Abstract
Background
Insufficient sleep increases the risk for insulin resistance, type 2 diabetes, and obesity, suggesting that sleep restriction may impair peripheral metabolic pathways. Yet, a direct link between sleep restriction and alterations in molecular metabolic pathways in any peripheral human tissue has not been shown.
Objective
To determine whether sleep restriction results in reduced insulin sensitivity in subcutaneous fat, a peripheral tissue that plays a pivotal role in energy metabolism and balance.
Design
Randomized, 2-period, 2-condition, crossover clinical study.
Setting
University of Chicago Clinical Resource Center.
Participants
Seven healthy adults (1 woman, 6 men) with a mean age of 23.7 years (SD, 3.8) and mean body mass index of 22.8 kg/m2 (SD, 1.6).
Intervention
Four days of 4.5 hours in bed or 8.5 hours in bed under controlled conditions of caloric intake and physical activity.
Measurements
Adipocytes collected from subcutaneous fat biopsy samples after normal and restricted sleep conditions were exposed to incremental insulin concentrations. The ability of insulin to increase levels of phosphorylated Akt (pAkt), a crucial step in the insulin-signaling pathway, was assessed. Total Akt (tAkt) served as a loading control. The insulin concentration for the half-maximal stimulation of the pAkt–tAkt ratio was used as a measure of cellular insulin sensitivity. Total body insulin sensitivity was assessed using a frequently sampled intravenous glucose tolerance test.
Results
The insulin concentration for the half-maximal pAkt–tAkt response was nearly 3-fold higher (mean, 0.71 nM [SD, 0.27] vs. 0.24 nM [SD, 0.24]; P = 0.01; mean difference, 0.47 nM [SD, 0.33]; P = 0.01), and the total area under the receiver-operating characteristic curve of the pAkt–tAkt response was 30% lower (P = 0.01) during sleep restriction than during normal sleep. A reduction in total body insulin sensitivity (P = 0.02) paralleled this impaired cellular insulin sensitivity.
Limitation
This was a single-center study with a small sample size.
Conclusion
Sleep restriction results in an insulin-resistant state in human adipocytes. Sleep may be an important regulator of energy metabolism in peripheral tissues.
Primary Funding Source
National Institutes of Health.
Humans spend up to one third of their lives asleep, yet the function of sleep remains a topic of intense debate. Although evidence supports a role for sleep in learning, memory, and other central nervous system functions (1–3), prospective epidemiologic studies have found that insufficient sleep may increase the risk for metabolic disturbances, including insulin resistance, obesity, and type 2 diabetes (4–10). Consistent with the epidemiologic evidence, well-controlled laboratory studies in healthy adults have shown that repeated partial sleep restriction has adverse effects on systemic insulin sensitivity and glucose tolerance, further supporting the hypothesis that sleep may be important for peripheral metabolism (11–17).
To our knowledge, no studies to date have linked sleep restriction to alterations in molecular metabolic pathways in any peripheral human tissue. In our study, we tested the hypothesis that experimental sleep restriction in young, healthy adults results in reduced insulin sensitivity in subcutaneous fat, a peripheral tissue that is a key site of insulin action and plays a pivotal role in energy metabolism, as well as in the communication of energy balance to the brain (18, 19). To test this hypothesis, we assessed the ability of insulin to increase the phosphorylation of Akt, a crucial early step in the insulin-signaling pathway, in adipocytes collected from subcutaneous fat biopsies after normal and restricted sleep conditions.
Methods
Participants were tested under 2 experimental conditions in randomized order—after 4 consecutive nights of 8.5 hours in bed (normal sleep) and 4 consecutive nights of 4.5 hours in bed (sleep restriction). At the end of both conditions, biopsy samples of abdominal subcutaneous adipose tissue were collected. Insulin sensitivity in adipocytes was determined in vitro by measuring relative levels of phosphorylated Akt (pAkt), and total body insulin sensitivity was derived from frequently sampled intravenous glucose tolerance tests (FSIVGTTs).
Participants
Healthy, lean men and women aged 18 to 30 years were recruited from the community in response to advertisements. Exclusion criteria were a history of any chronic medical condition, any acute illness, shift work, travel across time zones during the past 4 weeks, depressed mood (as assessed by a score on the Center for Epidemiologic Studies of Depression Scale >16), use of any prescription or over-the-counter medications or supplements known to affect sleep or glucose metabolism, current smoking, substantial consumption of alcohol (>2 drinks per day) or caffeine (>300 mg per day), or abnormal findings on physical examination or routine laboratory testing. All participants had an overnight laboratory polysomnography to exclude sleep disorders, as well as a standard 75-g oral glucose tolerance test and fasting blood sample collection for routine laboratory tests, including complete blood counts, a comprehensive metabolic panel, thyroid function tests, a lipid panel, and hemoglobin A1c measurement. A 12-lead electrocardiogram was also obtained. Healthy participants who had normal glucose tolerance and no current or previous sleep disorders were included. All participants had regular self-reported nocturnal time in bed of 7.5 to 8.5 hours.
Experimental Protocol
The Institutional Review Board of the University of Chicago approved the protocol, and written informed consent was obtained from each participant. Each participant was tested under 2 conditions at the University of Chicago Clinical Resource Center in randomized order spaced at least 4 weeks apart. During the week preceding both conditions, the participants were asked to maintain a standardized schedule of bedtimes and meal times in accordance with their usual habits. They were asked not to deviate from this schedule by more than 30 minutes. Naps were not allowed. Wrist activity was monitored continuously by using Actiwatch (Philips/Respironics, Andover, Massachusetts) to verify adherence to standardized bedtime schedules.
Both conditions involved 4 consecutive inpatient days with either 8.5 hours in bed (11:00 p.m. to 7:30 a.m., or normal sleep) or 4.5 hours in bed (1:00 a.m. to 5:30 a.m., or sleep restriction). Three of the 7 participants were tested under the sleep restriction condition first, and the remaining 4 participants began with the normal sleep condition. Random assignments (ordering of the sleep conditions) were made using computer-generated random numbers and concealed with sealed envelopes. All participants were under sedentary laboratory conditions during waking hours.
Sleep was recorded by polysomnography (Neurofax EEG-1100A, Nihon Kohden, Foothill Ranch, California) each night. The recordings were visually scored in 30-second epochs as rapid eye movement (REM) sleep, non-REM sleep, and wake according to standard criteria (20).
Caloric intake was strictly controlled and identical under the 2 sleep conditions. Participants were not allowed to consume any foods or beverages that were not provided by the metabolic kitchen. The study diet consisted of a 2-day cycle menu of 3 isocaloric meals per day. Each meal was consumed in its entirety within 20 minutes. No caffeinated beverages were allowed. A registered dietitian in the University of Chicago Clinical Resource Center Metabolic Kitchen supervised the preparation of all meals.
At 10:00 a.m. on the fourth day of both conditions, FSIVGTTs were performed after an overnight fast to estimate total body insulin sensitivity by using the Bergman Minimal Model (21). After completion of these tests, a sample of subcutaneous abdominal fat was collected by needle biopsy.
Biopsy of Subcutaneous Abdominal Fat
Subcutaneous abdominal fat biopsies were performed after the participants received detailed information on the procedure. Under both study conditions, tissue biopsy was performed within a 9-cm radius of the umbilicus. The second biopsy was performed at the opposite side of the first biopsy (also within a 9-cm radius of the umbilicus) to obviate the potential effect of local inflammation on insulin sensitivity.
The area was prepared with povidone-iodine solution, air-dried, and cleaned with isopropyl alcohol. Local anesthesia was obtained by using 1% plain lidocaine. A 0.5-cm incision was made parallel to the participants’ waistlines through the dermis. Sixty-mL syringes containing 10 mL of saline buffer were used to create suction to aspirate adipose tissue by using a 13-gauge, 3-inch hypodermic needle. Up to 6 syringes were used per abdominal site.
Steri-Strips (3M, St. Paul, Minnesota) were used to close the wound site, and gauze pads were secured using Elastoplast (Beiersdorf, Hamburg, Germany). Direct pressure was applied for 20 minutes, then ice packs were applied for 4 hours. The procedure was well-tolerated by all participants, and no side effects or complications occurred. None of the participants declined the second biopsy.
Insulin-Signaling Assay
Collected tissue samples were washed with DMEM+ (Dulbecco’s Modified Eagle Medium, which contains 5 mM of glucose, 10 mM of 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid at a pH of 7.4, sodium bicarbonate, 10 mM of phenylisopropyl adenosine and 2% bovine serum albumin [Life Technologies, Grand Island, New York]). Samples were incubated with 3.5 mL of DMEM+ and 1 mg of type II collagenase per 1 mL of adipose tissue for 20 to 30 minutes at 37 °C with periodic swirling to remove any connective tissue. Total processing time was routinely less than 1 hour from performance of the needle biopsy.
Adipocytes were rinsed with DMEM+ and stimulated in duplicate with porcine insulin (Sigma-Aldrich, St. Louis, Missouri) of 8 increasing concentrations (0.00, 0.10, 0.25, 0.50, 0.75, 1.00, 5.00, and 10.00 nM) for 10 minutes at 37 °C. Cells were rinsed with DMEM+ (lacking glucose and bovine serum albumin). An equal volume of 2X Laemmli buffer was added, and samples were vortexed, boiled for 2 minutes, and centrifuged to pellet nuclei and to form a consolidated lipid layer at the top of the tube.
Infranatants containing cellular proteins were carefully removed and transferred to new tubes and frozen at −80 °C. Lysates collected from the same participant under both sleep conditions were simultaneously separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred to a nitrocellulose membrane. Membranes containing all samples from each participant were blocked in TBST+ (Tris-buffered saline, containing 0.1% Tween [Sigma-Aldrich], and 5% nonfat dried milk) and immunoblotted in the same container by using a 1:1000 dilution of Ser473 rabbit polyclonal anti-pAkt antibody (Cell Signaling Technology, Danvers, Massachusetts) overnight at 4 °C.
The nitrocellulose membranes were extensively washed and then were incubated with horseradish peroxide–conjugated goat anti-rabbit antibody (Bio-Rad Laboratories, Hercules, California) in TBST+ for 30 minutes at room temperature. After membranes were washed with TBST+, antibody binding was visualized using the Pierce chemiluminescent Western blotting detection system (Thermo Fisher Scientific, Rockford, Illinois) and identical exposure times. Membranes were then stripped and reprobed with rabbit polyclonal anti-tAkt antibody (Cell Signaling Technology).
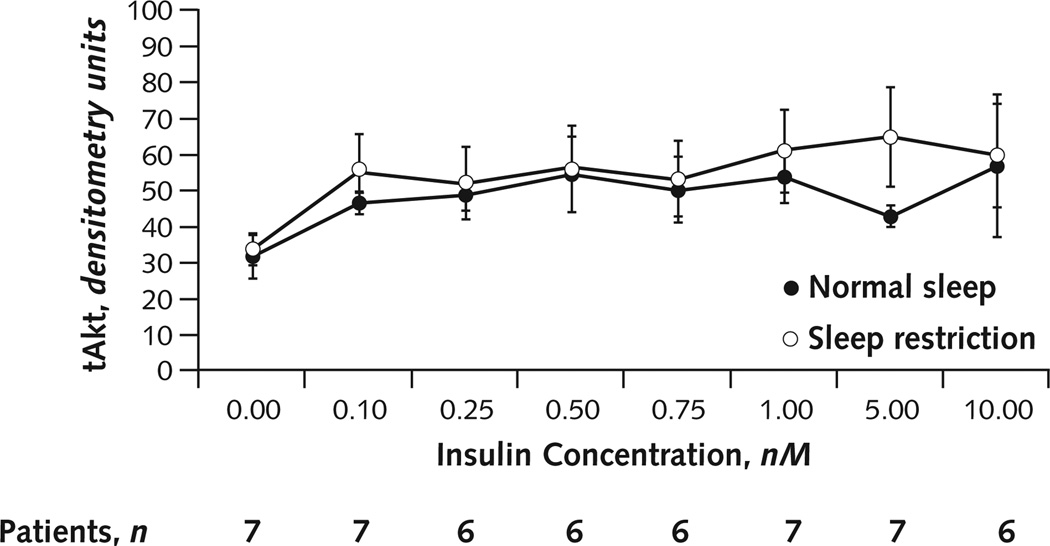
Densitometry was performed for immunoblots by using Image J, version 1.44 (National Institutes of Health, Bethesda, Maryland) to quantitate the densities of the pAkt and tAkt bands for each insulin concentration used. Total Akt levels were used as a protein-loading control to normalize for any variation in protein concentration between the samples, as in a previous study of insulin signaling in primary human adipocytes (22). Indeed, 10 minutes of insulin exposure did not change tAkt levels (Appendix Figure, available at www.annals.org).
Appendix Figure. tAkt response across all insulin concentrations.
Average densitometric quantification of the tAkt densities across all insulin concentrations under normal sleep and sleep restriction. Because of insufficient volume of biopsy samples, insulin concentrations of 0.25, 0.50, and 0.75 nM (under sleep restriction) and 0.50 nM (under normal sleep) in participant 6 and 10.00 nM (under normal sleep) in participant 3 were omitted. Because the volume of fat biopsy from our lean participants was limited, we first filled the sample tubes that were going to be exposed to the nonzero insulin concentrations (0.10–10.00 nM). The sample exposed to zero insulin concentration therefore tended to have a lower cell volume, resulting in a lower tAkt value than that of all nonzero concentrations. At the zero insulin concentrations, 10 of the 14 sample tubes had 0 pAkt values. No significant changes occurred in tAkt levels across all 8 insulin concentrations for either sleep condition (analysis of variance for repeated measures with insulin concentration as factor: normal sleep, P = 0.32; sleep restriction, P = 0.23; Greenhouse–Geisser adjusted F test). In contrast, pAkt was significantly increased with increasing insulin concentrations under both sleep conditions (normal sleep, P = 0.038; restricted sleep, P = 0.014; Greenhouse–Geisser adjusted F test). Differences between sleep conditions in mean tAkt levels (across all insulin concentrations, including the zero concentration) were also nonsignificant (tAkt level, 47.7 [SD, 14.3] during normal sleep vs. 54.7 [SD, 23.6] during restricted sleep; mean difference, +6.96 [SD, 10.9]; P = 0.14. Error bars are SEs of the mean. pAkt = phosphorylated Akt; tAkt = total Akt.
The insulin effect was then determined by dividing the density of the pAkt value (insulin effect) by the tAkt value (loading control). An increase in the pAkt–tAkt ratio indicates a greater cellular response for a given dose of insulin, whereas a decrease indicates a lower sensitivity of the cells to insulin. The pAkt and tAkt measurements do not use the same antibody, and the pAkt antibody produces a stronger signal than the tAkt antibody. Thus, the pAkt–tAkt ratio can be greater than 100%.
The volume of fat biopsy from these lean participants was limited. Thus, the sample tubes that were going to be exposed to the nonzero insulin concentrations (0.1 to 10 nM) were filled first because in the absence of insulin, pAkt levels were very low (undetectable in 10 of the 14 samples tested). The sample exposed to zero insulin concentration therefore tended to have a lower tAkt value than the samples exposed to all nonzero concentrations.
In the leanest participant of the group (participant 6; body mass index, 20.7 kg/m2), the volume of adipose tissue that could be collected was insufficient to allow for testing of all 8 insulin concentrations under both sleep conditions. In this participant, under the normal sleep condition, the adipocytes were exposed to 5 rather than 8 insulin concentrations (omitting stimulations with 0.25, 0.50, and 0.75 nM); under the sleep restriction condition, they were exposed to 7 rather than 8 concentrations (omitting 0.50 nM). In 1 other participant (participant 3), stimulation with maximum insulin concentration (10.00 nM) under the normal sleep condition was not possible because of insufficient volume of the biopsy sample. In total, the pAkt–tAkt ratio could thus be estimated for 107 of the 112 determinations specified by our protocol (7 participants × 2 sleep conditions × 8 insulin concentrations).
FSIVGTT
The FSIVGTT was used to estimate total body insulin sensitivity (21). This test has been used to show an adverse effect of sleep disturbances on human glucose metabolism and insulin sensitivity in multiple previous reports (12, 15,17, 23, 24). In 1 study that used both the FSIVGTT and the euglycemic hyperinsulinemic clamp to assess changes in insulin sensitivity after experimental sleep restriction in healthy participants, both methods provided similar results (12). Starting at 10:00 a.m. after an overnight fast, 1-mL blood samples were drawn every 5 minutes for 15 minutes (3 baseline samples), at which time glucose was administered as an intravenous bolus (0.3 g/kg of body weight). Blood samples were then taken at 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 21, 22, 24, 26, 28, 30, 40, 50, 60, 70, 90, 100, 120, 140, 180, 210, and 240 min. At 20 minutes, intravenous insulin (0.02 U/kg of body weight) was administered.
In participants 3, 5, and 7, technical difficulties invalidated the estimation of total body insulin sensitivity from the FSIVGTT under the normal sleep condition and these tests were repeated on a separate day under similar experimental conditions. Participants 3 and 7 had 1 night and participant 5 had 2 nights of laboratory polysomnography before the repeated FSIVGTT. Normal sleep duration was verified by actigraphy before the repeated sessions.
Statistical Analysis
Group values are expressed as means (SDs). All comparisons were performed by using the paired t test, and the mean (SD) difference between the 2 conditions was calculated. The insulin concentration corresponding to the half-maximal pAkt–tAkt response (identified as the first measured insulin concentration that had a pAkt–tAkt ratio greater than the half-maximal response) was used as a measure of cellular insulin sensitivity. In addition, the total area under the receiver-operating characteristic curve (AUC) of the pAkt–tAkt ratio between the lowest insulin dose (0.00 nM) and the maximum insulin dose (10.00 nM) was calculated in each participant for both sleep conditions by using the trapezoidal method as previously described (25). Statistical analyses were conducted using JMP statistical software, version 9.0.2 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
The National Institutes of Health provided funding for the study. The funding source had no role in the design, conduct, or reporting of this study or in the decision to submit the manuscript for publication.
Results
Six men and 1 woman participated in the study. Mean age was 23.7 years (SD, 3.8). Mean body mass index was 22.8 kg/m2 (SD, 1.6). Figure 1 shows individual values for age, body mass index, and percentage of body fat (determined by an impedance technique). No significant changes occurred in body weight (mean body weight, 72.6 kg [SD, 10.5] under the normal sleep condition vs. 72.4 kg [SD, 9.8] under the sleep restriction condition; mean difference, 0.3 kg [SD, 1.5]; P = 0.67) or percentage of body fat (mean percentage of body fat, 22.3% [SD, 5.5%]; vs. 21.8% [SD, 5.0%]; mean difference, 0.5% [SD, 2.9%]; P = 0.66) across the entire protocol.
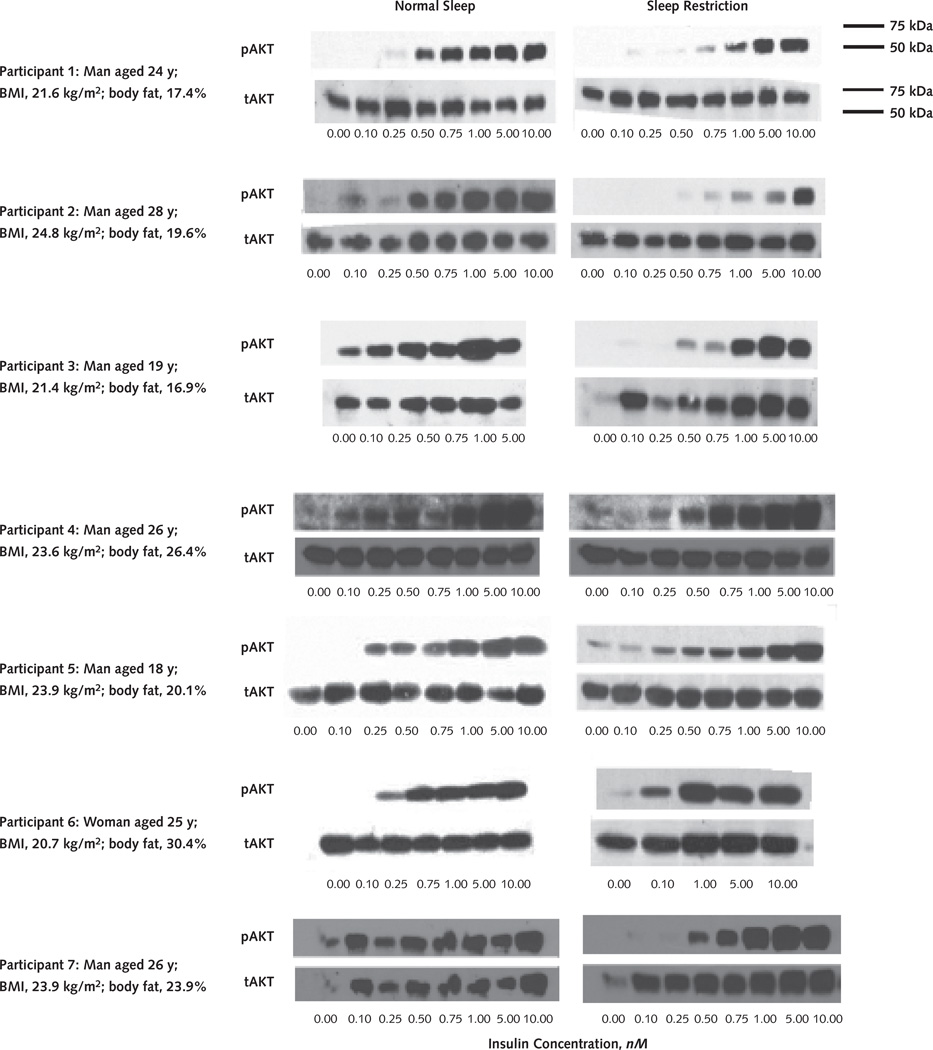
Figure 1. Insulin signaling in the adipocytes.
Individual Western blot tests show anti-pAkt and anti-tAkt responses to incremental increases in insulin concentrations in adipocytes under normal sleep and sleep restriction. Subcutaneous fat biopsies were performed on the same participants after normal sleep or sleep restriction conditions. Adipocytes were isolated by collagenase digestion, and isolated adipocytes were incubated in duplicate with increasing concentrations of insulin (0.00–10.00 nM). Samples were then frozen. All samples from the same participant from both sleep conditions were analyzed simultaneously by pAkt immunoblotting using identical exposure times. The same membranes were then stripped and reprobed with tAkt antibodies. The samples were not run on the same gel because of size constraints, but the 2 gels were transferred at the same time and the 2 membranes were processed in the same container. Molecular weight markers are shown on the upper right. Individual values for sex, age, BMI, and percentage of body fat (determined by an impedance technique) are provided for each participant. BMI = body mass index; pAkt = phosphorylated Akt; tAkt = total Akt.
Polysomnography
Over the 4 nights, participants slept an average of 7.87 hours (SD, 0.31) under the normal sleep condition versus 4.35 hours (SD, 0.12) under the sleep restriction condition (mean difference, 3.53 hours [SD, 0.24]; P < 0.001). Overall, the amount of REM sleep was reduced by 56.8% (SD, 9.5%) under the sleep restriction compared with normal sleep (51 min [SD, 16] vs. 117 min [SD, 21]; mean difference, −66 min; P < 0.001), whereas no change occurred in slow-wave sleep (99 min [SD, 25] vs. 102 min [SD, 31]; mean difference, −3 min; P = 0.39). The Appendix Table (available at www.annals.org) lists the mean total sleep time and the amounts of non-REM (stages N1, N2, and slow-wave sleep) and REM sleep for each day of each condition.
Appendix Table.
Sleep Stages Under Normal Sleep and Sleep Restriction
| Variable | Night 1 | Night 2 | Night 3 | Night 4 |
|---|---|---|---|---|
| Normal sleep | ||||
| Mean total sleep time (SD), min | 469.3 (23.7) | 480.9 (15.2) | 466.6 (29.2) | 472.7 (16.2) |
| Mean N1 (SD), min | 16.5 (7.1) | 15.0 (4.7) | 24.6 (15.1) | 15.9 (4.1) |
| Mean N2 (SD), min | 231.8 (23.9) | 240.1 (22.0) | 236.4 (23.1) | 232.1 (29.3) |
| Mean slow-wave sleep (SD), min | 103.5 (36.2) | 105.9 (30.8) | 94.0 (36.6) | 105.1 (26.9) |
| Mean REM sleep (SD), min | 117.6 (22.0) | 119.9 (21.5) | 111.6 (27.4) | 119.5 (19.1) |
| Sleep restriction | ||||
| Mean total sleep time (SD), min | 263.2 (11.3) | 258.5 (13.1) | 260.1 (9.9) | 261.5 (6.7) |
| Mean N1 (SD), min | 5.9 (2.4) | 4.9 (2.7) | 4.4 (2.1) | 3.8 (1.2) |
| Mean N2 (SD), min | 119.4 (20.7) | 101.8 (21.4) | 100.8 (22.4) | 103.0 (16.4) |
| Mean slow-wave sleep (SD), min | 91.5 (33.3) | 102.4 (18.6) | 92.6 (28.9) | 108.8 (24.2) |
| Mean REM sleep (SD), min | 46.6 (14.3) | 49.3 (22.8) | 62.2 (23.7) | 45.9 (13.1) |
N1 = stage 1 non-REM sleep; N2 = stage 2 non-REM sleep; REM = rapid eye movement.
Relative to normal sleep, cumulative sleep loss for 4 nights of sleep restriction amounted to 14.10 hours (SD, 0.97). Over the 4 nights of sleep restriction, the cumulative loss of REM sleep was 4.41 hours (SD, 0.90) and that of slow-wave sleep was 0.22 hours (SD, 0.63).
Cellular Insulin Sensitivity in Adipocytes
Figure 1 demonstrates insulin signaling in adipocytes by Western blot analysis, showing the pAkt response to incremental increases in insulin concentrations in adipocytes from each participant under normal sleep and sleep restriction. After normal sleep, insulin caused a dosedependent increase in pAkt levels that varied across participants (Figure 1, left column). Sleep restriction consistently induced a marked reduction in the pAkt response to insulin (Figure 1, right column). In contrast, tAkt levels were essentially constant across all insulin concentrations and sleep conditions and thus served as a loading control.
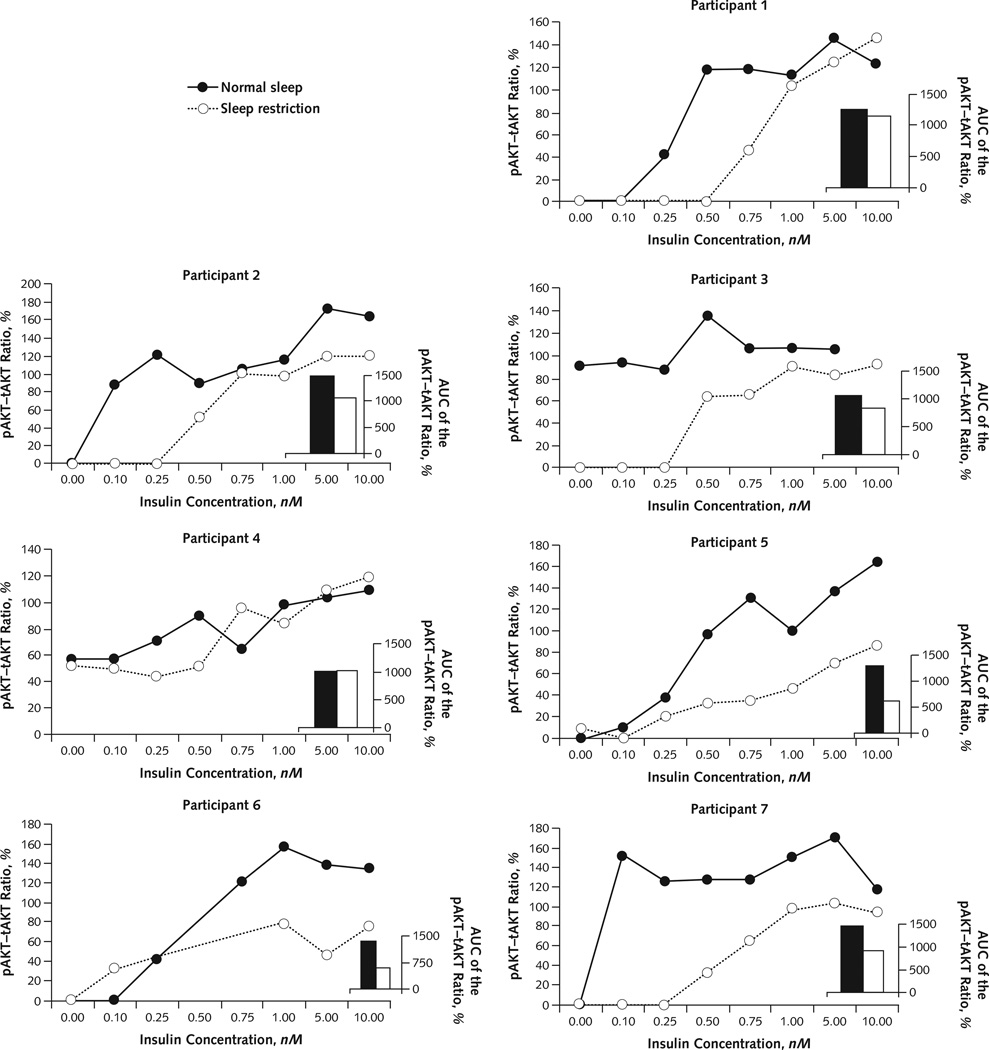
Figure 2 shows densitometric quantification of the dose-dependent responses of the pAkt–tAkt ratio to insulin stimulation in each participant under both sleep conditions. In all but 1 participant, the insulin-dependent response curve was shifted to the right or the maximal pAkt–tAkt ratio was decreased after sleep restriction compared with normal sleep. In contrast, tAkt levels did not change across study conditions or insulin concentrations (Appendix Figure).
Figure 2. Densitometric quantification of pAkt–tAkt response.
Individual densitometric quantification of the dose-dependent responses of the pAkt–tAkt ratio to insulin stimulation in each participant under normal sleep and sleep restriction. Because of insufficient volume of biopsy samples, insulin concentrations of 0.25, 0.50, and 0.75 nM (under sleep restriction) and 0.50 nM (under normal sleep) in participant 6 and 10.00 nM (under normal sleep) in participant 3 were omitted. At an insulin concentration of 0.00 nM in participants 1, 2, 6, and 7 and 0.10 nM in participant 1, there was no measurable pAkt activation under either sleep condition; therefore, the data shown are superimposed. Note that pAkt and tAkt are measured using different antibodies; thus, the pAkt–tAkt ratio can vary outside of the range of 0% to 100%. AUC = area under the receiver-operating characteristic curve; pAkt = phosphorylated Akt; tAkt = total Akt.
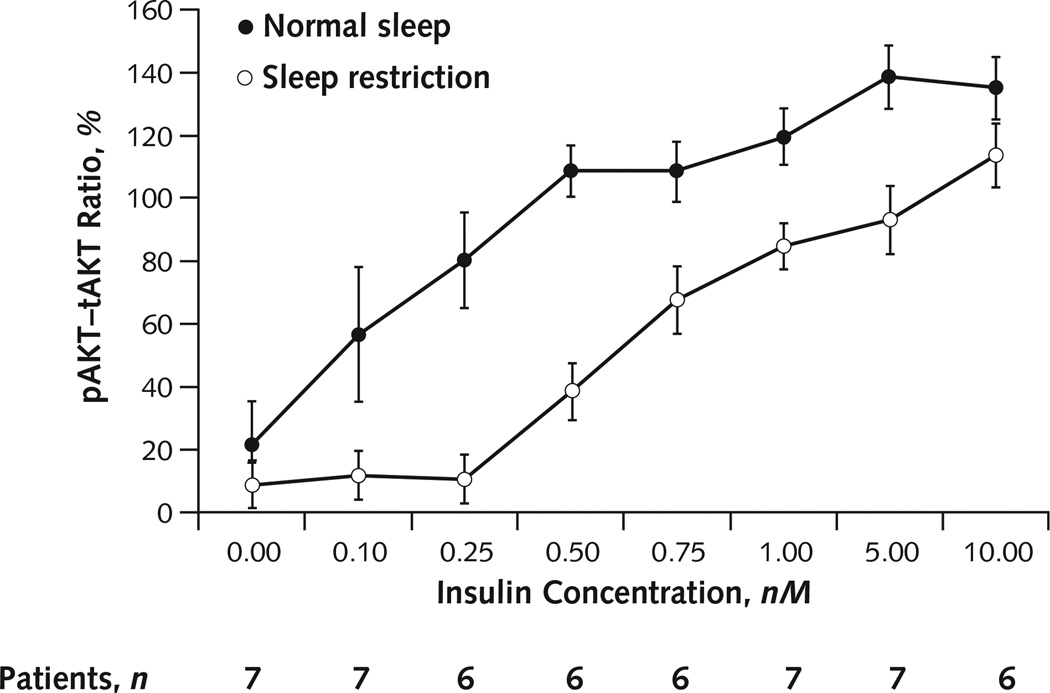
Figure 3 shows mean response curves for the pAkt–tAkt ratio versus insulin concentration. The total AUC of the pAkt–tAkt response to insulin was 30% lower after sleep restriction than after normal sleep (mean AUC of the pAkt–tAkt response to insulin, 893.00 [SD, 208.00] vs. 1280.00 [SD, 190.00]; mean difference, −387.00 [SD, 284.00]; P = 0.01). In addition, the insulin concentration corresponding to the half-maximal pAkt–tAkt response was nearly tripled after sleep restriction than after normal sleep (0.71 nM [SD, 0.27] vs. 0.24 nM [SD, 0.24]; mean difference, 0.47 nM [SD, 0.33]; P = 0.01). Finally, the maximum insulin-induced pAkt–tAkt response was reduced by 29% (106 [SD, 23] vs. 150 [SD, 23]; mean difference, −44 [SD, 36]; P = 0.02) during sleep restriction compared with normal sleep.
Figure 3. Dose–response effects of insulin on pAkt–tAkt response.
Average densitometric quantification of the dose-dependent responses of the pAkt–tAkt ratio to insulin stimulation under normal sleep and sleep restriction. Because of insufficient volume of biopsy sample, insulin concentrations of 0.25, 0.50, and 0.75 nM (under sleep restriction) and 0.50 nM (under normal sleep) in participant 6 and 10.00 nM (under normal sleep) in participant 3 were omitted. Note that pAkt and tAkt are measured using different antibodies; thus, the pAkt–tAkt ratio can vary outside of the range of 0% to 100%. Error bars are SEs of the mean. pAkt = phosphorylated Akt; tAkt = total Akt.
Total Body Insulin Sensitivity
Total body insulin sensitivity was reduced by 16% after 4 days of sleep restriction compared with normal sleep (3.77 [mU/L]−1 per min−1 [SD, 1.06] vs. 3.22 [mU/L]−1 per min−1 [SD, 1.38]; mean difference, −0.58 [mU/L]−1 per min−1 [SD, 0.51]; P = 0.02).
Discussion
We observed an approximate 30% reduction of cellular insulin sensitivity in adipocytes from subcutaneous fat samples collected in healthy, young, lean adults after 4 nights of sleep restriction compared with 4 nights of normal sleep in a randomized, crossover study. Phosphorylation of Akt, a crucial step of the phosphatidylinositol 3-kinase (PI3K) pathway that mediates most metabolic actions of insulin, was markedly impaired after sleep restriction. To our knowledge, this finding identifies for the first time a molecular mechanism that may be involved in the reduction in total body insulin sensitivity consistently observed in multiple laboratory studies of partial sleep deprivation in healthy adults (11–17), as well as in the participants of our study. Further, our finding of marked alterations in adipocyte function after experimental sleep restriction challenges the widely held belief that the primary function of sleep is the restoration of central nervous system function (26–29) and suggests that sleep may play an equally important role in peripheral energy metabolism.
The approximate 30% reduction in cellular insulin signaling in adipocytes induced by 4 nights of sleep restriction lies within the range of the difference in insulin sensitivity in adipocytes from obese versus lean participants (30) and from diabetic patients versus nondiabetic participants (31). Thus, the impairment of insulin signaling in adipocytes from persons who are chronically sleep-deprived or have sleep disorders is likely to have important metabolic consequences. The intracellular actions of insulin that are mediated by the PI3K–Akt pathway in adipose tissue include leptin secretion and lipid metabolism. Both reduced leptin levels (32, 33) and elevated free fatty acid concentrations (13) have been observed in healthy persons after experimental sleep restriction and could conceivably contribute to increased risk for obesity and diabetes.
Changes in autonomic nervous system inputs and increased activity of the hypothalamic–pituitary–adrenal axis, both of which occur after insufficient sleep (15, 17, 34, 35), are 2 mechanisms that may be responsible for the reduction in cellular insulin sensitivity in the adipocytes that we observed after experimental sleep restriction in our participants. Indeed, exogenous administration of cortisol or norepinephrine, 2 hormones that are hypersecreted when the hypothalamic–pituitary–adrenal axis and sympathetic nervous system are respectively activated, has been shown to induce total body (36–38) and cellular (39) insulin resistance.
Changes in sympathetic nervous system activity are readily transmitted to adipose tissue by means of direct innervation (40), whereas increases in circulating cortisol and norepinephrine levels result in increased binding to specific cellular receptors (41, 42). For these reasons, the adipocyte could be a particularly vulnerable cell type with respect to the deleterious effects of sleep loss on insulin sensitivity. Whether a reduction in vagal tone may also be involved is uncertain, because the evidence for parasympathetic innervation of adipocytes is still controversial (43).
Our study involved the simultaneous assessments of cellular insulin sensitivity in fat cells, total body insulin sensitivity, and sleep duration and quality over 4 nights of sleep restriction or normal sleep in young, healthy, lean participants studied under rigorously controlled caloric intake and activity conditions. Our study has several limitations. This was a single-center study with a small sample size, and our sample included only 1 woman. The findings will therefore need to be replicated in a larger and more diverse population. Sleep restriction in our study was relatively severe and maintained over a short period of 4 days. However, prospective, large epidemiologic studies (4–8, 10), as well as small laboratory experimental studies (11–17) of short duration, have been remarkably concordant in identifying insufficient sleep duration as a predictor of insulin resistance, risk for weight gain, and glucose intolerance. We assessed cellular insulin sensitivity in adipocytes by examining only pAkt. Because of the limited yield of adipose tissue from our lean participants, we could not assess other components of the insulin-signaling cascade.
The findings of our study suggest several lines of future investigation in healthy persons exposed to sleep restriction, as well as in patient populations with sleep disorders, such as sleep apnea. These include metabolic assays to examine whether sleep restriction adversely affects insulin-stimulated glucose uptake or suppression of lipolysis; assessments of components of the PI3K–Akt pathway that are upstream from the phosphorylation of Akt; assessment of the integrity of a second insulin-signaling pathway, such as mitogen-activated protein kinase activation; and measurement of insulin action in other peripheral cell types, such as liver and muscle.
In conclusion, our finding of a robust alteration in intracellular insulin signaling in a peripheral tissue that is pivotal in regulating energy balance and metabolism identifies a molecular mechanism underlying the adverse effect of sleep disturbances on insulin sensitivity. Our findings also shed novel light on the still-elusive function of sleep, traditionally conceptualized as necessary only for the brain, because they suggest that sleep plays an important role for the functional integrity of multiple peripheral cell types, as well as for whole-body energy homeostasis. From a clinical standpoint, our study provides additional evidence that insufficient sleep may contribute to the development of or exacerbate metabolic disorders. Future studies are needed to determine whether optimizing sleep duration may delay the development or reduce the severity of metabolic alterations in persons who are at increased risk for diabetes and whether maintaining sufficient sleep duration may be an important behavioral modification, in conjunction with a healthy diet and exercise, to prevent and treat obesity and diabetes.
Context
The molecular mechanisms by which impaired sleep increases insulin resistance are not known.
Contribution
In this study, an essential, early molecular step in insulin signaling was found to be reduced in the peripheral adipose tissue of volunteers whose sleep was restricted.
Implication
Sleep seems to play an important role in regulating metabolism in peripheral tissues. Understanding the molecular mechanisms by which sleep disruption increases the risk for insulin resistance may help to prevent diabetes.
—The Editors
Acknowledgment
The authors thank the nursing and dietary staff of the University of Chicago General Clinical Research Center for their expert assistance and the volunteers for participating in this study. The authors also thank Theodore Karrison, PhD, and Kristen Knutson, PhD, for their expertise and assistance in the statistical analysis of this study.
Grant Support: This research was supported by National Institutes of Health grants R01-HL086459, 5T32-HL07909, CTSA UL1-RR024999, P60-DK020595, and P50 HD-057796 and P01-AG11412 and Society in Science—The Branco Weiss Fellowship awarded to Dr. Broussard.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum_M12-0056.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Tasali (etasalimedicine.bsd.uchicago.edu) or Dr. Brady (mbradymedicine.bsd.uchicago.edu).
Author Contributions: Conception and design: J.L. Broussard, D.A. Ehrmann, E. Van Cauter, E. Tasali, M.J. Brady.
Analysis and interpretation of the data: J.L. Broussard, E. Van Cauter, E. Tasali, M.J. Brady.
Drafting of the article: J.L. Broussard, E. Van Cauter, E. Tasali, M.J. Brady.
Critical revision of the article for important intellectual content: J.L. Broussard, D.A. Ehrmann, E. Van Cauter, E. Tasali, M.J. Brady.
Final approval of the article: J.L. Broussard, D.A. Ehrmann, E. Van Cauter, E. Tasali, M.J. Brady.
Provision of study materials or patients: D.A. Ehrmann, E. Tasali. Statistical expertise: E. Van Cauter, E. Tasali.
Obtaining of funding: J.L. Broussard, D.A. Ehrmann, E. Van Cauter, E. Tasali, M.J. Brady.
Administrative, technical, or logistic support: J.L. Broussard, D.A. Ehrmann, E. Van Cauter, E. Tasali, M. Brady.
Collection and assembly of data: J.L. Broussard, D.A. Ehrmann, E. Tasali.
References
- 1.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [PMID: 12209117] [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [PMID: 16251952] [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34:452–463. doi: 10.1016/j.tins.2011.07.005. [PMID: 21840068] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–357. doi: 10.1016/j.annepidem.2008.12.001. [PMID: 19362278] [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [PMID: 19910503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao CY, Wu JS, Yang YC, Shih CC, Wang RH, Lu FH, et al. Sleep duration is a potential risk factor for newly diagnosed type 2 diabetes mellitus. Metabolism. 2011;60:799–804. doi: 10.1016/j.metabol.2010.07.031. [PMID: 20846701] [DOI] [PubMed] [Google Scholar]
- 7.Chaput JP, Després JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10:919–924. doi: 10.1016/j.sleep.2008.09.016. [PMID: 19332380] [DOI] [PubMed] [Google Scholar]
- 8.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–743. doi: 10.1016/j.beem.2010.07.001. [PMID: 21112022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [PMID: 18239586] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafalson L, Donahue RP, Stranges S, Lamonte MJ, Dmochowski J, Dorn J, et al. Short sleep duration is associated with the development of impaired fasting glucose: the Western New York Health Study. Ann Epidemiol. 2010;20:883–889. doi: 10.1016/j.annepidem.2010.05.002. [PMID: 20620078] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–273. doi: 10.1159/000158874. [PMID: 20054188] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [PMID: 20585000] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–2968. doi: 10.1210/jc.2009-2430. [PMID: 20371664] [DOI] [PubMed] [Google Scholar]
- 14.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [PMID: 19955752] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–3250. doi: 10.1210/jc.2009-0483. [PMID: 19567526] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–377. doi: 10.1093/sleep/34.3.371. [PMID: 21358855] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [PMID: 10543671] [DOI] [PubMed] [Google Scholar]
- 18.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [PMID: 18202144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [PMID: 21527729] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Weschester, IL: American Acad Sleep Medicine; 2007. [Google Scholar]
- 21.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [PMID: 2684710] [DOI] [PubMed] [Google Scholar]
- 22.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [PMID: 16531507] [DOI] [PubMed] [Google Scholar]
- 23.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [PMID: 18172212] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [PMID: 19542260] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elashoff JD. Down with multiple t-tests. Gastroenterology. 1981;80:615–620. [PMID: 7450453] [PubMed] [Google Scholar]
- 26.Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–1256. doi: 10.1038/nature04283. [PMID: 16251949] [DOI] [PubMed] [Google Scholar]
- 27.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [PMID: 16251951] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [PMID: 16376591] [DOI] [PubMed] [Google Scholar]
- 29.Vassalli A, Dijk DJ. Sleep function: current questions and new approaches. Eur J Neurosci. 2009;29:1830–1841. doi: 10.1111/j.1460-9568.2009.06767.x. [PMID: 19473236] [DOI] [PubMed] [Google Scholar]
- 30.Björnholm M, Al-Khalili L, Dicker A, Näslund E, Rössner S, Zierath JR, et al. Insulin signal transduction and glucose transport in human adipocytes: effects of obesity and low calorie diet. Diabetologia. 2002;45:1128–1135. doi: 10.1007/s00125-002-0875-9. [PMID: 12189443] [DOI] [PubMed] [Google Scholar]
- 31.Kashiwagi A, Verso MA, Andrews J, Vasquez B, Reaven G, Foley JE. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983;72:1246–1254. doi: 10.1172/JCI111080. [PMID: 6355180] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [PMID: 15531540] [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [PMID: 15583226] [DOI] [PubMed] [Google Scholar]
- 34.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [PMID: 19850688] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PMID: 9415946] [PubMed] [Google Scholar]
- 36.Darmon P, Dadoun F, Boullu-Ciocca S, Grino M, Alessi MC, Dutour A. Insulin resistance induced by hydrocortisone is increased in patients with abdominal obesity. Am J Physiol Endocrinol Metab. 2006;291:E995–E1002. doi: 10.1152/ajpendo.00654.2005. [PMID: 16772320] [DOI] [PubMed] [Google Scholar]
- 37.Fantus IG, Ryan J, Hizuka N, Gorden P. The effect of glucocorticoids on the insulin receptor: an in vivo and in vitro study. J Clin Endocrinol Metab. 1981;52:953–960. doi: 10.1210/jcem-52-5-953. [PMID: 7014590] [DOI] [PubMed] [Google Scholar]
- 38.Marangou AG, Alford FP, Ward G, Liskaser F, Aitken PM, Weber KM, et al. Hormonal effects of norepinephrine on acute glucose disposal in humans: a minimal model analysis. Metabolism. 1988;37:885–891. doi: 10.1016/0026-0495(88)90124-2. [PMID: 3047523] [DOI] [PubMed] [Google Scholar]
- 39.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [PMID: 16612386] [DOI] [PubMed] [Google Scholar]
- 40.Hücking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest. 2003;111:257–264. doi: 10.1172/JCI14466. [PMID: 12531882] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Björntorp P. Adipose tissue distribution and function. Int J Obes. 1991;15(Suppl 2):67–81. [PMID: 1794941] [PubMed] [Google Scholar]
- 42.Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, et al. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem. 1996;271:5301–5304. doi: 10.1074/jbc.271.10.5301. [PMID: 8621378] [DOI] [PubMed] [Google Scholar]
- 43.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [PMID: 20935665] [DOI] [PMC free article] [PubMed] [Google Scholar]