun 5;266(16):10196–10200. [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993 Jun;3(3):291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Ziegra C. J., Oswald R. E., Bass A. H. [3H]kainate localization in goldfish brain: receptor autoradiography and membrane binding. Brain Res. 1990 Sep 17;527(2):308–317. doi: 10.1016/0006-8993(90)91151-6. [DOI] [PubMed] [Google Scholar]
- Ziegra C. J., Willard J. M., Oswald R. E. Biochemical characterization of kainate receptors from goldfish brain. Mol Pharmacol. 1992 Aug;42(2):203–209. [PubMed] [Google Scholar]
- Ziegra C. J., Willard J. M., Oswald R. E. Coupling of a purified goldfish brain kainate receptor with a pertussis toxin-sensitive G protein. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4134–4138. doi: 10.1073/pnas.89.9.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]