Abstract
Objective
Novel treatments such as natalizumab and fingolimod achieve their therapeutic efficacy in multiple sclerosis (MS) by blocking access of subsets of immune cells into the central nervous system, thus creating nonphysiological intrathecal immunity. In contrast, daclizumab, a humanized monoclonal antibody against the alpha chain of the IL-2 receptor, has a unique mechanism of action with multiple direct effects on innate immunity. As cellular intrathecal abnormalities corresponding to MS have been well defined, we asked how daclizumab therapy affects these immunological hallmarks of the MS disease process.
Methods
Nineteen subpopulations of immune cells were assessed in a blinded fashion in the blood and 50-fold concentrated cerebrospinal fluid (CSF) cell pellet in 32 patients with untreated relapsing-remitting MS (RRMS), 22 daclizumab-treated RRMS patients, and 11 healthy donors (HDs) using 12-color flow cytometry.
Results
Long-term daclizumab therapy normalized all immunophenotyping abnormalities differentiating untreated RRMS patients from HDs. Specifically, strong enrichment of adaptive immune cells (CD4+ and CD8+ T cells and B cells) in the CSF was reversed. Similarly, daclizumab controlled MS-related increases in the innate lymphoid cells (ILCs) and lymphoid tissue inducer cells in the blood and CSF, and reverted the diminished proportion of intrathecal monocytes. The only marker that distinguished daclizumab-treated MS patients from HDs was the expansion of immunoregulatory CD56bright NK cells.
Interpretation
Normalization of immunological abnormalities associated with MS by long-term daclizumab therapy suggests that this drug's effects on ILCs, NK cells, and dendritic cell-mediated antigen presentation to CD4+ and CD8+ T cells are critical in regulating the MS disease process.
Introduction
While there have been major advances in the treatment of relapsing-remitting multiple sclerosis (RRMS) in the last decade, neither the cause nor the underlying pathogenic mechanisms of this neuroinflammatory demyelinating disorder have been fully defined. Nevertheless, strong over-representation of immune genes in the susceptible genetic background,1,2 therapeutic success of immunomodulatory treatments, and consistent immunophenotyping abnormalities observed in the cerebrospinal fluid (CSF) of RRMS patients3–5 leave little doubt that the defective immunoregulation of adaptive immunity plays a crucial role in this disease.
Daclizumab, a humanized monoclonal antibody (Ab) against CD25, the alpha chain of the high-affinity IL-2 receptor (IL-2R), was originally designed as a therapeutic to selectively block activated T cells.6 Unexpectedly, T cells that lack CD25, either via genetic deletion or daclizumab blockade, were shown to not only proliferate and produce cytokines normally upon polyclonal stimulation,7,8 but paradoxically survive longer. This presumably occurs due to inhibited activation-induced cell death (AICD)9–11 in the absence of CD25.12 Consequently, both CD25-deficient mice and humans suffer from lymphoproliferation,13–15 while only CD25-deficient humans are also immunocompromised. The latter phenomenon can be accounted for by the behavior of human dendritic cells (DCs) during the maturation process. The DCs utilize the upregulated CD25 to trans-present IL-2 to primed T cells across the immune synapse when naïve T cells do not yet express high-affinity IL-2R.8 This early IL-2 signal is essential for the development of antigen-specific T-cell effectors. Its relevance to daclizumab's mechanism of action (MOA) is substantiated by mild but reproducible increases in infection rates observed in Phase II/III trials.16,17 Daclizumab also has unanticipated effects on innate lymphoid cells (ILCs), promoting differentiation of common ILC precursors away from pro-inflammatory lymphoid tissue inducer cells (LTis), and toward immunoregulatory CD56bright NK cells.18,19 Significant correlations between the expansion of CD56bright NK cells and therapeutic responses to daclizumab,19,20 as confirmed in double-blind Phase II trials, indicate that the composition of ILC subpopulations is likewise important for daclizumab's efficacy in MS.
These surprising observations suggest a fundamental involvement of the innate immune system in the MS disease process. Indeed, while most are eager to ascribe a pathogenic role to adaptive immunity in auto-immune diseases based on experimentations with T- and B-cell receptor transgenics21,22 or adoptive transfers, the fact that innate immunity shapes the extent and phenotype of T and B lymphocyte activation is generally overlooked. Therefore, the goal of the current study was to investigate the link between components of the innate immune system altered by daclizumab and the MS disease process by measuring the effects of long-term daclizumab therapy on the characteristic intrathecal immunophenotyping abnormalities reproducibly described in multiple cohorts of untreated RRMS.
Material and Methods
Subjects
The study was approved by the NIH Institutional Review Board and all patients provided written consent. Twenty-two RRMS patients participated in the NIH clinical trial 10-N-0125: “Investigating mechanism of action of DAC HYP in the treatment of high-inflammatory multiple sclerosis (MS)” (ClinicalTrials.gov identifier NCT01143441) and received daclizumab high yield process (Dac-HYP, 150 mg subcutaneously every 4 weeks) for a minimum of 36 months. 71% of Dac-HYP-treated patients were also treated with a previous formulation of daclizumab (Zenapax, Hoffmann-La Roche Inc.Nutley, NJ, USA) for up to 6 years prior to enrollment in the 10-N-0125 protocol. Dac-HYP has the identical amino sequence of Zenapax®, but due to expression in a different cell type and different expression system, Dac-HYP has glycosylation changes that affect its binding to Fc receptors.10
Control subjects (32 untreated RRMS patients and 11 healthy donors, [HDs]) were prospectively recruited from the natural history protocol 09-N-0032. Diagnosis of RRMS was based on the 2010 revisions to the McDonald diagnostic criteria.23 All eligible control subjects studied with the modified flow cytometry immunophenotyping panel (see below) acquired between December 2012 and May 2014 were included in this study. Patients' demographic data are provided in Table1.
Table 1.
Patient demographics and diagnoses
| Demographics | RR-MS (n = 32) | Dac-treated RRMS (n = 22) | Health donor (n = 11) |
|---|---|---|---|
| Age (year) | 38.0/38.3 [18.30–65.50]a | 44.10/44.40 [25.90–62.00]a | 37.9/39.00 [23.30–56.20]a |
| Disease duration | 1.20/5.60 [0.10–36.30]a | 10.50/10.80 [4.60–20.30]a | N/A |
| EDSS | 1.50/1.90 [0.00–6.00]a | 1.50/1.90 [1.00–2.50]a | 0.00/0.30 [0.00–1.00]b |
| SNRS | 95.00/91.20 [71.00–100.00]a | 91.00/89.20 [70.00–97.00]a | 100.00/98.70 [96.00–100.00]b |
| CEL | 0.00/0.90 [0.00–15.00]a | 0.00/0.10 [0.00–2.00]b | N/A |
| CSF WBC (per mL) | 3.00/4.56 [0.00–17.00]a | 2.00/3.62 [0.00–17.00]a | 2.00/2.00 [1.00–4.00]a |
| Oligoclonal band pattern % (I/II/III/IV)1 | 3.13/87.50/9.38/0.00a | 4.55/40.91/36.36/18.19b | 72.73/9.09/0.00/18.18c |
| IgG index | 0.72/0.86 [0.53–2.48]a | 0.69/0.83 [0.44–1.29]ab | 0.52/0.50[0.37–0.70]b |
| Treatment | Untreated | Dac-HYP | N/A |
Data are represented as median/mean [range]. Means with different superscript letters (a, b, and c) are significantly different with P < 0.001. RRMS, relapsing-remitting multiple sclerosis; EDSS, Expanded Disability Status Scale; SNRS, Scripps Neurologic Rating Scale; CEL, Contrast Enhancing Lesion; CSF, cerebrospinal fluid.
I – no bands in CSF and Serum; normal profile/II – oligoclonal bands in CSF only; consistent with intrathecal IgG synthesis/III – partially identical oligoclonal bands in CSF and serum; consistent with systemic disease/IV – identical oligoclonal bands in CSF and serum; consistent with systemic inflammation.
Sample preparation and flow cytometry
Specimen collection, handling, and processing were performed according to written standard operating procedures (SOPs).5 All samples (N = 65) were assigned an alpha-numeric code and personnel performing the studies were blinded to the subjects' diagnoses. Immunophenotyping of peripheral blood cells was performed on anticoagulated blood within 60 min of ex vivo collection following osmotic lysis of erythrocytes. CSF samples (20 mL) were placed on ice immediately after collection and spun within 15 min. Cell pellets were resuspended in 400 μL ice-cold X-Vivo media (Lonza, Walkersville, MD, USA) to produce a 50-fold concentration, and CSF cells were counted by hemocytometer (Neubauer; Hausser Scientific, Horsham, PA, USA) at high magnification to enable differentiation between erythrocytes and nucleated cells. Concentrations of CSF leukocytes per 1 mL of CSF were calculated by dividing the total number of CSF leukocytes by the volume of CSF collected.
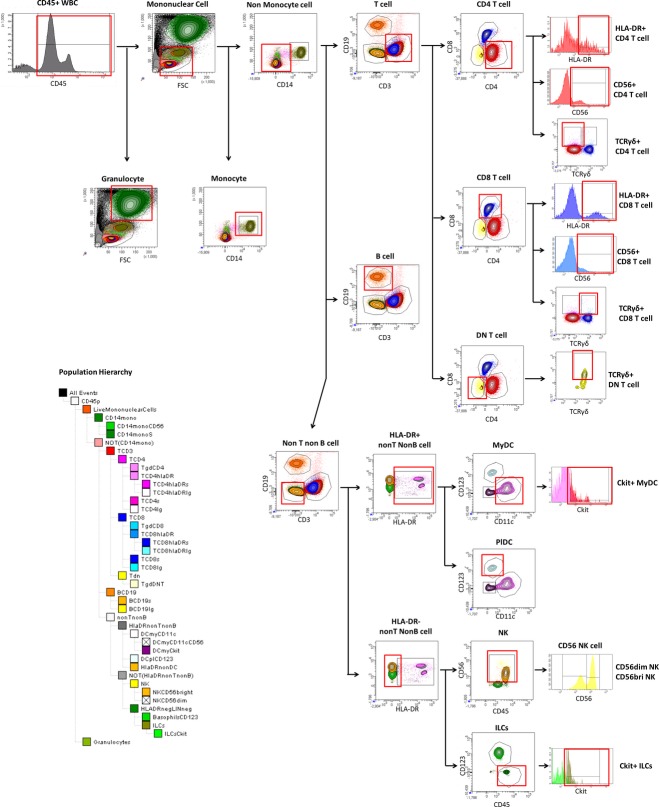
The 12-color immunophenotyping panel used is described in Table2. This panel was modified from a previously reported immunophenotyping panel5 to enable quantification of ILCs, LTi cells, and γ/δ T cells. Our preliminary experiments demonstrated that out of all cell surface markers (OX-40L, CD30L, c-kit, CD127) reported to be upregulated on the human pro-inflammatory subgroup of ILCs called LTi cells, the stem cell growth factor receptor, otherwise called tyrosine protein kinase kit (c-kit; CD117) has most consistent expression on the proportion of ILCs in the blood and CSF. C-kit is also expressed on the proportion of immature DCs, and our 12-color panel could differentiate LTi cells from other c-kit-expressing immune cells (see gating strategy in Fig.1). A minimum of 106 blood cells and 5000 CSF cells were stained according to a previously established protocol5 that blocks Fc receptors by 2% intravenous immunoglobulin (IVIg). Cells were immediately acquired on a BD LSR II with a High Throughput Sampler delivery system and analyzed with FACSDiva 6.1 software (all BD Biosciences, San Jose, CA, USA). Gating was based on isotype controls.
Table 2.
Optimized combination of 12 commercially available flourochrome-conjugated antibodies to reliably quantify 14 subpopulations of immune cells
| Conjugation | Name | Company (clone) |
|---|---|---|
| FITC | Anti-human CD56 antibody | BD (clone: MEM188) |
| PE | Anti-human SCF R/c-kit antibody | R&D (clone: 47233) |
| PerCP-Cy5.5 | Anti-human CD123 antibody | eBioscience (clone: 7G3) |
| PE-Cy7 | Anti-human CD11c antibody | eBioscience (clone: 3.9) |
| V450 | Anti-human CD45 antibody | BD (clone: HI30) |
| AmCyan | Anti-human CD8 antibody | BD (clone: SK1) |
| eFluor 605 Nanocrystal | Anti-human CD19 antibody | eBioscience (clone: HIB19) |
| eFluor 655 Nanocrystal | Anti-human CD3 antibody | eBioscience (clone: OKT3) |
| Qdot 705 | Anti-human CD4 antibody | Invitrogen (clone: S3.5) |
| APC | Anti-human TCRγδ antibody | BD (clone: B1) |
| Alexa Fluor 700 | Anti-human CD14 antibody | BioLegend (clone: HCD14) |
| APC-Cy7 | Anti-human HLA-DR antibody | eBioscience (clone: LN3) |
Figure 1.
Gating strategy. Live leukocytes were first identified based on CD45 expression. Mononuclear cells were differentiated from granulocytes based on forward (size) and side (granularity) scatter characteristics. T cells were identified as mononuclear cells expressing CD3 and lacking CD14, and were further subdivided based on CD4 and CD8 expression into CD4+ T cells, CD8+ T cells and double negative T cells (DnT). Expression of HLA-DR and CD56 identified the proportions of “effector” T cells (HLA-DR+) and “cytotoxic” T cells (CD56+). Expression of TCRγ/δ subdivided TCRγ/δ into CD4+, CD8+ and double negative (CD4-CD8-) TCRγ/δ T cells. Monocytes were gated as mononuclear cells that do not express CD3 but express CD14 (as well as HLA-DR and intermediate levels of CD45). NK cells were gated as mononuclear cells with CD56 expression that do not express CD3, CD14 or CD19. These were further subdivided into CD56dim and CD56bright NK cells, identified as distinct subpopulations in the CD56 mean fluorescent intensity (MFI) histogram. B cells were identified as mononuclear cells that did not express CD3, CD14, or CD56, but did express CD19 (and HLA-DR). Dendritic cells were identified as mononuclear cells expressing HLA-DR but lacking expression of lineage markers (CD3, CD4, CD8, CD14, and CD19). They were further subdivided into myeloid DCs expressing CD11c and plasmacytoid DCs expressing CD123. Cells lacking lineage markers (including CD56), HLA-DR, CD11c, and CD123 were gated as ILCs. Expression of tyrosine protein kinase kit (c-kit; CD117) identified proportions of ILCs as LTi cells and proportion of MyDCs as more immature MyDCs that may have an anti-inflammatory role.
Statistical analysis
One-way ANOVA (analysis of variance) was performed to explore the association of explanatory variable (diagnosis: untreated RRMS, Dac-treated RRMS, and HDs) with 80 response variables which are the absolute numbers or proportions of adaptive and innate immune cells collected from CSF and blood staining samples. Since most of these response variables did not follow the normal distribution, Box-Cox transformation was applied (log- transformation for most of the variables) prior to ANOVA. Tukey's method was used for pair-wise multiple comparisons. P-value <0.01 was considered significant to adjust for multiple tests (a total of 160 tests). SAS (SAS, SAS Institute Inc, Cary, NC, USA) version 9.3 was used for the above analyses.
Results
Dac-HYP exhibits differential effects on subpopulations of innate immune system, restoring intrathecal abnormalities observed in untreated RRMS patients to HD levels
Multiple investigators reported proportional decreases in CSF monocytes,3,5 while our group also reported unexpected increases in the population of ILCs in the blood of untreated RRMS patients. Although the latter was normalized by daclizumab treatment,18 no previous studies have investigated ILCs and LTi cells in the CSF. Therefore, we implemented modifications to a previously reported 12-color immunophenotyping protocol5 (Table2 and Fig.1) that allowed us to study these specific cell types of interest.
The effects of Dac-HYP on innate immune cells are depicted in Figure2. While we reported all statistically significant differences in the figure, we considered only those with P-values <0.01 as reliable due to multiple comparisons. The only differences in the blood (Fig.2, left columns) were the previously reported Dac-HYP-induced expansion of CD56bright NK cells distinguishing Dac-HYP-treated patients from untreated RRMS and HDs, and a proportional increase in ILCs in the untreated RRMS cohort which was normalized by Dac-HYP therapy.
Figure 2.
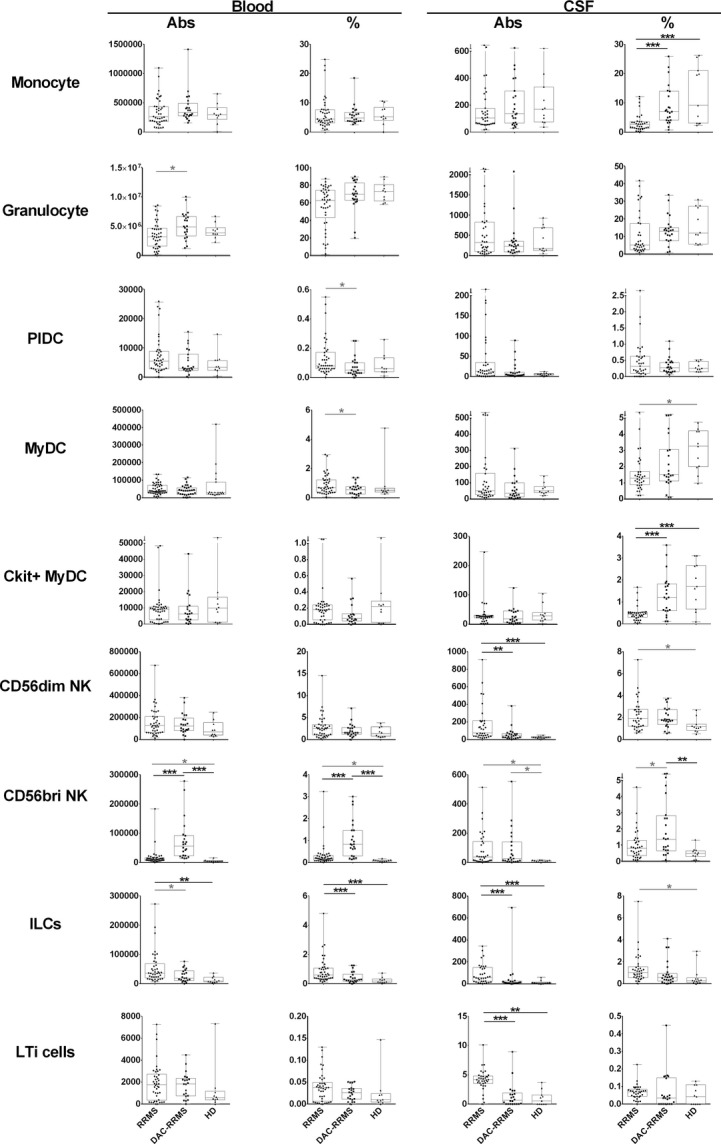
Differences in proportions and absolute numbers of innate immune cells: monocytes, granulocytes, plasmacytoid dendritic cells (PlDCs), myeloid DCs (MyDCs), CD56dim, and CD56bright NK cells, innate lymphoid cells (ILCs), tyrosine protein kinase kit (c-kit)+ ILCs, and CKit+ MyDCs. The two left panels in each row represent proportions and absolute numbers of specific cell populations in the blood, while the two right panels represent proportions and absolute numbers of the same cell populations in the cerebrospinal fluid. Each diagnostic category corresponds to one vertical box-and-whisker plot. The boxes show the median and 25–75% range, while the whiskers indicate minimum and maximum values for each diagnostic category. The scatter dot plots represent data from individual patients. *P < 0.05, **0.001 < P < 0.05, ***P < 0.001.
In the intrathecal compartment (Fig.2, right columns), Dac-HYP therapy completely normalized a proportional decrease in CSF monocytes. We also observed a trend with marginal statistical significance in the proportional decrease in myeloid DCs in the untreated RRMS patients as compared to HDs. When evaluating developmentally less-differentiated C-kit+ myeloid DCs, their proportional decrease in RRMS was highly statistically significant and Dac-HYP therapy again fully normalized this abnormality.
Untreated RRMS patients had robust expansion of absolute numbers of cytotoxic CD56dim NK cells in the CSF, which was normalized in the Dac-HYP cohort. At the same time, Dac-HYP treatment resulted in a significant expansion of immunoregulatory CD56bright NK cells in the blood, both in terms of absolute numbers and proportions. Only the proportional increase in these cells in the intrathecal compartment achieved statistical significance in comparison to HDs.
Finally, the absolute numbers and the proportions of ILCs were significantly increased in the blood and CSF of untreated RRMS patients as compared to HDs. While similar trends were observed for LTi cells (enumerated in this study as c-kit+ ILCs), they did not reach statistical significance in the blood due to a single HD outlier. Nevertheless, we still observed a strong, statistically significant reduction in absolute numbers of intrathecal LTi cells in the Dac-HYP-treated MS patients as compared to untreated RRMS controls.
Dac-HYP treatment has profound inhibitory effect on abnormally activated intrathecal adaptive immunity
In the blood, we observed a statistically significant expansion in absolute numbers of CD4+ T cells, along with a trend toward expansion of CD8+ T cells as well as their more cytotoxic counterparts (CD56+ CD8 T cells) in untreated RRMS patients in comparison to HDs. All of these abnormalities were normalized in the Dac-HYP cohort (Fig.3, left columns). Because we have previously observed a trend toward expansion of T-cell receptor (TCR) γ/δ+ T cells in the patients treated long-term with intravenous administration of previous formulation of daclizumab (Zenapax©), we aimed to investigate effect of Dac-HYP on different subpopulations of TCR γ/δ+ T cells more formally. We again only observed a trend for the expansion of these cells in the blood of the Dac-HYP cohort, which did not reach predetermined levels of statistical significance after adjustment for multiple comparisons. Only the proportions of CD4+ TCR γ/δ+ were significantly higher in the Dac-HYP cohort as compared to HDs.
Figure 3.

Differences in proportions and absolute numbers of adaptive immune cells: CD4+ and CD8+ T cells and their subsets (HLA-DR+ effector cells and CD56+ cytotoxic cells), CD19+ B cells, TCRγ/δ T cells, TCRγ/δ CD4+ T cells, TCRγ/δ CD8+ T cells, and TCRγ/δ double negative T cells. The two left panels in each row represent proportions and absolute numbers of specific cell populations in the blood, while the two right panels represent proportions and absolute numbers of the same cell populations in the cerebrospinal fluid. Each diagnostic category corresponds to one vertical box-and-whisker plot. The boxes show the median and 25–75% range, while the whiskers indicate minimum and maximum values for each diagnostic category. The scatter dot plots represent data from individual patients. *P < 0.05, **0.001 < P < 0.05, ***P < 0.001.
In the intrathecal compartment, we reproduced previously reported increases in CD4+ T cells and B cells in the CSF of untreated RRMS patients (Fig.3, right columns), which were normalized by Dac-HYP therapy. We also observed increases in newly activated (HLA-DR+) CD4+ T cells in the CSF of untreated RRMS patients, which was likewise normalized in the Dac-HYP cohort. In terms of CD8+ T cells, we only observed a trend toward intrathecal expansion in the untreated RRMS cohort, which reached statistical significance only for the subpopulation of CD56+ CD8+ cytotoxic T cells. In contrast to blood, we observed highly statistically significant expansion of both CD4+ and CD8+ TCR γ/δ+ T cells in the CSF of untreated RRMS patients. However, Dac-HYP treatment, which marginally expanded absolute numbers of these cells in the blood, had the opposite effect in the CSF, where Dac-HYP patients had comparable absolute numbers and proportions of these cells to HD controls. The situation was slightly different for the most prominent subpopulation of TCR γ/δ T cells called “double negative” (DN) TCR γ/δ T cells based on the lack of either CD4 or CD8 co-receptors. These cells were only marginally increased in absolute numbers but decreased in proportion in untreated RRMS patients compared to the Dac-HYP-treated cohort.
Dac-HYP therapy does not alter CSF CD4/CD8 ratios, and normalizes abnormal proportional increases in intrathecal T cells
Because CSF CD4/CD8 ratios were found to be altered beyond physiological levels by natalizumab24 and fingolimod therapies and abnormally high CSF B cell/monocyte ratios are associated with aggressive MS,3 we also investigated the effect of Dac-HYP therapy on these combinatorial intrathecal biomarkers (Fig.4). We observed a nonsignificant trend toward increased CD4/CD8 ratios in untreated RRMS patients, which was completely normalized within (but not below) physiological (HD) range in Dac-HYP cohort. Dac-HYP also raised abnormally low CD4/B cell and CD8/B-cell ratios in comparison to the untreated RRMS cohort. Finally, we again observed previously reported strong elevation of CSF B-cell/monocyte ratios in the untreated RRMS cohort, which was returned entirely to HD levels in Dac-HYP-treated MS patients.
Figure 4.
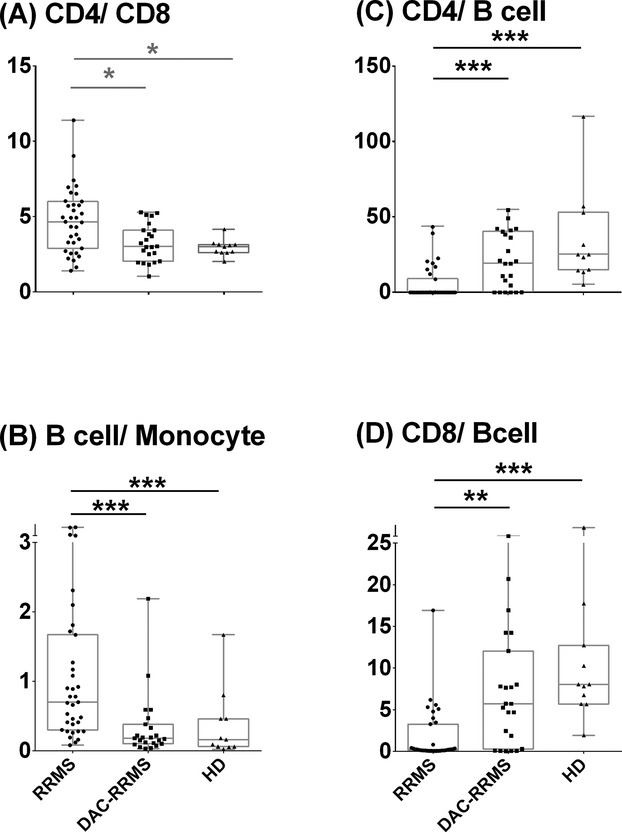
Differences in intrathecal ratios of (A) CD4 to CD8, (B) B cell to monocyte, (C) CD4 to B cell, and (D) CD8 to B cell in CSF among diagnostic categories. The boxes show the median and 25–75% range, while the whiskers indicate minimum and maximum values for each diagnostic category. The scatter dot plots represent data from individual patients. *P < 0.05, **0.001 < P < 0.05, ***P < 0.001.
Discussion
During the period in which regulatory approval for Dac-HYP is pending, pivotal insights into its MOA continue to emerge. Although future studies will likely expand upon the current understanding of daclizumab's MOA, data reported thus far have revealed surprisingly limited (and potentially deleterious) direct effects on adaptive immune cells,10,25 but unanticipated effects on multiple subpopulations of innate immune cells.8,18,19,26,27 Indeed, by blocking CD25, daclizumab reduces IL-2 consumption by FoxP3+ regulatory T cells (T-regs) and effector T cells.28 This results in reduced T-reg numbers29 and their in-vivo proliferation,28 but also potentially promotes survival of activated, effector T cells by inhibiting AICD.9,15,10 These effects are thought to underlie abnormal lymphoproliferation and autoimmunity observed in mice and humans with genetically deleted CD2513–15 and would be expected to exacerbate putative T-cell-mediated autoimmune diseases such as MS. In contrast, daclizumab16,17,20,30 or Dac-HYP31,32 potently inhibit MS disease activity, as evidenced by imaging and clinical disability measures.
We have partially explained this paradox by demonstrating that increased bio-availability of IL-2 upon daclizumab treatment predicted by mechanistic in-vitro studies28 activates CD56bright NK cells19,28 and common ILC precursors18 expressing high levels of intermediate affinity IL-2R unhindered by daclizumab. This leads to dramatic expansion of CD56bright NK cells19 and activation of their immunoregulatory functions,26 as well as contraction of pro-inflammatory LTi cells due to their diminished differentiation from common ILC precursor.18 The initial finding that expanded CD56bright NK cells kill autologous activated (but not resting) T cells19 via perforin/granzyme-K cytotoxicity,26 leading to a mild but statistically significant decrease in absolute peripheral T-cell counts,19 was reproduced in the current cohorts. Additionally, daclizumab inhibits antigen-specific activation of T cells by blocking the trans-presentation of IL-2 by DCs.8
While fetal LTi cells play pivotal role in the development of secondary lymphoid tissue, LTi cells in adult animals retain lymphoid tissue inducing capacity (at least in the gastro-intestinal tract) and may play important role in evolution of T-cell memory and T-cell-dependent high-affinity antibody responses.33,34 We reported previously that concomitantly with daclizumab-mediated inhibition of differentiation of ILC precursors toward LTi lineage, daclizumab therapy also decreases CSF levels of CXCL13 and IgG index,18 and development of gray matter brain atrophy.35 Current observations of daclizumab-mediated normalization of CSF immune cell composition substantiates these mechanistic studies and is consistent with either active efflux of inflammatory cells from CSF back to periphery or their death in the intrathecal compartment. Interestingly, although similar proportion of untreated RRMS patients and Dac-HYP-treated patients had CSF oligoclonal bands (OCB), type II OCBs (i.e., present only in the CSF but not in serum) predominated in untreated cohort (85.71%), while Dac-HYP patients had equal proportion (46.15%) of Type II and Type III OCBs (i.e., partially identical bands between CSF and serum). This observation supports possibility of daclizumab-driven active efflux of immune cells from the intrathecal compartment to periphery.
It is important to note that a previous CSF immunophenotyping study on MS patients36 did not observe significant changes in the numbers of intrathecal T cells following short-term (6 months) Zenapax® therapy. However, we acknowledged that the major drawback of the previous study was the execution of cell counting in un-spun CSF samples (as is traditional in clinical laboratories). These counts are highly unreliable and insensitive to detecting mild-to-moderate levels of CSF pleiocytosis such as those observed in untreated MS.5 We therefore maximized the accuracy of CSF cell counts in 50-fold concentrated cell samples, allowing us to determine that Dac-HYP decreases absolute numbers of CSF T and B cells to normal but not below physiological values. We also reproduced our previous observation of proportional enrichment of immunoregulatory CD56bright NK cells in both blood and CSF.36 However, the absolute numbers of these cells were expanded only in the blood, but not in the CSF. This seemingly paradoxical observation can be logically explained by the fact that the major determinant of immunoregulatory capacity of CD56bright NK cells is their ratio to the activated T cells (as well as their functional ability to kill activated autologous T cells, which, as we previously reported, is also enhanced by daclizumab26). As Dac-HYP therapy lead to significant decrease in absolute numbers of T cells in the intrathecal compartment, the ratio of T cells/CD56bright NK cells also decreased significantly (from 200.51 in RRMS to 85.07 in Dac-HYP MS; P < 0.05) without continuous increase in the absolute numbers of regulatory NK cells. The augmented chemotactic cues that lead to enhanced recruitment of immunoregulatory NK cells to the intrathecal compartment under inflammatory conditions also normalized when central nervous system inflammation is diminished.18
In addition to demonstrating this normalizing effect on traditionally studied α/β TCR T cells representing the majority of T cells in the blood and CSF, we also found that Dac-HYP normalized elevated numbers of CSF γ/δ TCR+ T cells, especially those co-expressing CD8. While the biology of γ/δ T cells is not completely elucidated, animal models have suggested their importance in regulating certain infectious agents including neurotrophic viruses such as herpes simplex virus,37 ability to participate in autoimmunity,38 and concurrent regulation of their more abundant counterparts, the α/β T cells.39,40
It remains unclear whether the afore-mentioned indirect effects on the T cells comprise the only, or even the most fundamental mechanism(s) by which daclizumab impedes the MS disease process. While we do not yet understand the roles of ILCs and LTi cells in MS, their expanded absolute and proportional levels in the blood18 and CSF (current results) of untreated RRMS patients suggest that they may be crucial to MS pathogenesis. Unfortunately, due to their infrequency in the blood and CSF and a lack of strong surface markers, extensive developmental work is required before we can study the function(s) of these cells and understand their potential role(s) in autoimmunity. Nevertheless, our results provide an impetus for such future mechanistic studies. Similarly, the role monocytes play in MS is currently unknown. Based on an association between monocytes and acutely demyelinating MS lesions, some authors have concluded that this cell population is a causal contributor to the demyelinating process. An alternative explanation is that they strip and phagocytose already dysfunctional myelin, thus promoting repair.41,42 Dac-HYP-induced reversals of proportional declines in CSF monocytes provide indirect support to the latter hypothesis, especially in light of the reported inhibitory effect of long-term daclizumab treatment on the development of MS-related brain atrophy.35
Our observations that daclizumab does not indiscriminately inhibit intrathecal immune responses, but instead induces selective changes that restore abnormal proportions and absolute numbers of innate and adaptive immune cells observed in untreated RRMS back to physiological levels, support the notion that the IL-2R system is fundamental to immune dysregulation characteristic of MS. In this regard, daclizumab therapy provides a unique window to a greater understanding of MS pathogenesis.
Acknowledgments
The study was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke (NINDS) and collaborative agreement (CRADA) between NINDS and Abbvie/Biogen-IDEC. The authors thank Anne Mayfield for scheduling patients and Jenifer Dwyer, Jamie Cherup, and the clinical team for sample collection.
Conflicts of Interest
Dr. Bielekova is co-inventor on several NIH patents related to daclizumab therapy for MS, and as such, has received patent royalty payments from NIH.
References
- Compston A, Sawcer S. Genetic analysis of multiple sclerosis. Curr Neurol Neurosci Rep. 2002;2:259–266. doi: 10.1007/s11910-002-0085-3. [DOI] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics C. Beecham AH, Patsopoulos NA, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepok S, Jacobsen M, Schock S, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(Pt 11):2169–2176. doi: 10.1093/brain/124.11.2169. [DOI] [PubMed] [Google Scholar]
- Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- Han S, Lin YC, Wu T, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol. 2014;192:2551–2563. doi: 10.4049/jimmunol.1302884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Goldman CK, Bongiovanni KF, et al. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988;72:1805–1816. [PubMed] [Google Scholar]
- Caudy AA, Reddy ST, Chatila T, et al. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan CC, Balk AH, van Riemsdijk IC, et al. Anti-CD25 monoclonal antibody therapy affects the death signals of graft-infiltrating cells after clinical heart transplantation. Transplantation. 2003;75:1704–1710. doi: 10.1097/01.TP.0000063937.53702.97. [DOI] [PubMed] [Google Scholar]
- Bielekova B. Daclizumab therapy for multiple sclerosis. Neurotherapeutics. 2013;10:55–67. doi: 10.1007/s13311-012-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo MJ. Interleukin-2 programs mouse T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Richter GH, Mollweide A, Hanewinkel K, et al. CD25 blockade protects T cells from activation-induced cell death (AICD) via maintenance of TOSO expression. Scand J Immunol. 2009;70:206–215. doi: 10.1111/j.1365-3083.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Lian ZX, Moritoki Y, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon-beta. Proc Natl Acad Sci USA. 2004;101:8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 2010;9:381–390. doi: 10.1016/S1474-4422(10)70033-8. [DOI] [PubMed] [Google Scholar]
- Perry JS, Han S, Xu Q, et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci Transl Med. 2012;4:145ra06. doi: 10.1126/scitranslmed.3004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R-alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol. 2009;66:483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Baeten D, Jager A, et al. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63:1383–1387. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- Martin D, Near SL. Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chai NR, Maric D, Bielekova B. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol. 2011;187:781–790. doi: 10.4049/jimmunol.1100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McClellan M, Efros L, et al. Daclizumab reduces CD25 levels on T cells through monocyte-mediated trogocytosis. Mult Scler. 2014;20:156–164. doi: 10.1177/1352458513494488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JF, Perry JS, Jakhete NR, et al. An IL-2 paradox: blocking CD25 on T cells induces IL-2-driven activation of CD56(bright) NK cells. J Immunol. 2010;185:1311–1320. doi: 10.4049/jimmunol.0902238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol. 2009;66:471–479. doi: 10.1001/archneurol.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JW, Burns JB, Bjorklund J, et al. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–2175. doi: 10.1016/S0140-6736(12)62190-4. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Gold R, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. Lancet Neurol. 2014;13:472–481. doi: 10.1016/S1474-4422(14)70039-0. [DOI] [PubMed] [Google Scholar]
- Lane PJ, McConnell FM, Withers D, et al. Lymphoid tissue inducer cells: bridges between the ancient innate and the modern adaptive immune systems. Mucosal Immunol. 2009;2:472–477. doi: 10.1038/mi.2009.111. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Borges IT, Shea CD, Ohayon J, et al. The effect of daclizumab on brain atrophy in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2013;2:133–140. doi: 10.1016/j.msard.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Richert N, Herman ML, et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology. 2011;77:1877–1886. doi: 10.1212/WNL.0b013e318239f7ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciammas R, Kodukula P, Tang Q, et al. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa A, Lahn M, Pflum EK, et al. Evidence that the same gamma delta T cells respond during infection-induced and autoimmune inflammation. J Immunol. 1997;159:5787–5794. [PubMed] [Google Scholar]
- Mukasa A, Hiromatsu K, Matsuzaki G, et al. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995;155:2047–2056. [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Yassai M, et al. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1587–1595. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–175. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]