Abstract
Articular cartilage has poor capacity of self-renewal and repair. Insufficient number and activity of resident mesenchymal (connective tissue) progenitors is likely one of the underlying reasons. Chondroprogenitors reside not only in the superficial zone of articular cartilage but also in other zones of articular cartilage and in the neighboring tissues, including perichondrium (groove of Ranvier), synovium and fat pad. These cells may respond to injury and contribute to articular cartilage healing. In addition, marrow stromal cells can migrate through subchondral bone when articular cartilage is damaged. We should develop drugs and methods that correctly stimulate resident progenitors for improvement of repair and inhibition of degenerative changes in articular cartilage.
Keywords: Progenitors, chondroprogenitors, articular cartilage, injury, repair
1. Introduction
Articular cartilage does not possess a satisfactory ability for self-renewal and repair, especially at middle and elderly ages. Once articular cartilage is damaged by injury, overload or wasting over age, the defect site does not usually regain original structure and function and may undergo degeneration, leading to chronic joint degenerative disorders such as osteoarthritis. Limited regenerative capacity of articular cartilage is likely due to low metabolic activity of articular chondrocytes and scarcity of resident mesenchymal (connective tissue) progenitors. Marrow stimulation techniques have been established to induce blood supply and recruit mesenchymal stem cells into the affected lesion from bone marrow through the subchondral bone. The procedures for marrow stimulation include transcortical Pridie drilling, abrasion arthroplasty and microfracture (Schindler, 2011;Hunziker, 2002). The autologous chondrocyte implantation (ACI) and ACI with biomaterials (MACI) are other techniques to supply autologous chondrocytes and/or chondrogenic progenitors to a focal lesion from the healthy unloading site of articular cartilage (Schindler, 2011;Oldershaw, 2012). Recently tissue engineering approaches using adult mesenchymal cells and progenitors derived from several types of tissues have been intensively studied. In many cases, the cells are grown under various stimuli and led to express a chondrogenic phenotype in culture prior to transplantion to the defect with biomaterials (Oldershaw, 2012;Tuan et al., 2013). These approaches provide favorable outcomes in developing an effective therapy for articular cartilage injury at young ages although long-term observation are required for reaching conclusion (Pastides et al., 2013). In addition, transplantation or injection of undifferentiated mesenchymal stem cells, bone marrow stromal cells or synovial mesenchymal cells into cartilage defects has been attempted in preclinical and clinical studies (Koga et al., 2008;Filardo et al., 2013;Kim et al., 2013) and demonstrated improvement of macro or micro-appearance in defects or better clinical outcome compared to the control without cell transplantation. Nevertheless, successful activation of resident progenitors must be an ideal solution for maintenance of homeostasis and repair of minor injury in articular cartilage. Furthermore, it would enhance repair of big defects in conjunction with transplantation of extrinsic chondrocytes or stem/progenitor cells. In this review, we summarize current information on resident progenitor cells that could contribute to development, maintenance and repair of articular cartilage, and discuss their potential and limitation.
2. Progenitor cells during articular cartilage development
2.1. Interzone cells
Articular cartilage is an essential component of synovial joints and has unique structure, biomechanical properties and function comparing to those of transient growth plate cartilage (Williams J.A. et al., 2010;Wardale et al., 1994;Cohen et al., 1988). How does articular cartilage originate and how is it organized? There are still many issues to be clarified in understanding the entire mechanisms. However, it is now recognized that articular cartilage and other synovial joint components share cell origin and are derived from a unique mesenchymal cell population, called interzone. Interzone appears at the future joint site between two cartilaginous skeletal elements and shows distinct histology and gene expression profile from those of adjacent cartilage elements (Khan et al., 2007;Pacifici et al., 2006;Archer et al., 2003). The two independent studies using a cell lineage tracing technique indicate that interzone cells constitute synovial joint components including joint capsule and articular cartilage, but not majority of the growth plate. Koyama et al. (2008) have generated the compound transgenic mice of Gdf5-Cre (Rountree et al., 2004) with RosaR26R-LacZ reporter mice and examined the fate of the resulting LacZ-positive cells. The labeled cells first constituted the entire interzone at an early stage, gave rise to the articular cartilage and synovial capsule at later stages but were mostly absent in the growth plate. Hyde et al. (2007) have used the transgenic mice that encode Cre-recombinase in the Matrillin 1 allele in RosaR26R-LacZ background. The LacZ-positive chondrocytes appear in the growth plate, but not in articular cartilage in these mice. These findings indicate that interzone is a source of progenitors for articular cartilage and synovial joint components at embryonic stages, and that the interzone-derived cell population constitutes articular cartilage through life, and may supply progenitor cells to articular cartilage for its renewal.
2.2. Slow-cycling cells in synovial joints
Articular cartilage development is tightly synchronized with the development of other synovial joint structures (Hunziker et al., 2007;Las Heras et al., 2012) and the formation of the secondary ossification center (Blumer et al., 2008;Blumer et al., 2007). The mature articular cartilage is divided into the following zones, beginning at surface: the superficial zone, the transition or mid zone, the deep or radial zone and the calcified zone, and is lined by subchondral bone (Hunziker et al., 2007;Las Heras et al., 2012;Becerra et al., 2010;Poole, 2003). Which mechanism supports growth of articular cartilage and organization of the zonal structure? Where do articular cartilage and other synovial joint tissues obtain stem/progenitors to organize and maintain their structure and function?
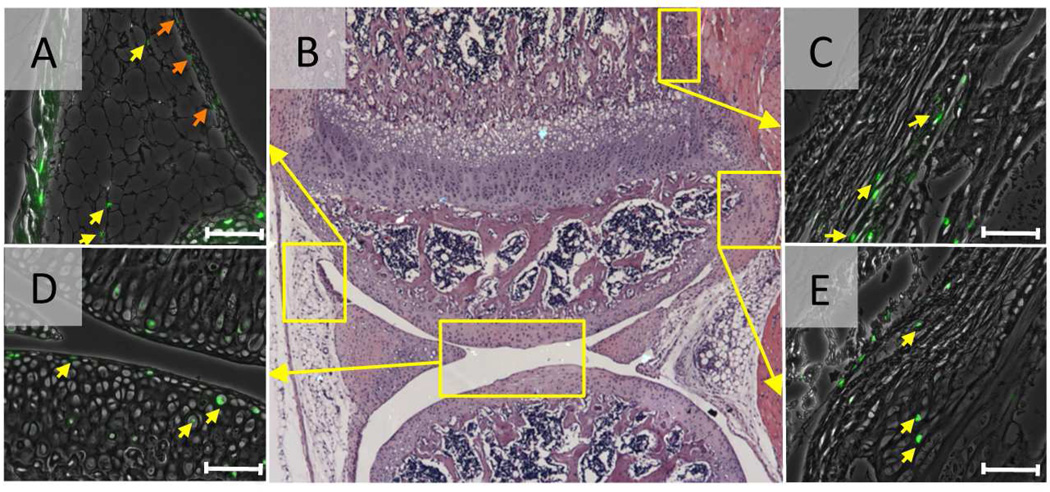
The cell labeling studies with tritium thymidine or bromodeoxyuridine have been performed in the developing synovial joints in animals such as rats, opossums and rabbits, and attempt to identify localization of stem cells since the long-term labeling of cells is one of the characteristics of stem cells (Hunziker et al., 2007;Ohlsson et al., 1992;Hayes et al., 2001). Ohlsson et al. (1992) have administrated the tritiated thymidine starting in embryo or young rats and examined the distribution of labeled cells 2 to 4 weeks after isotope administration was stopped. They have found that the long-term labeled cells are present in the proximal portion of growth plate, the perichondrial ring and the surface of articular cartilage, suggesting that surface of articular cartilage would provide stem cells to articular cartilage. Karlsson et al (Karlsson et al., 2009) have demonstrated that the groove of Ranvier contains long-term labeled cells with bromodeoxyuridine (BrdU) in the knee joint of 3-month-old rabbits. Along with the finding that this region shows immunoreactivity of the antibodies against progenitor markers Stro-1 and Jagged1, they conclude that the groove of Ranvier has the properties of a stem cell niche and that the groove of Ranvier would directly or indirectly support renewal of articular cartilage. As there is very limited information on the slow-cycling cells in developing synovial joint in mice, we performed cell labeling experiments with a new nucleoside derivative, 5-ethynyl-2'-deoxyuridine (EdU) in mice. Chemical detection of EdU makes the specificity higher and reduces the background. The mice received daily intraperitoneal injections of EdU from postnatal day 4 to day 7 or from E12 to E15. One day after the last EdU injection, a large population of cells in synovial joint cells including articular chondrocytes was labeled. Six weeks after the last injection, the number of EdU-labeled cells dramatically decreased, but a small number of them were still clearly detectable in synovium (Fig. 1A, yellow arrows), infrapatellar fat pad (Fig. 1A, orange arrows), perichondrium/periosteum (Fig. 1B) and ligament attachment sites (Fig. 1E). In articular cartilage, the labeled cells are dominantly present in the articular surface, but also detectable in the other zones (Fig. 1D). These data suggest that slow-cycling cells are present in articular cartilage surface and the adjacent tissues to articular cartilage such as synovium and infrapatella fat pad. Further investigation is required to define whether these EdU-labeled cells are the progenitors that support renewal of articular cartilage and exert a role for joint tissue repair.
Figure 1.
Slow-cycling cells in synovial joints. The C57BL6/j mice received 4 daily intraperitoneal injections of EdU (5-ethynyl-2'-deoxyuridine, 5 µg/10 µl/ mouse) from postnatal day 4. Scale bars represent 50 µm. Six weeks after the last EdU injection at P7, the knee joints were harvested and subjected to EdU staining. The EdU fluorescence images (green) were superimposed to the phase contrast images. B, Low magnification hematoxyline-eosin staining image of the knee joint. Small number of EdU-labeled cells were detected in the synovium (A, orange arrows) and fat pad (A, yellow arrows), perichondrium/periosteum (C), articular cartilage (D) and ligament attachment site (E). Scale bars represent 50 µm.
2.3 Chondroprogenitors in superficial zone
Several independent research groups including our group have demonstrated that the cells isolated from the superficial zone of postnatal bovine or mouse articular cartilage have progenitor characteristics including high colony formation capacity and expression of mesenchymal stem cell markers and can acquire and express a chondrogenic phenotype after multiple passages (Dowthwaite et al., 2004;Hattori et al., 2007;Yasuhara et al., 2011). Furthermore, presence of stem/progenitor cells in the superficial layer has been reported in human articular cartilage (Muinos-Lopez et al., 2012;Tallheden et al., 2006). These cells respond to transforming growth factor βs (TGFβs) and enhance synthesis of proteoglycan 4 proteins (also called superficial zone proteins or lubricin) and stimulate expression of cartilage matrix such as aggrecan and collagen 2B in micromass culture (Dowthwaite et al., 2004;Hattori et al., 2007). Using a mouse system, we have demonstrated that treatment of Wnt3a maintains expression of Prg4 and Erg in the superficial layer of cells in culture, while ablation of β-catenin strongly impairs proliferation and expression of these genes in the cultured cells and stimulates chondrogenesis in the transplants (Yasuhara et al., 2011). High Mobility Group Proteins 2 (HMGB2) has been shown to be restrictedly expressed in superficial zone and involved in chondrocyte survival and functional maintenance in articular cartilage through the Wnt/β-catenin signaling pathway (Taniguchi et al., 2009;Taniguchi et al., 2009). These findings indicate that TGFβ and Wnt/β-catenin signaling are key regulators of proliferation and differentiation of the superficial cells and may be important for articular cartilage long-term function.
3. Contribution of resident progenitors to articular cartilage repair
3.1 Superficial zone
In osteoarthritic joints, degenerative changes start from cellular disorganization, gradual stiffening and irregular surface of superficial zone followed by loss of matrix, appearance of proliferative chondrocyte clones of fibrillation zones, clefts and osteophyte formation in articular cartilage (Pritzker et al., 2006;Glasson et al., 2010). The superficial zone exhibits reorganization of cell arrangement and cell proliferation at an early osteoarthritis stage (Rolauffs et al., 2011;Rolauffs et al., 2010). Incidence of apoptosis induced by blunt impact, experimental wound or reactive oxygen species (H2O2 treatment) was higher in superficial zone than that in deeper zone in immature or mature articular cartilage in explant culture of bovine articular cartilage (Tew et al., 2000;Gilbert et al., 2009;Khan et al., 2008). When such cell death was inhibited by Necrostatin-1 or a pan-caspase inhibitor, tissue deformation and matrix loss were greatly reduced (Gilbert et al., 2009). These findings strongly indicate that superficial zone is an onset of osteoarthritis and thereby a critical target for prevention of osteoarthritis. As we described above, superficial zone of articular cartilage contains chondroprogenitor cells. Interestingly, Seol et al. (2012) have shown that chondrogenic progenitors have superior migration abilities compared to chondrocytes and migrate to injured sites in response to blunt impact. Thus, chondroprogenitors in superficial zone could play a role in articular cartilage repair. Once articular cartilage is damaged, chondroprogenitors present in the superficial zone may respond to biomechanical and chemical stress and undergo cell death, resulting in poor healing and degeneration of articular cartilage. Further studies are required to clarify how these cells respond to injury, mechanical or chemical stress or inflammation and alter their proliferative and differentiation ability and activity.
3.2 Articular cartilage
Articular cartilage by itself may contain more chondroprogenitor cells than we expected. Several membrane-associated proteins including Notch-1, CD44, CD151, CD105 and CD166 have been selected as biomarkers of mesenchymal progenitor cells in cartilage (Dowthwaite et al., 2004;Grogan et al., 2007;Alsalameh et al., 2004). Interestingly both normal and osteoarthritic articular cartilage contain high number of CD105+/CD166+ cells in the radial (deep) zone, and the cell population containing CD166-positive cells has strong chondrogenic potential (Pretzel et al., 2011). Furthermore, the cells positive to Notch-1, Stro-1, and VCAM-1 -markers of mesenchymal progenitors-have been broadly found throughout normal human articular cartilage from the superficial zone to deep zone and that the number of these cells increased at the middle zone in the non-fibrillated OA (Grogan et al., 2009). The authors emphasize that widely accepted markers may not be applicable for detection of progenitors in articular cartilage. Interestingly the articular cartilage progenitors have a phenotype distinct from bone marrow stromal cells. The former can organize chondrogenic induction maintaining chondrogenicity without calcification or expression of hypertrophic traits in 3D-pellet culture system (Williams R. et al., 2010;McCarthy et al., 2012). The autologous chondrocyte implantation (ACI) (Schindler, 2011;Mastbergen et al., 2013) could be dependent on chondrogenic differentiation of such cell populations as well as proliferation of articular chondrocytes.
3.3 Synovium and infrapatellar fat pad
Recent studies have demonstrated that synovium and infrapatellar fat pad contain mesenchymal stem cells in humans (De Bari et al., 2001;Dragoo et al., 2003;Wickham et al., 2003) as well as in animals (Futami et al., 2012;Koga et al., 2008;Yoshimura et al., 2007;Jones et al., 2008), and that these cells can be utilized for cell-based tissue engineering and treatment for cartilage regeneration (Tuan et al., 2013;Filardo et al., 2013). Interestingly, these cells have superior chondrogenic ability compared to the mesenchymal stem cells derived from other tissues such as bone marrow and muscles (Futami et al., 2012;Yoshimura et al., 2007;Segawa et al., 2009). Mesenchymal stem cells have also been found in synovial fluid in the normal knee joint and their number increase in the affected knee joints (Jones et al., 2008;Morito et al., 2008). The source of these mesenchymal stem cells may be synovium or infrapattelar fat pad because their nature resembles that of the mesenchymal cells isolated from these tissues (Futami et al., 2012;Yoshimura et al., 2007;Segawa et al., 2009). Kurth et al. (Kurth et al., 2011) have demonstrated that slow-cycle cells in the synovium rapidly and strongly respond to the joint injury in an articular cartilage injury model in rabbits. These findings suggest contribution of endogenous progenitors in synovium and infrapaterllar pad to renew and repair articular cartilage.
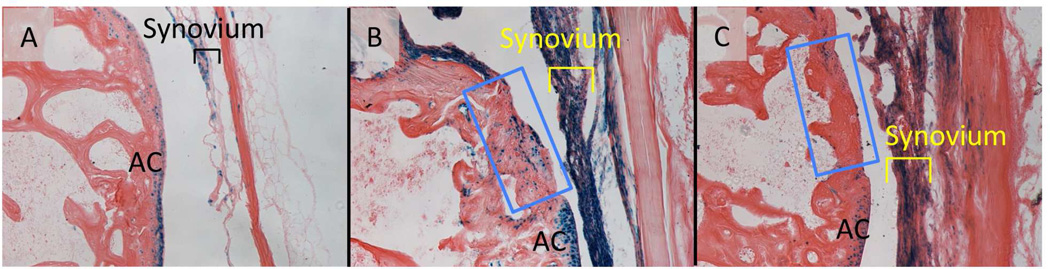
Recent studies have shown that the LacZ-labeled cells are detected in synovial fibroblasts in Col2a1-Cre;ROSA26RLacZ mice and these cells are expanded in the antigen-induced arthritis model of inflammatory arthritis without up-regulation of Cre expression (Fosang et al., 2013). We performed articular injury surgery in the femoral condyle of the knee joint of the GDF5Cre;ROSA26RLacZ mice and chased the LacZ-labeled cells. In the control mouse that had received a skin incision only, the LacZ-labeled cells were found in articular cartilage and synovium (Fig. 2A, AC and Synovium, respectively), suggesting these cells are interzone-derived cells (Koyama et al., 2008). One week after surgery, the synovium was thickened and contained a large number of the LacZ-positive cells (Fig. 2B) while the injured site in articular cartilage had less number of the LacZ-positive cells (Fig. 2B, blue box). Three weeks after surgery, the number of positive cells was decreased in synovium (Fig. 2C, synovium). These results suggest that interzone-derived cells rapidly respond to inflammation and injury in synovium. It is important to clarify whether these interzone-derived cells contain progenitor population and how they participate in articular cartilage repair.
Figure 2.
The LacZ-labeled cells in synovium in the GDF5Cre; ROSA26RLacZ mice after articular cartilage injury. Joint surface injury was made in distal femoral articular groove in GDF5Cre; ROSA26RLacZ mice. The mice were sacrificed 1-week (B) or 3-weeks (C) after the surgery. The injured (B and C) or sham-operated articular cartilage (A) sections were subjected to β-galactosidase. Injured areas were indicated by blue boxes. AC, articular cartilage.
3.4 Bone marrow
Bone marrow is rich in adult hematopoietic stem cells and mesenchymal stem cells (Li et al., 2005). The surgical procedures for articular cartilage repair such as transcortical Pridie drilling, abrasion arthroplasty and microfracture (Schindler, 2011) largely rely on mesenchymal stem cells and growth factors coming from bone marrow. It has been demonstrated that chondrogenic progenitors migrate to damaged articular cartilage from the bone marrow cavity underneath the subchondral bone and have stem cell characteristics (Koelling et al., 2009). It is not clear whether these cells contribute to intrinsic repair of damaged articular cartilage. On the other hand, mesenchymal stem cells in bone marrow may impair homeostasis of articular cartilage receiving TGF-β signaling. Zhen et al. (2013) have shown that TGFβ signaling activates nestin-positive mesenchymal stem cells to make clusters and that inactivation of this signaling pathway attenuates osteoarthritic changes in articular cartilage. It is suggested that bone marrow stromal cells can be utilized for osteoarthritis and articular cartilage regeneration, but inadvertent stimulation of these cells could worsen degeneration of articular cartilage.
4. Concluding remarks
Chondroprogenitors are present in articular cartilage, especially in the superficial zone and its neighboring tissues including synovium, fad pad and perichondrium (Ranvier groove). At this moment, we do not have direct evidence that these progenitors support self-renewal and healing of articular cartilage. They may do so in some cases, but not always. Obviously resident chondroprogenitors are attractive therapeutic targets to develop drugs and cell-based tissue engineering for articular cartilage repair. How can we stimulate their potential and increase their life span? The candidate signaling pathways that regulate resident mesenchymal (connective tissue) progenitors include TGFβ signaling (Hattori et al., 2007;Embree et al., 2010) and Wnt/β-catenin signaling (Yasuhara et al., 2011;Taniguchi et al., 2009;Taniguchi et al., 2009;Yuasa et al., 2009). Recently an axis of CBFβ-Runx1 is highlighted for articular cartilage repair (Johnson et al., 2012). In addition, EGFR signaling might be involved in regulation of proliferation of progenitors in articular cartilage because deficiency of Mig-6, a negative regulator of EGFR signaling increases the number of cells that express putative progenitor cell markers in articular cartilage (Shepard et al., 2013). However, we should keep in mind that these signaling pathways also do induce inhibition of chondrogenic differentiation and/or catabolic actions of cartilage matrix (Zhen et al., 2013;Yuasa et al., 2008;Enomoto-Iwamoto et al., 2002;Zhang et al., 2013;Zhang et al., 2011). Thus stimulation of resident chondroprogenitors requires special cautions. If chondroprogenitors proceed toward differentiation of transient cartilage or fail or stop to express chondrogenic phenotype, it would induce ectopic bone formation or fibrous tissue formation, leading to further degeneration of articular cartilage and deformity of joints. We should continue investigating the mechanisms that support survival and maintenance of these cells and carefully consider how to modulate their proliferation, migration and differentiation regarding level, location, time and/or duration.
Acknowledgements
We thank Ms. Leslie Cantley for editorial assistance and continuous help. This work was supported by the grants form National Institute of Health (Grant numbers AR46000 and AG025868), Internal fund of Children’s Hospital of Philadelphia, and Grants-in-Aid for Scientific Research and Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS) (Grant Number 24791983).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schindler OS. Current concepts of articular cartilage repair. Acta Orthop Belg. 2011;77(6):709–726. [PubMed] [Google Scholar]
- 2.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 3.Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012;93(6):389–400. doi: 10.1111/j.1365-2613.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21(5):303–311. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastides P, Chimutengwende-Gordon M, Maffulli N, Khan W. Stem cell therapy for human cartilage defects: a systematic review. Osteoarthritis Cartilage. 2013;21(5):646–654. doi: 10.1016/j.joca.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Koga H, Shimaya M, Muneta T, Nimura A, Morito T, Hayashi M, Suzuki S, Ju YJ, Mochizuki T, Sekiya I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10(4):R84. doi: 10.1186/ar2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, Kon E. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1717–1729. doi: 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim YS, Park EH, Kim YC, Koh YG. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090–1099. doi: 10.1177/0363546513479018. [DOI] [PubMed] [Google Scholar]
- 9.Williams JA, Kane M, Okabe T, Enomoto-Iwamoto M, Napoli JL, Pacifici M, Iwamoto M. Endogenous retinoids in mammalian growth plate cartilage: analysis and roles in matrix homeostasis and turnover. J Biol Chem. 2010;285(47):36674–36681. doi: 10.1074/jbc.M110.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardale RJ, Duance VC. Characterisation of articular and growth plate cartilage collagens in porcine osteochondrosis. J Cell Sci. 1994;107(Pt 1):47–59. doi: 10.1242/jcs.107.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Cohen EK, Kressel HY, Frank TS, Fallon M, Burk DJ, Dalinka MK, Schiebler ML. Hyaline cartilage-origin bone and soft-tissue neoplasms: MR appearance and histologic correlation. Radiology. 1988;167(2):477–481. doi: 10.1148/radiology.167.2.3162774. [DOI] [PubMed] [Google Scholar]
- 12.Khan IM, Redman SN, Williams R, Dowthwaite GP, Oldfield SF, Archer CW. The development of synovial joints. Curr Top Dev Biol. 2007;79:1–36. doi: 10.1016/S0070-2153(06)79001-9. [DOI] [PubMed] [Google Scholar]
- 13.Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann N Y Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69(2):144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 15.Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto-Iwamoto M, Pacifici M. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2(11):e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyde G, Dover S, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304(2):825–833. doi: 10.1016/j.ydbio.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15(4):403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Las Heras F, Gahunia HK, Pritzker KP. Articular cartilage development: a molecular perspective. Orthop Clin North Am. 2012;43(2):155–171. v. doi: 10.1016/j.ocl.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190(4):305–315. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Blumer MJ, Longato S, Schwarzer C, Fritsch H. Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes. Dev Dyn. 2007;236(8):2077–2088. doi: 10.1002/dvdy.21228. [DOI] [PubMed] [Google Scholar]
- 22.Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16(6):617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 23.Poole AR. What type of cartilage repair are we attempting to attain? J Bone Joint Surg Am. 2003;85-A(Suppl 2):40–44. doi: 10.2106/00004623-200300002-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci U S A. 1992;89(20):9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203(6):469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson C, Thornemo M, Henriksson HB, Lindahl A. Identification of a stem cell niche in the zone of Ranvier within the knee joint. J Anat. 2009;215(3):355–363. doi: 10.1111/j.1469-7580.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 28.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocytes stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91(12):1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muinos-Lopez E, Rendal-Vazquez ME, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Cryopreservation effect on proliferative and chondrogenic potential of human chondrocytes isolated from superficial and deep cartilage. Open Orthop J. 2012;6:150–159. doi: 10.2174/1874325001206010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallheden T, Brittberg M, Peterson L, Lindahl A. Human articular chondrocytes--plasticity and differentiation potential. Cells Tissues Organs. 2006;184(2):55–67. doi: 10.1159/000098947. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, Lotz M. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106(4):1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi N, Carames B, Kawakami Y, Amendt BA, Komiya S, Lotz M. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc Natl Acad Sci U S A. 2009;106(39):16817–16822. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Rolauffs B, Rothdiener M, Bahrs C, Badke A, Weise K, Kuettner KE, Kurz B, Aurich M, Grodzinsky AJ, Aicher WK. Onset of preclinical osteoarthritis: the angular spatial organization permits early diagnosis. Arthritis Rheum. 2011;63(6):1637–1647. doi: 10.1002/art.30217. [DOI] [PubMed] [Google Scholar]
- 37.Rolauffs B, Williams JM, Aurich M, Grodzinsky AJ, Kuettner KE, Cole AA. Proliferative remodeling of the spatial organization of human superficial chondrocytes distant from focal early osteoarthritis. Arthritis Rheum. 2010;62(2):489–498. doi: 10.1002/art.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43(1):215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert SJ, Singhrao SK, Khan IM, Gonzalez LG, Thomson BM, Burdon D, Duance VC, Archer CW. Enhanced tissue integration during cartilage repair in vitro can be achieved by inhibiting chondrocyte death at the wound edge. Tissue Eng Part A. 2009;15(7):1739–1749. doi: 10.1089/ten.tea.2008.0361. [DOI] [PubMed] [Google Scholar]
- 40.Khan IM, Gilbert SJ, Caterson B, Sandell LJ, Archer CW. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(−) in adult bovine articular cartilage. Osteoarthritis Cartilage. 2008;16(6):698–707. doi: 10.1016/j.joca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA, Martin JA. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64(11):3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grogan SP, Barbero A, Diaz-Romero J, Cleton-Jansen AM, Soeder S, Whiteside R, Hogendoorn PC, Farhadi J, Aigner T, Martin I, Mainil-Varlet P. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007;56(2):586–595. doi: 10.1002/art.22408. [DOI] [PubMed] [Google Scholar]
- 43.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 44.Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13(2):R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, Lewis H, Roberts S, Shaw HM, Dudhia J, Fairclough J, Briggs T, Archer CW. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE. 2010;5(10):e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192(3):345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 48.Mastbergen SC, Saris DB, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9(5):277–290. doi: 10.1038/nrrheum.2013.29. [DOI] [PubMed] [Google Scholar]
- 49.De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 50.Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, Hedrick MH, Benhaim P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85(5):740–747. [PubMed] [Google Scholar]
- 51.Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 52.Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE. 2012;7(9):e45517. doi: 10.1371/journal.pone.0045517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333(2):207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 55.Jones EA, Crawford A, English A, Henshaw K, Mundy J, Corscadden D, Chapman T, Emery P, Hatton P, McGonagle D. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58(6):1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
- 56.Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27(4):435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 57.Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology (Oxford) 2008;47(8):1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 58.Kurth TB, Dell'accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63(5):1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 59.Fosang AJ, Golub SB, East CJ, Rogerson FM. Abundant LacZ activity in the absence of Cre expression in the normal and inflamed synovium of adult Col2a1-Cre; ROSA26RLacZ reporter mice. Osteoarthritis Cartilage. 2013;21(2):401–404. doi: 10.1016/j.joca.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 61.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Embree MC, Kilts TM, Ono M, Inkson CA, Syed-Picard F, Karsdal MA, Oldberg A, Bi Y, Young MF. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176(2):812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175(5):1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 66.Shepard JB, Jeong JW, Maihle NJ, O'Brien S, Dealy CN. Transient anabolic effects accompany epidermal growth factor receptor signal activation in articular cartilage in vivo. Arthritis Res Ther. 2013;15(3):R60. doi: 10.1186/ar4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 68.Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol. 2002;251(1):142–156. doi: 10.1006/dbio.2002.0802. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Zhu J, Li Y, Lin T, Siclari VA, Chandra A, Elena CM, Koyama E, Enomoto-Iwamoto M, Qin L. Epidermal growth factor receptor (EGFR) signaling regulates epiphyseal cartilage development through beta-catenin-dependent and - independent pathways. J Biol Chem. 2013 doi: 10.1074/jbc.M113.463554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Siclari VA, Lan S, Zhu J, Koyama E, Dupuis HL, Enomoto-Iwamoto M, Beier F, Qin L. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res. 2011;26(11):2622–2633. doi: 10.1002/jbmr.502. [DOI] [PMC free article] [PubMed] [Google Scholar]