Abstract
A large-size blanket composed of the parallel catheters and silica core side glowing fiber is designed to substitute the hand-held point source in the photodynamic therapy treatment (PDT) of the malignant pleural or intraperitoneal diseases. It produces a reasonably uniform field for effective light coverage and is flexible to conform to anatomic structures in intraoperative PDT. The size of the blanket is 30cm×20cm. The light blanket composed of several PVC layers and a series of parallel catheters attached on both sides of the intralipid layer of 0.2% concentration. On one side of the intralipid layer, the parallel fiber catheters were attached using thermal sealing technique. On the other side, the parallel detect catheters were attached along the perpendicular direction. 0.1mm aluminum foil was used to construct the reflection layer to enhance the efficiency of light delivery. The long single side-glowing fiber goes through the fiber catheters according to the specific fiber pattern design. Compared with the prototype of the first generation, the new design is more cost-efficient and more applicable for clinical applications. The light distribution of the blanket was characterized by scanning experiments which were performed in flatness and on the curved surface of tissue body phantom. The fluence rate generated by the blanket can meet requirements for the light delivery in pleural or intraperitoneal (IP) PDT. Taking the advantage of large coverage and flexible conformity, it has great value to increase the reliability and consistency of PDT.
Keywords: Photodynamic therapy, intraperitoneal PDT, pleural PDT, light blanket
I. INTRODUCTION
Photodynamic therapy (PDT) is a treatment modality for cancer that requires light delivery, photosensitizer agent under the appropriate tumor oxygenation condition. As photosensitizer only works after they have been activated or "turned on" by certain light with localized distribution, the light delivery needs to be closely controlled to provide an efficacious treatment. PDT is used in a number of medical fields including oncology (cancer), dermatology (skin disease), and cosmetic surgery in nowadays. In oncology, it is FDA approved for non-small cell lung cancer, esophageal cancer and precancerous changes of Barrett's esophagus. Its use is also being further investigated through clinical trials in general oncology for the conditions including cancers of the cervix (mouth of uterus), prostate gland, brain, and peritoneal cavity. Adequate and homogenous light coverage of the entire tumor area can be difficult and requires, at a minimum, patient restraint. Light delivery to irregularly shaped cavities or surfaces of high curvature still remains a challenge and several approaches have been proposed [1–5].
In our pleural and intraperitoneal (IP) PDT clinical trials, a point source/balloon constructed from a modified endotracheal tube filled with intralipid scattering liquid is used to delivery light to the tissue cavities. Seven photodiode detectors were sewn to the wall of the pleural cavity for monitoring the light dose in the following sites inside of the abdominal or chest volume: Apex, anterior medial chest wall (ACW), posterior chest wall (PCW), posterior diaphragmatic sulcus (PS), anterior diaphragmatic sulcus (AS), posterior mediastinum (PM) and pericardium (Peri). The illumination was delivered under manual control until the prescribed dose was reached at all sites. The current method of light delivery and dosimetry method are highly dependent on the trace of light source movement, which comes from the experience of the surgeon.
The new applicator for light delivery needs to be investigated substitute the hand-held point source in the PDT treatment of the malignant pleural or intraperitoneal disease with more uniformity of light distribution and better conformity with the interior side of the surgical cavity. The luminous texture in literatures [4 –5], however, are currently not suitable for use in pleural and IP PDT for the several reasons, such as power energy, heat effect, water-proof design and light distribution. A 10×10cm2 light blanket composed of a series of parallel cylindrical diffusing fibers (CDF) was designed. The preliminary experiments were carried out for its characterization using motorized dosimetry system and tissue mimicking phantom [6]. The advantages are: (1) Thermal effect under high laser power input is reduced since there is negligible thermal loss at the output end of CDFs. (2) The light blanket is watertight with the polymer composite materials and thermal sealing technique. The output is independent of pressure. (3) The light distribution profile of each individual CDF can be quantified individually. The main disadvantage of the previous generation is some difficulties of fabricating a larger light blanket for the clinical treatment area, which is usually more than 8 times of the 10×10cm2. The cost will increase with the expense of the additional linear diffusers and diffusers length extension. The beam splitter with more channels and more powerful laser source are needed, correspondingly.
Based on the fiber pattern of previous generation and the characteristics of silica core side-glowing optical fiber, a new design for the optical blanket is proposed in this paper. A prototype was manufactured in cooperation with commercial companies. The mathematic model for the light intensity distributed on the surface of the blanket can be developed using the fiber side-emitting parameters. The 3D light distribution was characterized in the experiments of flatness scanning and curved/body phantom scanning. The new design is more cost-efficient and more applicable in clinical settings, compared with the previous generation. The blanket will generate the homogeneous illumination which is adaptable for the treatment area in clinic. It shows the great value for the clinical application when the treatment area is not easily accessible by collimated light sources.
II. METHODS AND MATERIALS
1. Blanket design and fabrication
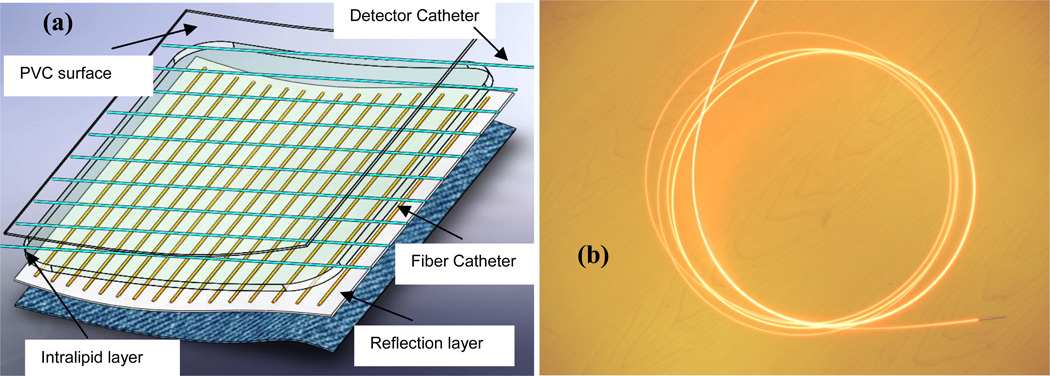
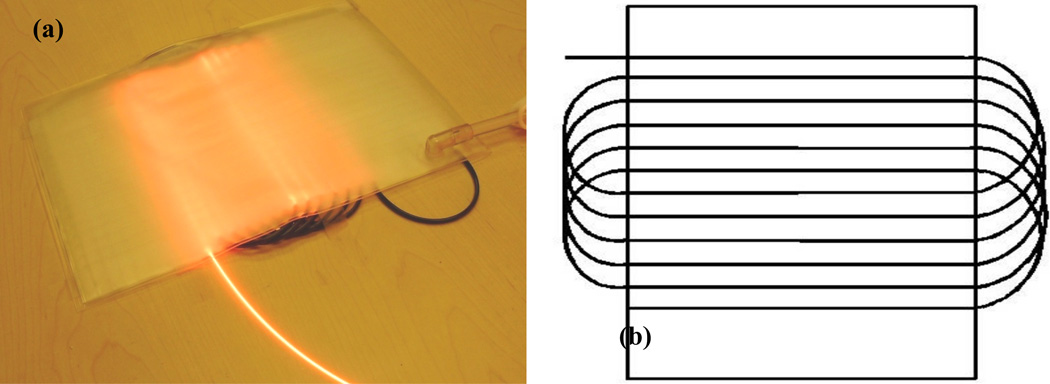
The light blanket composed of several PVC layers and a series of parallel catheters is designed (Figure 1(a)). The intralipid layer is filled with 0.2 % intralipid scattering medium to improve the uniformity of light distribution. On one side of the intralipid layer, the parallel fiber catheters (18 gage) were attached using thermal sealing technique, on the other side of the intralipid layer, the parallel detect catheters (18 gage) were attached along the perpendicular direction. 0.1mm aluminum foil was used to construct the reflection layer for the light transmission to enhance the efficiency of light delivery. The current side-glowing fiber has 400 micron core size, 16 feet length, 0.3– 0.4 numerical aperture and 20mm minimum bending radius. The reflecting end mirror enhances the linear uniformity and side-glowing intense of the fiber. A prototype blanket of 30cm×20cm was fabricated according to the design above by Bionix Co. (Toledo, OH) (Figure 2(a)). The long side-glowing fiber goes through the fiber catheters as the pattern shown in Figure 2(b). The spacing distance between adjacent catheters is 1 cm. The current active area of the blanket was ~15×20 cm2, which can be enlarged if longer side glowing fiber are used. The blanket is water-proofed, and the PVC cover is specialized for clinic. The PVC material is feasible for the sterilization in Operation Room (OR). The linear side-glowing fiber and flexible polymer materials provide the capability for the conformation with the surface of anatomic structures.
Figure 1.
(a) Schematics of light blanket design and (b) a picture of the side-glowing fiber
Figure 2.
(a) a picture of the blanket prototype (b) fiber pattern of light blanket design
2. Experimental setup to characterize light distribution of the blanket in flatness
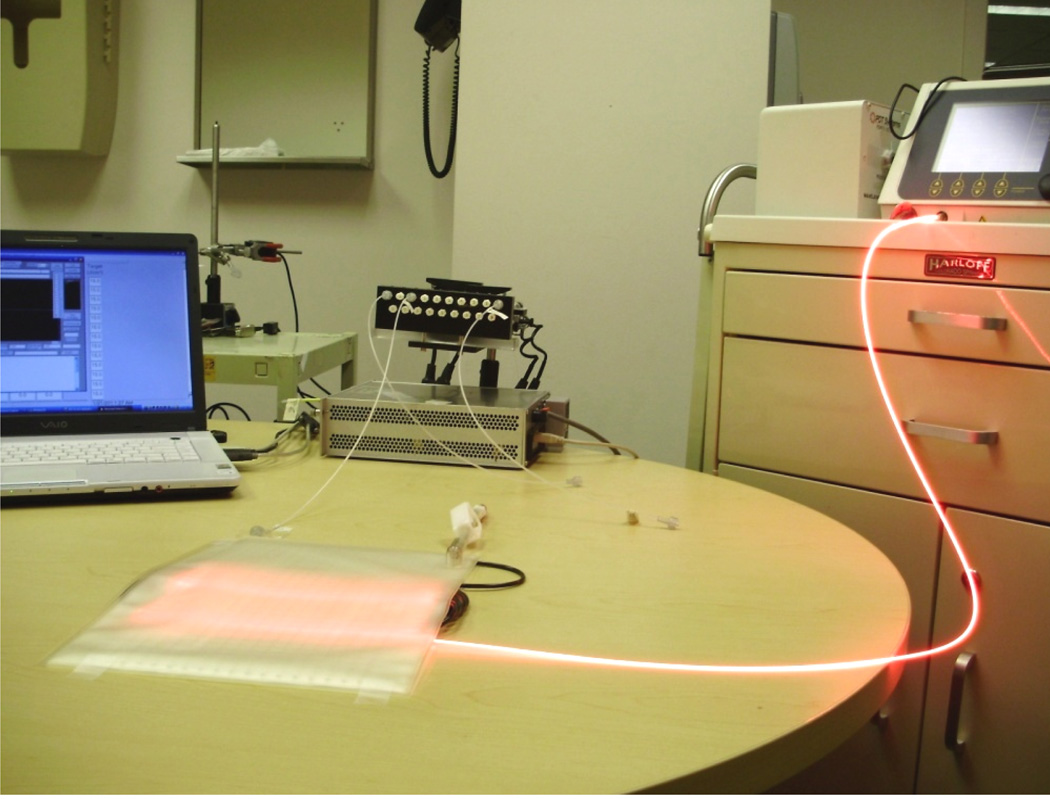
The light distribution was measured using isotropic detectors on the entire 2D plane of the light blanket in air and on the entire 2D plan with intralipid solution layer of certain concentration. 100ml intralipid with 0.2% concentration was injected into the intralipid layer. The experimental setup is shown in Figure 3. The maximum output level of 633nm laser machine is 8W. The power can be adjusted according to different conditions.
Figure 3.
Characterize light distribution in flatness
The isotropic detectors were placed in the detector catheters for the scan in air and the scan with intralipid solution layer. The scan was performed across the active area, line by line perpendicular to the fiber catheters, with the separation of 1 cm. The motorize platform pulls the detector along the catheter with the speed up to 25mm/s. There was the 0.05mm spacing between two sampling points, The whole travel range was 180mm. The detector is connected to the dosimetry system, which has the sampling rate of 1000/s. The laptop is used for the control of the motor and sampling the data which come from the dosimetry system.
3. Experimental setup to characterize light fluence rate distribution of the blanket with body phantom
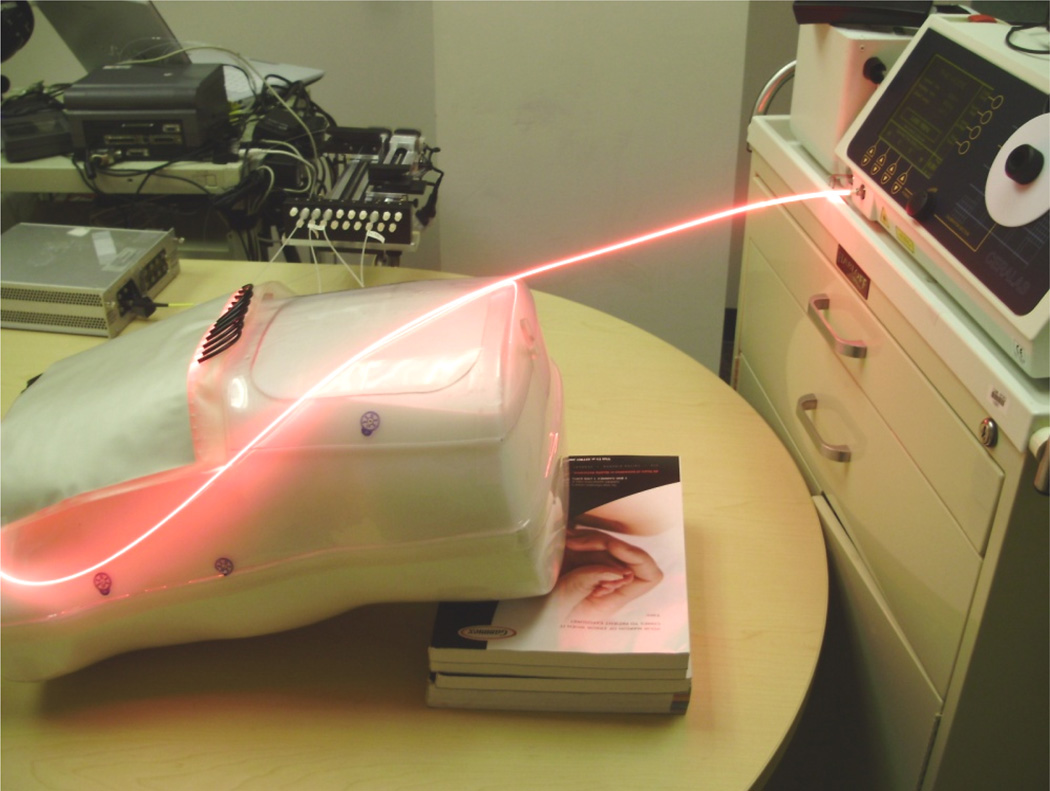
Active area of the blanket was contacting the exterior wall of the thoracic cavity. The first scan was performed with the empty body phantom as the base line for comparison. In the second scan, the body phantom was filled with 1% intralipid solution to simulate the optical properties of tissue (µa and µs´) (Figure 4). It was scanned by the isotropic detector to determine the light distribution close to the body surface of normal tissue, with full backscatter of light. As the blanket conforms to the curved surface of the thoracic cavity, it needs more intralipid to produce the scattering of uniform light coverage. 200ml intralipid with 0.2% concentration was injected into the intralipid layer in this experiment.
Figure 4.
Characterize light distribution with body phantom
III. RESULTS AND DISCUSSIONS
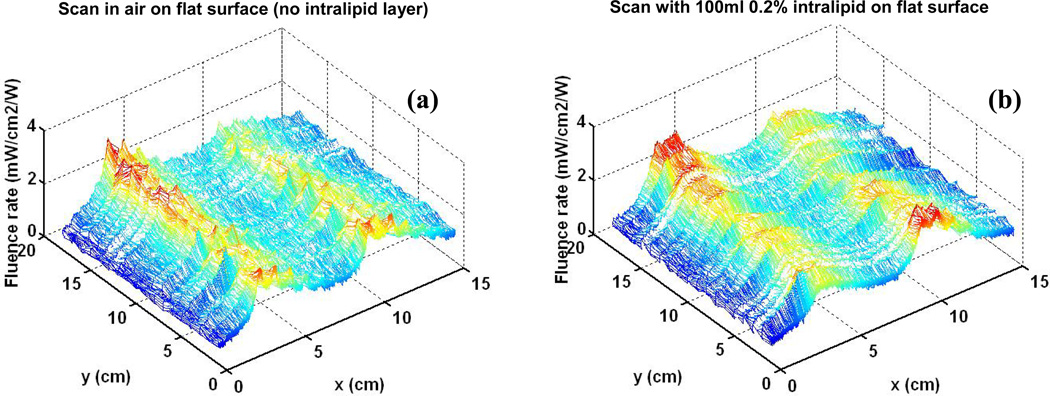
Figure 5 shows the 3D characterization for the blanket light distribution on a flat surface in air (Figure 5(a)) and with 0.2% intralipid layer (Fgure 5(b)). The average fluence rate and standard deviation are calculated using equation (1) and (2), based on the statistics of the data acquired from the dosimetry system. For the scan in air, the average of fluence rate per input power (ϕ̄) is 1.562mW/cm2/W, and the standard deviation (Dev) of fluence rate is 0.451mW/cm2/W. For the scan with intralipid layer, the the average of fluence rate per input power (ϕ̄) is 1.689mW/cm2/W, and the standard deviation (Dev) of fluence rate is 0.447mW/cm2/W.
| (1) |
| (2) |
Figure. 5.
(a) Light distribution for scan on flat surface in air (b) Scan on flat surface with introlipid
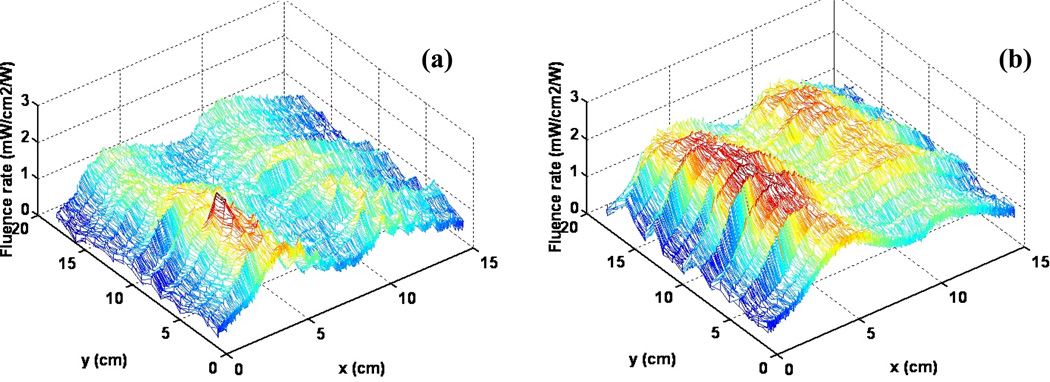
Figure 6 shows the 3D characterization for the blanket light distribution on a curved surface of the empty body phantom (Figure 6(a)) and the body phantom filled 1% intralipid (Figure 6(b)). The average fluence rate and standard deviation of the light distribution are as follows. For the scan on the surfac, the average of fluence rate per input power (ϕ̄) is 1.068mW/cm2/W, and the standard deviation (Dev) of fluence rate is 0.372mW/cm2/W. For the scan with intralipid layer, the average of fluence rate per input power (ϕ̄) is 1.671mW/cm2/W, and the standard deviation (Dev) of fluence rate is 0.505mW/cm2/W.
Figure 6.
(a) Light distribution for scan on the body phantom (b) Scan on the body phantom filled with 1% intralipid
The fluence rate generated by the blanket can meet requirements for the light delivery in pleural or intraperitoneal PDT with 1W input power under all the above experimental conditions. As shown in Figure 6(b), the light distribution is more uniform compared with the hand-held point source currently used in clinic, under the same conditions of full light backscatter of tissue.
The side-emitting uniformity of the fiber is critical to the light blanket design. A mathematic model to describe the emission of the glowing fiber was developed in literature [7]. Expressions for the emission parameters as functions of the input radiation intensity, distance along the fibre axis and scattering efficiency longitudinal distribution were given for the specific fiber fabrication. Based on the fiber model, a mathematic model for blanket light distribution will be investigated. Fiber pattern is another important factor of the light blanket design. Helical circular fiber pattern has all the fibers go through the blanket, saving more space and cost. Light distribution can also be adjusted by changing the spacing distance between the adjacent catheters in different fiber patterns.
The intralipid increase the light scattering and improves the uniform coverage of light, at the same time the concentration and amount of the intralipid will have influence on the output power of light delivered to some extent, because of absorption. 0.2% is a reasonable intralipid concentration to compromise the output power level and light uniformity for the blanket design. The amount of the intralipid will be further optimized to be adaptable to conform to the curved surface of tissue in clinical settings.
IV. CONCLUSION
Base on the previous study, a blanket composed of the parallel catheters and side glowing fiber is designed to substitute the hand-held point source in the photodynamic therapy treatment of the malignant pleural or intraperitoneal diseases. Compared with the prototype of the first generation, the new design is more cost-efficient and more applicable for clinical applications. The light blanket produces a reasonably uniform field for effective coverage and is flexible to confirm to anatomic structures. It has great value to increase the reliability and consistency in pleural and IP PDT.
In the future, various fiber patterns will be designed based on the mathematic model for the light intensity using the fiber side-emitting parameters. The thickness of the intralipid layer and concentration of the intralipid medium will be specified with the curved surface of the different anatomic structures.
ACKNOWLEDGMENT
The authors would like to thank Dr. Jarod C Finlay and Dr. Jun Li for their help in making the prototype device and for setting up measurement instruments. This work is supported by grants from National Institute of Health (NIH) P01 CA87971.
REFERENCES
- 1.Dwyer PJ, White WM, Fabian RL, Anderson RR. Optical integrating balloon device for photodynamic therapy. Lasers Surg Med. 2000;26(1):58–66. doi: 10.1002/(sici)1096-9101(2000)26:1<58::aid-lsm9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Zhu TC, Hahn SM, Kapatkin Amy S, Dimofte A, Rodriguez CE, Vulcan TG, Glaststein E, Hsir Alex. In vivo Optical Properties of Normal Canine Prostate at 732 nm Using Motexafin Lutetium– mediated Photodynamic Therapy. Photochemistry and Photobiology. 2003;77(1):81–88. doi: 10.1562/0031-8655(2003)077<0081:ivopon>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Benthem HE, Sterenborg HJ, Meulen FW, Gemert MJ. Performance of a light applicator for photodynamic therapy in the oral cavity: Calculations and measurements. Phys Med Biol. 1997;42(9):1689–700. doi: 10.1088/0031-9155/42/9/002. [DOI] [PubMed] [Google Scholar]
- 4.Selm B, Camenzind M. Flexible textile light diffuser for photodynamic therapy". SPIE proceedings: Optical Fibers and Sensors for Medical Applications. 2005:95–103. [Google Scholar]
- 5.Khana T, Unternährera M, Buchholza J, Kaser-Hotzc B, Selmd B, Rothmaierd M, Walt H. Performance of a contact textile-based light diffuser for photodynamic therapy. Photodiagnosis and Photodynamic Therapy. 2006;3(1):51–60. doi: 10.1016/S1572-1000(05)00182-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Wang K, Zhu TC. A light blanket for intraoperative photodynamic therapy. Photodynamic Therapy: Back to the Future. Proceedings of the SPIE. 2009;7380:73801W1–73801W9. doi: 10.1117/12.823064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spigulis J, Pfafrods D, Stafeckis M, Jelinska-Platace W. The glowing optical fiber designs and parameters. Proceedings of SPIE, International Society for Optical Engineering. 1997;2967:231–236. [Google Scholar]